Introduction
Immunosuppressants are usually used to prevent the
occurrence of acute rejection following organ transplantation
(1). To reduce the adverse events
associated with immunosuppressants, it is helpful to preserve the
immune system of recipients, to reduce the dose of
immunosuppressive drugs and/or to induce immune tolerance (2–5). In
order to extend the duration of graft survival and to maximally
reduce the side-effects associated with immunosuppressive agents,
the doses of immunosuppressants can be optimized by monitoring the
immunological status of transplant recipients and rejection. A
variety of monitoring methods have been reported based on different
cells and molecules involved in immune responses, including:
Cell-mediated lymphotoxicity assay (6,7);
measurement of cytokines such as interleukin (IL)-2, IL-4, IL-10,
γ-interferon, tumor necrosis factor (TNF)-α and soluble CD30
(8–11); measurement of donor-specific
antibodies in recipients' blood (12,13);
measuring polyclonal T-cell responses to nonantigen-specific
stimulations (14,15); monitoring regulatory T-cells (Tregs)
(16,17); analysis of circulating mRNA
precursors for cytokines (18,19); and
searching for rejection-related biomarkers in blood, urine or
bronchoalveolar lavage fluid using proteomic methods (18,20–22).
However, these immunological detection methods each have their own
advantages, disadvantages and limitations due to the complexity of
immune responses. In certain cases, conflicting results are
obtained (23).
Currently, the evaluation of clinical symptoms
together with graft biopsy is the most commonly used method for
monitoring a recipient's immunological status in clinical practice.
However, biopsy is an invasive procedure that has inherent
limitations. To abrogate the need for biopsy, a simple in
vivo examination method was designed in the present study to
monitor the immunological status of New Zealand white rabbits after
skin grafting, inspired by the in vivo application of
lymphocyte-mediated cytotoxicity tests.
Materials and methods
Animals
Female and male New Zealand white rabbits weighing
between 1.9 and 2.5 kg served as donors and recipients,
respectively [certification No. SCXK (Yue)-0015]. All rabbits were
purchased from the Experimental Animal Center of Southern Medical
University (Guangzhou, China), and all animal experiments were
conducted according to the ethical guidelines of Southern Medical
University.
Establishment of the skin
transplantation model
Rabbits were randomly divided into five groups,
namely the allograft rejection group, autograft tolerance group,
nontransplant (control) group, allograft low-dose immunosuppressant
group and allograft high-dose immunosuppressant group. For rabbits
in the three allograft groups, a patch of skin (3×3 cm) was cut
from the back of the donor female rabbits, and the subcutaneous
tissue was trimmed cleanly with ophthalmic scissors. Next, a patch
of skin (3×3 cm) was obtained from the recipient male rabbits
without removing the subcutaneous tissue. The donor skin graft was
fixed onto the backs of the recipients with 5-0 noninvasive
synthetic sutures. Wounds were covered with gauze and fixed with
tapes. In the autograft tolerance group, a patch of skin (3×3 cm)
was grafted in situ onto the back of the male rabbits as
described above.
Rabbits in the allograft low-dose immunosuppressant
group were treated with 2 mg/kg cyclosporine A intravenously 8 h
prior to transplantation and once a day following transplantation
for 25 days. Rabbits in the allograft high-dose immunosuppressant
group were treated with 25 mg/kg cyclosporine A intravenously 8 h
prior to transplantation and once a day following transplantation
for 25 days.
Preparation of single-cell
suspensions
On day 12 after the transplantation, splenectomy was
performed on all rabbits. Standard layered abdominal closure was
performed and the rabbits recovered uneventfully. Fluid therapies
were administered to all rabbits undergoing surgery and penicillin
(80,000 U/kg) was administered intravenously following the surgery.
In addition, samples from all skin grafts, including the rejected
grafts, were collected at the time of surgery, stained with
hematoxylin and eosin (H&E), and examined under a
microscope.
