Introduction
As the second most common type of cancer in men
worldwide, prostate cancer mainly affects elderly male patients
(1). In the United States, prostate
cancer is the most frequently occurring tumor type in men, with
~217,730 new cases annually (2).
Prostate cancer accounts for ~11% of all types of tumor in European
men and is responsible for 9% of the cases of cancer-related
mortality (3). Patients with
prostate cancer lack specific clinical symptoms during the early
stages of the disease, meaning that, when a diagnosis of prostate
cancer is reached, the majority of patients are at an advanced
stage, when the tumor is no longer resectable. Despite the
improvement in chemotherapeutic agents, the outcome of patients
with prostate cancer remains poor, and it is therefore imperative
that new anticancer drugs are explored.
As a proteasome inhibitor, bortezomib was first
approved by the Food and Drug Administration for the treatment of
relapsed or refractory multiple myeloma (4). Bortezomib has additionally been
confirmed to elicit a good response in the treatment of certain
solid tumors (5). The drug
selectively binds to and inhibits the chymotryptic-like activity of
the proteasome at nanomolar concentrations. Proteasome inhibition
is associated with decreased tumor cell proliferation rates and
increased apoptosis (6). A previous
study indicated that Bcl-2-interacting killer (Bik)/natural born
killer (NBK) is one of the mediators of proteasome
inhibitor-induced apoptosis in several cell lines (7). Furthermore, bortezomib reverses the
proliferative and anti-apoptotic effect of neuropeptides,
particularly nuclear factor-κB (NF-κB), in prostate cancer cells
(8). By contrast, bortezomib
treatment alone is not effective for the treatment of metastatic
castration-resistant prostate cancer (9). The available results concerning the
effect of bortezomib on prostate cancer are therefore conflicting.
To help resolve this conflict, the present study further
investigated the inhibitory effect of bortezomib on prostate cancer
by treating DU145 prostate cancer cells with bortezomib and
observing the induced inhibitory effect and the rate of DU145 cell
apoptosis, with the aim of providing a theoretical basis for the
clinical treatment of prostate cancer with bortezomib.
Materials and methods
Drugs and reagents
Bortezomib was obtained from Xi'an Janssen
Pharmaceutical Co., Ltd. (Xi'an, China). MTT and propidium iodide
(PI) dye were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Fetal bovine serum and RPMI-1640 medium were obtained from
Evergreen Biotech Co., Ltd. (Hangzhou, China). The Annexin V
apoptosis kit was purchased from Unitech Biotechnology Co., Ltd.
(Nanjing, China). Rabbit monoclonal anti-human Bik (1:500),
active-caspase-3 (1:500) and β-actin (1:1,000) antibodies were
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA),
while goat anti-rabbit IgG secondary antibody (1:3,000) was
purchased from Boster Biological Technology Co., Ltd. (Wuhan,
China).
Cell culture
DU145 human prostate cancer cells were obtained from
the Chinese Academy of Medical Sciences (Beijing, China). The cells
were maintained in RPMI-1640 medium supplemented with 10% fetal
bovine serum in a chamber with 5% CO2 at 37°C.
Cell proliferation assay
DU145 cells in the logarithmic phase were seeded
into a 96-well, round-bottomed culture plate (2×104
cells per well) with the total volume of 100 µl. According to the
experimental design, the cells were divided into phosphate-buffered
saline (PBS)-treated and bortezomib-treated (0.2, 0.4, 0.8, 1.6,
3.2 and 6.4 µmol/l) groups. The cells were incubated for 0, 12, 24
and 48 h, respectively. After the indicated times, an MTT assay
microplate reader (Wuhan Boster Biological Technology Ltd., Wuhan,
China) was used to measure the absorbance value (A) at a wavelength
of 570 nm. The growth inhibition rate was calculated as follows:
Growth inhibition rate (%) = (Acontrol group -
Aexperimental group)/(Acontrol group -
Ablank group) × 100.
Cell cycle analysis
The DU145 cells were seeded into 12-well plates
(5×105 cells per well) with a 2-ml total volume and
treated with PBS or 1.6 µmol/l bortezomib. The morphology of the
anchorage-dependent cells was observed 24 h after exposure to
bortezomib. The cells were fixed with 70 ml/l cold ethanol,
digested with RNase (Wuhan Boster Biological Technology Ltd.), dyed
with PI and the cell cycle status was detected using flow cytometry
(FCM).
Cell apoptosis analysis
The DU145 cells were trypsinized 24 h after
treatment with 1.6 µmol/l bortezomib and then washed with PBS.
Those cells were then suspended in binding buffer and the number of
the cells was adjusted to 5×105/ml. Annexin
V-fluorescein isothiocyanate and 10 µl PI were added to 100 µl cell
suspension and mixed thoroughly; this mixture was then kept in the
dark for 15 min. Finally, FCM was used to detect the apoptosis rate
of the DU145 cell.
Western blot analysis
Following treatment with 1.6 µmol/l bortezomib for
24 h, the cells were washed with cold PBS and lysed in lysis buffer
containing 150 mM NaCl, 1% NP40, 50 mM Tris/HCl, 1 mM ethylene
glycol tetraacetic acid, 1 mM phenylmethanesulfonyl fluoride, 10 mM
Na4P2O7, 10 mM NaF, 1 mM
Na3VO5, 10 µg/ml leupeptin and 20 µg/ml
aprotinin (Wuhan Boster Biological Technology Ltd.). A
bicinchoninic acid assay (Pierce Biotechnology, Inc., Rockford, IL,
USA) was used to measure the total protein concentration in the
sample. Following the addition of the protein loading buffer (50 mM
Tris/HCl, 10% glycerol, 0.02% bromophenol blue, 2%
β-mercaptoethanol and 5% sodium dodecyl sulfate (SDS), pH 6.8) and
denaturation for 5 min at 95°C, 12% SDS-polyacrylamide gel
electrophoresis was used to separate 40 µg total protein from each
sample, prior to the protein being transferred onto nitrocellulose
membranes. The membranes were blocked in 5% non-fat milk for 1 h at
37°C and then incubated with the primary antibodies
(active-caspase-3, Bik and β-actin) for 24 h at 4°C. Following
incubation with the primary antibodies, the membranes were
incubated with the horseradish peroxidase-conjugated secondary
antibody for 1 h at room temperature in blocking buffer. Signal
visualization was performed using enhanced chemiluminescence, and
the data were quantified by scanning and analyzing the average
volume density of the hybridization signals, corrected against
β-actin, using Gel-Pro 3.0 image analysis software (Media
Cybernetics, Silverspring, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard error from
at least three independent experiments. Statistical analysis of the
data was performed with the Student's t-test for the
comparison of two groups or one-way analysis of variance for
multiple comparisons using SPSS 13.0 software (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Bortezomib inhibits the proliferative
activity of DU145 cells
The MTT assay results showed that the various
concentrations of bortezomib had no significant inhibitory effect
on the proliferation of DU145 cells within 12 h (P>0.05)
(Table I). After 24 h, bortezomib
inhibited the proliferation of the DU145 cells in a time- and
dose-dependent fashion (P<0.05) (Table I). A bortezomib concentration of 1.6
µmol/l was selected to perform the subsequent experiments, as the
half maximal inhibitory concentration of bortezomib against the
DU145 cells.
 | Table I.Effect of bortezomib on cell
proliferative activity. |
Table I.