Spleens of the male and female rabbits were crushed
in RPMI-1640 medium, and the cell suspension was filtered with a
400-mesh stainless steel filter. Red blood cells were lysed using
erythrocyte lysis buffer (BD Biosciences, San Jose, CA, USA), and a
single-cell suspension was prepared with 0.01 mol/l
phosphate-buffered saline. Cells from the recipient and donor
rabbits were labeled with 0.3 and 0.6 µM carboxy fluorescein
diacetate succinimidyl ester (Molecular Probes, Thermo Fisher
Scientific, Inc., Eugene, OR, USA), respectively, at 37°C for 15–20
min. Then, 5% fetal bovine serum was added to terminate the
reaction. The cells were then resuspended and washed in
phosphate-buffered saline. The cells labeled with 0.3 and 6 µM
carboxy fluorescein diacetate succinimidyl ester were mixed in 1:1
ratio, and counted after dilution to a final concentration of
5–7×107 cells/l. The cells were examined via
fluorescence microscopy, and a trypan blue exclusion test was
performed to ensure the proportion of viable cells was >95%.
Subsequently on day 12, the single-cell suspension
(20 ml) containing 1×109 cells was injected into
recipient male rabbits via the auricular vein.
H&E staining
Samples of skin grafts (0.5×0.5 cm) were obtained
during the surgery, fixed with formaldehyde and embedded with
paraffin wax for slicing. The 5-µm slices of skin grafts underwent
H&E staining; conventional glass slides were fixed with 95%
ethanol for at ≥15 min, and then treated with water for 1 min,
hematoxylin for 10 min, running water for 15 min, eosin for 30 sec,
95% ethanol for 1 min and 100% ethanol for 2 min. The
H&E-stained slices were then observed using an XSP-BM19A
optical microscope (Shanghai Optical Instrument Factory, Shanghai,
China).
Flow cytometry
Blood samples were collected via auricular vein at
1, 2, 4 and 8 h after the infusion of the single-cell suspension.
The ratio of the two types of labeled cells in 200 µl of peripheral
blood was determined by flow cytometry. Prior to analysis, red
blood cells were lysed using erythrocyte lysis buffer at room
temperature, and the sample was subsequently washed three times in
phosphate-buffered saline. Since the ratio of positive cells was
relatively small, 100,000 cells were measured for each sample.
Calculation of the cell death
rate
The degree of rejection of allogeneic donor cells
was defined as the cell death rate (R), which was calculated using
the following formula: R (%) = (1 - number of remaining allogeneic
spleen cells/number of remaining isogeneic spleen cells) × 100.
Statistical analysis
All data were analyzed using SPSS software, version
13.0 (SPSS Inc., Chicago, IL, USA). Values were presented as means
± standard deviation. One-way analysis of variance was applied for
comparing groups. The Dunnett t-test (2-sided) was used to compare
the allograft rejection group to the other four groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Low-dose immunosuppressant has no
significant effect on graft survival, but high-dose
immunosuppressant prolongs graft survival time
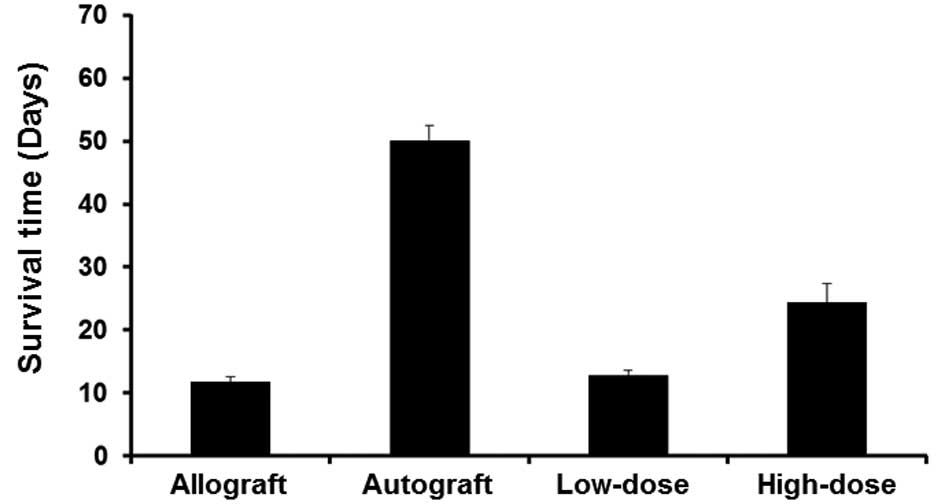
To investigate the effect of different doses of
immunosuppressant on graft survival, the number of days that the
grafts in each group survived were determined. In the allograft
rejection group, the graft turned black on day 9 after the
transplantation, indicating complete skin necrosis, with a mean
graft survival time of 13.0±1.0 days. In the low-dose
immunosuppressant group, rejection occurred on day 12 after the
transplantation with a mean survival time of 13.4±1.1 days, whereas
the mean survival time of skin grafts in the high-dose
immunosuppressant group was 23.2±1.5 days. In the autograft group,
the mean graft survival time was longer than 50 days (Fig. 1). These data indicated that low-dose
immunosuppressant had no significant effect on graft survival, but
high-dose immunosuppressant prolonged the survival time of the
graft.
High-dose immunosuppressant is more
effective than low-dose immunosuppressant in suppressing
histological changes of the skin grafts
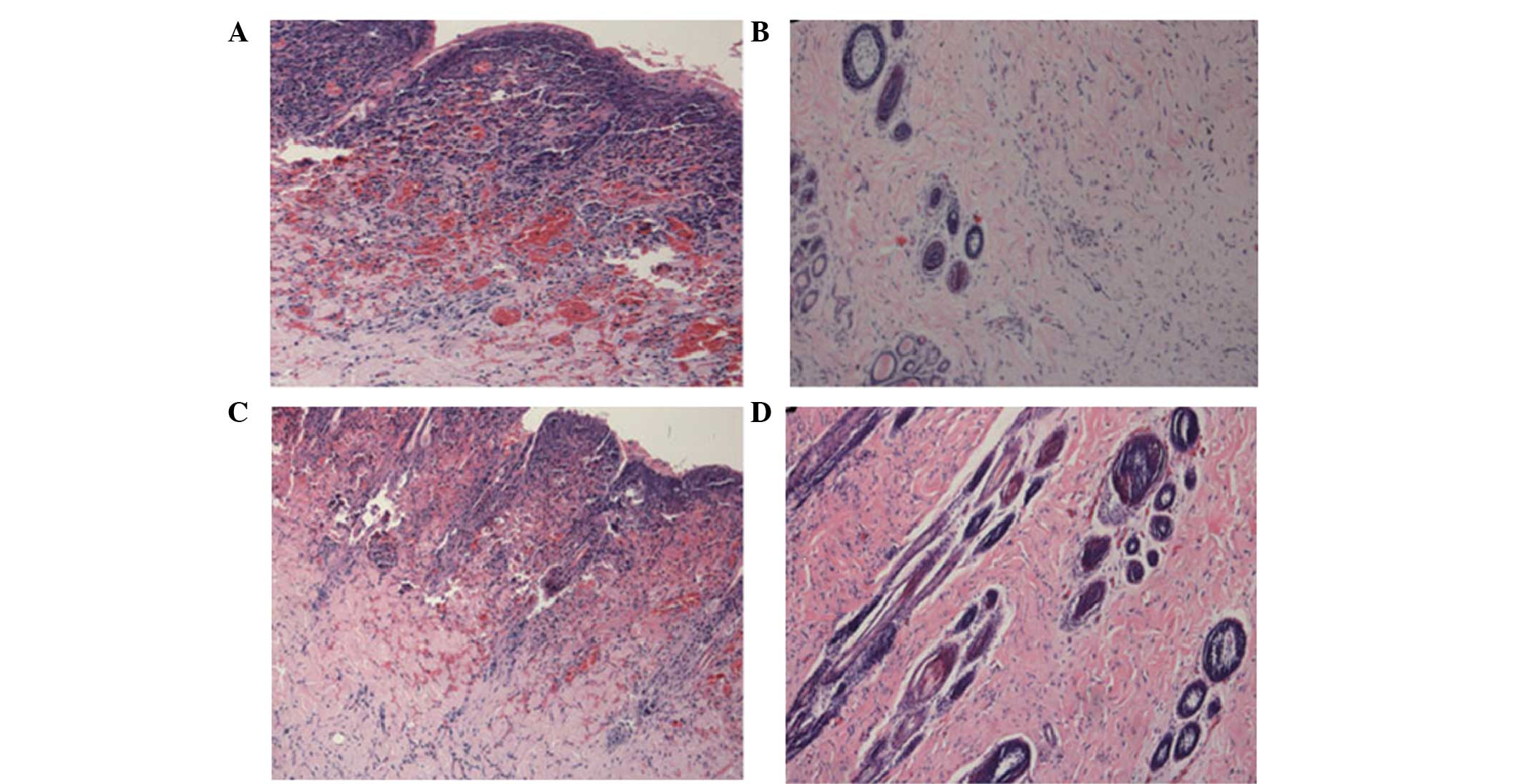
To visualize the effects of different doses of
immunosuppressant on the skin grafts, the tissues were stained with
H&E and observed under a microscope. In the allograft rejection
group and low-dose immunosuppressant group, necrosis of the skin
graft, damaged skin structure and massive lymphocytic infiltration
were observed. By contrast, in the autograft and high-dose
immunosuppressant groups, the skin grafts showed no histological
changes that were associated with necrosis. In addition, the skin
structure was normal, and no lymphocytic infiltration was observed
(Fig. 2). These data showed that
high-dose immunosuppressant was more effective than low-dose
immunosuppressant in suppressing histological changes in the skin
grafts.
Allogeneic splenic cells are
specifically destroyed in the recipients, but are rescued by
high-dose immunosuppressant
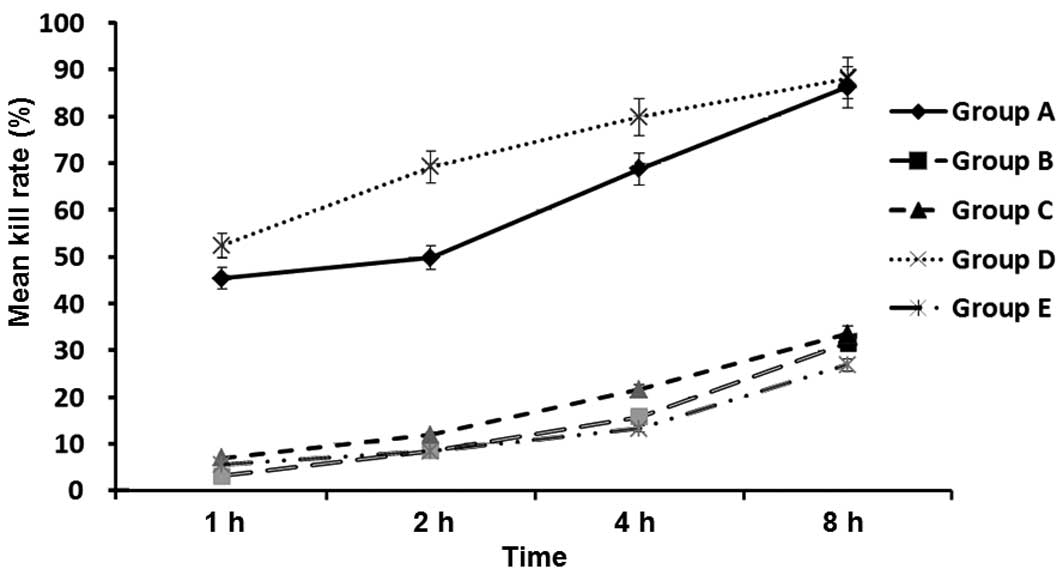
To test how different doses of immunosuppressant
affect the ratio of allogeneic splenocytes to isogeneic
splenocytes, flow cytometry was employed. In the allograft group,
the ratios of the remaining allogeneic splenocytes to the isogeneic
splenocytes (as ratios of percentages) at 1, 2, 4 and 8 h after the
injection were 3.9:7, 1.07:2.13, 0.35:1.12 and 0.09:0.41, with the
proportion of remaining allogeneic spleen cells decreasing rapidly.
In the autograft group, the ratios of the remaining allogeneic
splenocytes to the isogeneic splenocytes were 2.44:2.44, 1.50:1.64,
1.23:1.46 and 0.93:1.31, which were similar to the ratios in the
nontransplant group (3.67:3.45, 2.75:3.12, 1.92:2.45 and
1.21:1.72). In the low-dose immunosuppressant group, the results
were similar to those in the allograft group (1.65:4.52, 0.81:3.02,
0.28:1.39 and 0.11:1.07). Finally, the ratios of remaining
allogeneic spleen cells to isogeneic spleen cells in the high-dose
immunosuppressant group were 8.75:9.21, 2.65:2.86, 1.45:1.67, and
0.93:1.20, which were similar to those in the autograft and control
groups (Fig. 3). In addition,
statistical analysis showed that the cell death rate of the
allograft group was significantly different from those of all other
groups with the exception of the low-dose immunosuppressant group
at 1, 2, 4 and 8 h after injection (P<0.05), and no significant
differences were observed between the cell death rates of the
allograft and low-dose immunosuppressants group at 1, 2, 4 and 8 h
after injection (P>0.05; Fig. 4).
These data suggest that the allogeneic splenic cells were
specifically destroyed in the recipients, but were rescued by
treatment with a high dose of immunosuppressant.
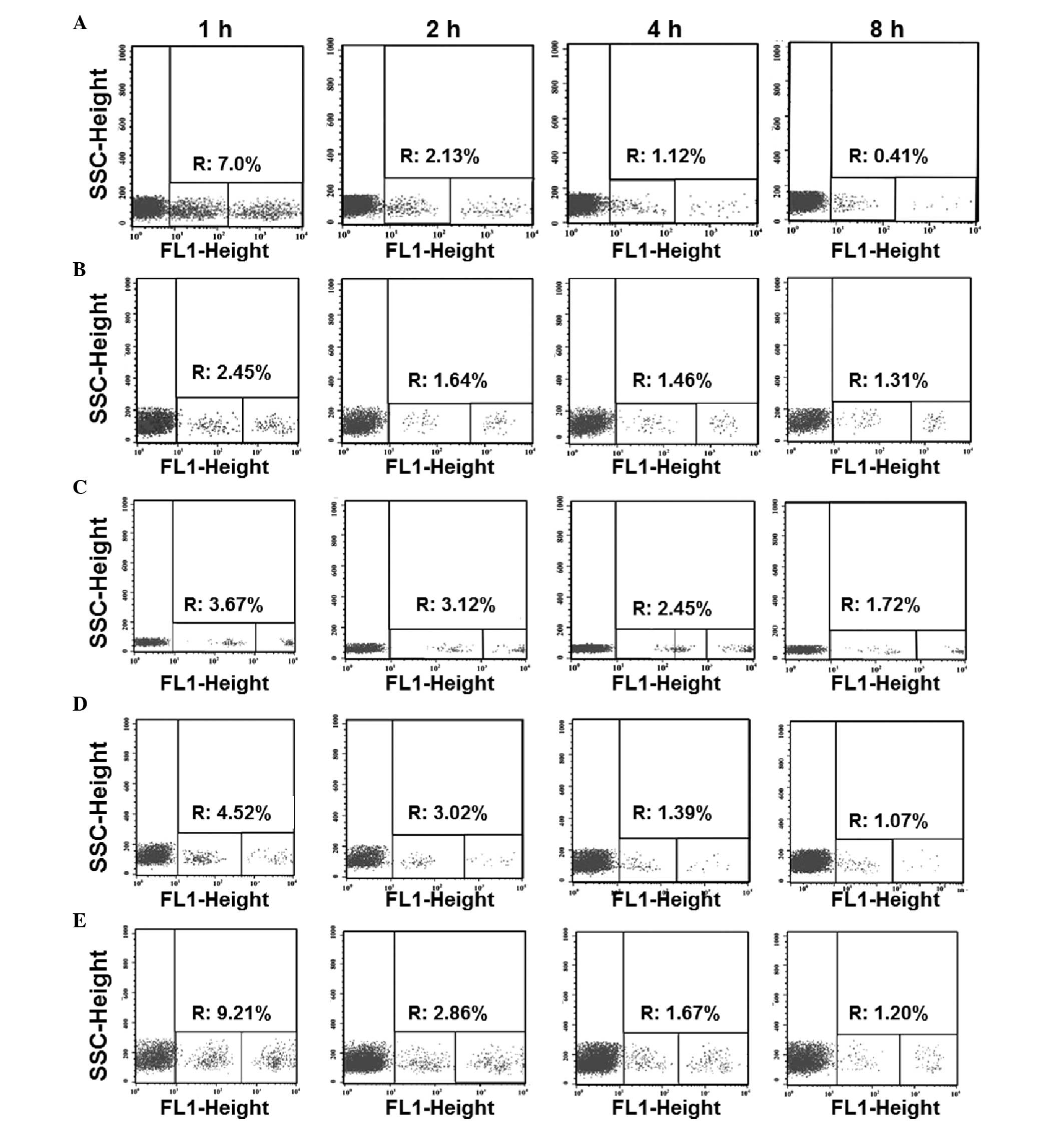 | Figure 3.Ratio of remaining allogeneic
splenocytes to isogeneic splenocytes in the (A) allograft, (B)
autograft, (C) control, (D) low-dose immunosuppressant and (E)
high-dose immunosuppressant groups at 1, 2, 4 and 8 h after
injection. Blood samples were collected via the auricular vein at
1, 2, 4 and 8 h after the infusion of a single-cell suspension of
labeled splenocytes. The ratio of the two types of labeled cells in
200 µl peripheral blood was determined by flow cytometry. For each
sample, 100,000 cells were measured. R, cell death rate; SSC, side
scatter; FL1, fluorescence intensity in X-axis. |
Discussion
The current study investigated a novel and simple
in vivo method to monitor the immunological status of skin
graft recipients using New Zealand white rabbits. Donor splenocytes
in the allograft rejection group and low-dose immunosuppressant
group were specifically destroyed by graft recipients. However,
specific loss of the labeled splenocytes was not evident in the
autograft, nontransplant or high-dose immunosuppressant groups.
Since skin transplantation induced strong rejection in the rabbits
of the allograft rejection group, the bodies maintained numerous
immune cells such as lymphocytes, natural killer (NK) cells,
antibodies and complements. When donor cells contacted the
sensitized recipient for a second time following injection of the
labeled splenocytes, cell-mediated immunity (involving lymphocytes,
NK cells, antibodies and complements) together with the humoral
immune responses, resulted in violent attacks on donor splenic
cells and severe rejection. As a result, the splenocytes were
rejected and almost completely removed by the rabbits within 8 h
following injection of the labeled splenocytes. The cell death rate
within 8 h was as high as 86.19±6.95%.
No specific destructive activity against the labeled
splenocytes occurred in the autograft and nontransplant groups,
with only gradual rejection being observed. In these groups, the
cell death rates were 31.58±3.41 and 33.51±3.49% at 8 h,
respectively. This was because the immune systems were not
‘activated’ in the two groups, and the recipients were not in a
state of sensitization, that is, no sensitized lymphocytes,
antibodies, and complements were present.
Similarly, in the low-dose immunosuppressant group,
the dosage of immunosuppressive agent was not sufficient to
effectively inhibit rejection of donor skin and splenocytes by the
recipient, resulting in the preservation of a large number of
sensitized immune mediators such as lymphocytes, complements and
antibodies. The cell death rate at 8 h was 88.14±4.21%, with no
statistically significant difference from that of the allograft
rejection group. By contrast, the recipients' immune systems were
inhibited in the high-dose immunosuppressant group, with a cell
death rate of 26.82±3.26% at 8 h, which was significantly different
from that in the allograft rejection group, but similar to those of
the nontransplant and autograft groups (P>0.05). Therefore, the
current study accurately reflected the immunological status of the
recipients following transplantation, and was able to clearly
distinguish between immune rejection and immune tolerance following
transplantation.
Fluorescence-based flow cytometry is advantageous
for use as a functional assay for specific cytotoxic T lymphocytes
(CTLs) (24,25), as it improves on the sensitivity and
specificity of in vitro CTL techniques. Fluorescence-based
flow cytometry is safe for use in clinical and laboratory
experiments and avoids radioactive contamination during
experimentation. Unfortunately, it is not able to simulate the
biological environment of the body during in vitro CTL
experiments due to numerous factors. Therefore, some researchers
have suggested using in vivo CTL experiments (26–28), in
which isogeneic target cells and antigen peptides are injected into
animal bodies to compare the in vivo CTL activity of these
‘treated’ animals with that of the controls. However, these
experiments are limited by the detection of reactions between
specific CTLs and simple antigens. Antigens for immune rejection
are complex antigens, including major histocompatibility antigen
(MHC), minor histocompatibility antigen (mH) and other antigens
that are involved in the rejection, such as ABO blood group
antigens and tissue-specific antigens. Therefore, transplantation
rejection is a complicated process involving both cell-mediated
immunity and humoral immunity against complex antigens. In
vivo experiments of lymphocyte toxicity reflect only the in
vivo destructive effect of CTL on certain specific antigens,
but cannot comprehensively reflect the rejection process.
The present study was inspired by the application of
an in vivo cytotoxicity test. Donor cell complex antigens
injected into the recipient not only reflect in vivo CTL
activity but also the effect of complicated cellular and humoral
immunity on in vivo transplantation rejection. As the
splenocytes were from donor rabbits, their antigenicity was nearly
the same as the that of the skin graft. Moreover, the number of
dead donor cells indirectly reflected the degree of transplantation
rejection, since the experiment was conducted in vivo. This
assay reflects the rejection and destructive activity of CTLs, as
well as a series of destructive effects on cellular and humoral
immunity mediated by a variety of immune molecules. Therefore, this
assay comprehensively monitors the immunological status of the
recipients. The detection method described herein proved to be
antigen-specific, and this technique appears to be an excellent
method of monitoring the immunological status of a recipient
following transplantation.
This in vivo experiment has the potential for
wider application. If a sufficient number of target cells and
isogeneic internal control cells can be obtained, the fluorescent
dye can stain all living cells in the animals (29).
In summary, the present study investigated an
experimental protocol that can accurately reflect the immunological
status of transplantation recipients and the intensity of
transplantation rejection. The method used in this study has
previously been demonstrated to be feasible in mouse skin
transplantation (30). However, the
protocol is invasive, as it requires a splenectomy in order to
create a single-cell suspension. Thus, the application of this
method remains relatively limited and further studies are
required.
Acknowledgements
This study was partly supported by the National
Natural Science Foundation of China (no.30972825).
References
|
1
|
Segoloni GP: New immunodepressant drugs
for the prevention and control of kidney transplant rejection. G
Ital Nefrol. 22:3–15. 2005.(In Italian). PubMed/NCBI
|
|
2
|
Cobbold SP, Adams E, Graca L, Daley S,
Yates S, Paterson A, et al: Immune privilege induced by regulatory
T cells in transplantation tolerance. Immunol Rev. 213:239–255.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pretagostini R, Cinti P, Lai Q, Poli L and
Berloco PB: Minimization of immunosuppressive therapy and
immunological monitoring of kidney transplant recipients with
long-term allograft survival. Transpl Immunol. 20:3–5. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quatra F, Lowenberg DW, Buncke HJ, Romeo
OM, Brooks D, Buntic RF and Baxter-Lowe LA: Induction of tolerance
to composite tissue allograft in a rat model. Microsurgery.
26:573–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tryphonopoulos P, Ruiz P, Weppler D,
Nishida S, Levi DM, Moon J, Tekin A, Velez M, Neuman DR, Island E,
Selvaggi and Tzakis AG: Long-term follow-up of 23 operational
tolerant liver transplant recipients. Transplantation.
90:1556–1561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ashokkumar C, Talukdar A, Sun Q, Higgs BW,
Janosky J, Wilson P, Mazariegos G, Jaffe R, Demetris A, Dobberstein
J, Soltys K, Bond G, Thomson AW, Zeevi A and Sindhi R: Allospecific
CD154+ T cells associate with rejection risk after
pediatric liver transplantation. Am J Transplant. 9:179–191. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weimar W, Rischen-Vos J, de Kuiper P,
Gregoor PJ, IJzermans N, van Besouw NM, et al: Tapering
immunosuppression in recipients of living donor kidney transplants.
Nephrol Dial Transplant. 19 (Suppl 4):iv61–iv63. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Amirzargar A, Lessanpezeshki M, Fathi A,
Amirzargar M, Khosravi F, Ansaripour B and Nikbin B: TH1/TH2
cytokine analysis in Iranian renal transplant recipients.
Transplant Proc. 37:2985–2987. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sengul S, Keven K, Gormez U, Kutlay S,
Erturk S and Erbay B: Identification of patients at risk of acute
rejection by pretransplantation and posttransplantation monitoring
of soluble CD30 levels in kidney transplantation. Transplantation.
81:1216–1219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cinti P, Pretagostini R, Arpino A,
Tamburro ML, Mengasini S, Lattanzi R, et al: Evaluation of
pretransplant immunologic status in kidney-transplant recipients by
panel reactive antibody and soluble CD30 determinations.
Transplantation. 79:1154–1156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Süsal C, Döhler B, Sadeghi M, Salmela KT,
Weimer R, Zeier M and Opelz G: Posttransplant sCD30 as a predictor
of kidney graft outcome. Transplantation. 91:1364–1369. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gebel HM, Bray RA and Nickerson P:
Pre-transplant assessment of donor-reactive, HLA-specific
antibodies in renal transplantation: Contraindication vs. risk. Am
J Transplant. 3:1488–1500. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Worthington JE, Martin S, Al-Husseini DM,
Dyer PA and Johnson RW: Posttransplantation production of donor
HLA-specific antibodies as a predictor of renal transplant outcome.
Transplantation. 75:1034–1040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Israeli M, Klein T, Sredni B, Avitzur Y,
Mor E, Bar-Nathen N, et al: A new parameter in immune monitoring of
pediatric liver transplantation recipients. Liver Transpl.
14:893–898. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kowalski RJ, Post DR, Mannon RB, Sebastian
A, Wright HI, Sigle G, Burdick J, Elmagd KA, Zeevi A, Lopez-Cepero
M, Daller JA, Gritsch HA, Reed EF, Jonsson J, Hawkins D and Britz
JA: Assessing relative risks of infection and rejection: A
meta-analysis using an immune function assay. Transplantation.
82:663–668. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Koshiba T, Yoshizawa A, Yonekawa Y,
Masuda K, Ito A, Ueda M, Mori T, Kawamoto H, Tanaka Y, Sakaguchi S,
Minato N, Wood KJ and Tanaka K: Analyses of peripheral blood
mononuclear cells in operational tolerance after pediatric living
donor liver transplantation. Am J Transplant. 4:2118–2125. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cortesini R, Renna-Molajoni E, Cinti P,
Pretagostini R, Ho E, Rossi P and Suciu-Foca Cortesini N: Tailoring
of immunosuppression in renal and liver allograft recipients
displaying donor specific T-suppressor cells. Hum Immunol.
63:1010–1018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wolf T, Oumeraci T, Gottlieb J, Pich A,
Brors B, Eils R, Haverich A, Schlegelberger B, Welte T, Zapatka M
and von Neuhoff N: Proteomic bronchiolitis obliterans syndrome risk
monitoring in lung transplant recipients. Transplantation.
92:477–485. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cookson S, Doherty DG, Todryk S, Gibbs P,
Portmann B, O'Grady J, et al: Hepatic expression of IL-15 mRNA is
associated with liver graft acceptance. Transpl Immunol. 11:39–48.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schaub S, Rush D, Wilkins J, Gibson IW,
Weiler T, Sangster K, et al: Proteomic-based detection of urine
proteins associated with acute renal allograft rejection. J Am Soc
Nephrol. 15:219–227. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El Essawy B, Otu HH, Choy B, Zheng XX,
Libermann TA and Strom TB: Proteomic analysis of the allograft
response. Transplantation. 82:267–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sigdel TK and Sarwal MM: The proteogenomic
path towards biomarker discovery. Pediatr Transplant. 12:737–747.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Truong DQ, Bourdeaux C, Wieërs G, Saussoy
P, Latinne D and Reding R: The immunological monitoring of kidney
and liver transplants in adult and pediatric recipients. Transpl
Immunol. 22:18–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodrigo E, López-Hoyos M, Corral M,
Fábrega E, Fernández-Fresnedo G, San Segundo D, et al: ImmuKnow as
a diagnostic tool for predicting infection and acute rejection in
adult liver transplant recipients: A systematic review and
meta-analysis. Liver Transpl. 18:1245–1253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li B, Tian L, Diao Y, Li X, Zhao L and
Wang X: Exogenous IL-10 induces corneal transplantation immune
tolerance by a mechanism associated with the altered Th1/Th2
cytokine ratio and the increased expression of TGF-β. Mol Med Rep.
9:2245–2250. 2014.PubMed/NCBI
|
|
26
|
Hermans IF, Silk JD, Yang J, Palmowski MJ,
Gileadi U, McCarthy C, et al: The VITAL assay: A versatile
fluorometric technique for assessing CTL- and NKT-mediated
cytotoxicity against multiple targets in vitro and in vivo. J
Immunol Methods. 285:25–40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ritchie DS, Hermans IF, Lumsden JM, Scanga
CB, Roberts JM, Yang J, et al: Dendritic cell elimination as an
assay of cytotoxic T lymphocyte activity in vivo. J Immunol
Methods. 246:109–117. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weist BM, Hernandez JB and Walsh CM: Loss
of DRAK2 signaling enhances allogeneic transplant survival by
limiting effector and memory T cell responses. Am J Transplant.
12:2220–2227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Barchet W, Oehen S, Klenerman P, Wodarz D,
Bocharov G, Lloyd AL, et al: Direct quantitation of rapid
elimination of viral antigen-positive lymphocytes by antiviral
CD8(+) T cells in vivo. Eur J Immunol. 30:1356–1363. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang Z, Gao Y, Pan M and Zhong L: Reagent
for monitoring immune state of rabit after skin grafting, and
preparation method thereof. Chinese Patent CN201210013937. Filed.
January 17–2012 Issued. July 18–2012
|