Effect of bortezomib on cell
proliferative activity.
| Concentration of
bortezomib (µmol/l) | Growth inhibition
rate (%) |
|---|
|
|---|
| 12 h | 24 h | 48 h |
|---|
| 0.2 |
3.26±0.82 |
4.17±1.64 |
5.13±0.16 |
| 0.4 |
5.32±2.12 |
9.65±1.50* |
11.48±1.23* |
| 0.8 |
6.59±2.43 |
21.31±1.51* |
27.88±2.46* |
| 1.6 |
7.29±4.30 |
53.13±8.84* |
58.66±8.14* |
| 3.2 |
6.98±5.13 |
61.36±7.24* |
72.17±8.72* |
| 6.4 |
8.63±5.81* |
66.25±9.15* |
76.57±9.06* |
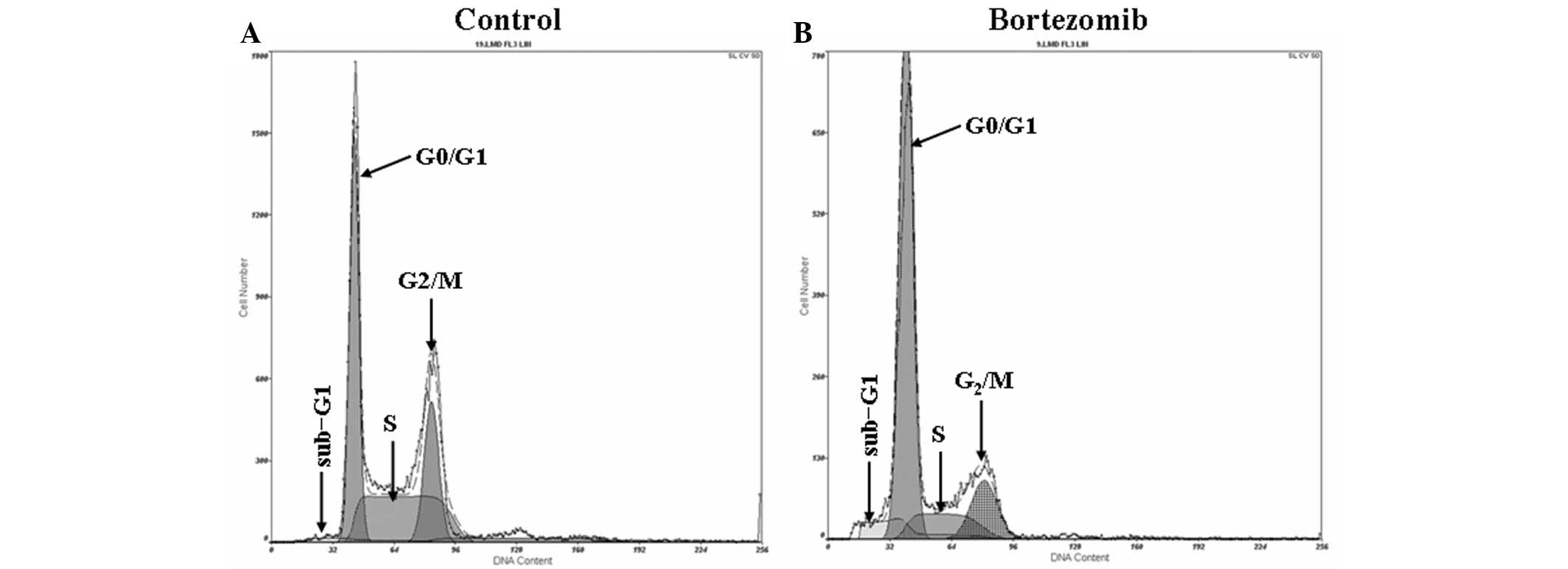
Bortezomib induces G1-phase cell cycle
arrest in DU145 cells
The results from the flow cytometric analysis are
shown in Fig. 1. After 24 h of
exposure to 1.6 µmol/l bortezomib, flow cytometric analysis
revealed a significant sub-G1 peak (apoptosis peak) in the cell
cycle of the DU145 cells (Fig. 1B).
In the control group, the proportion of cells in G0/G1, S and G2/M
phase was 48.15±4.3, 23.42±3.83 and 28.37±4.0%, respectively
(Fig. 1A), compared with 70.18±6.25,
12.60±1.72 and 17.28±6.25%, respectively, in the cells treated with
1.6 µmol/l bortezomib (Fig. 1B)
(P<0.05).
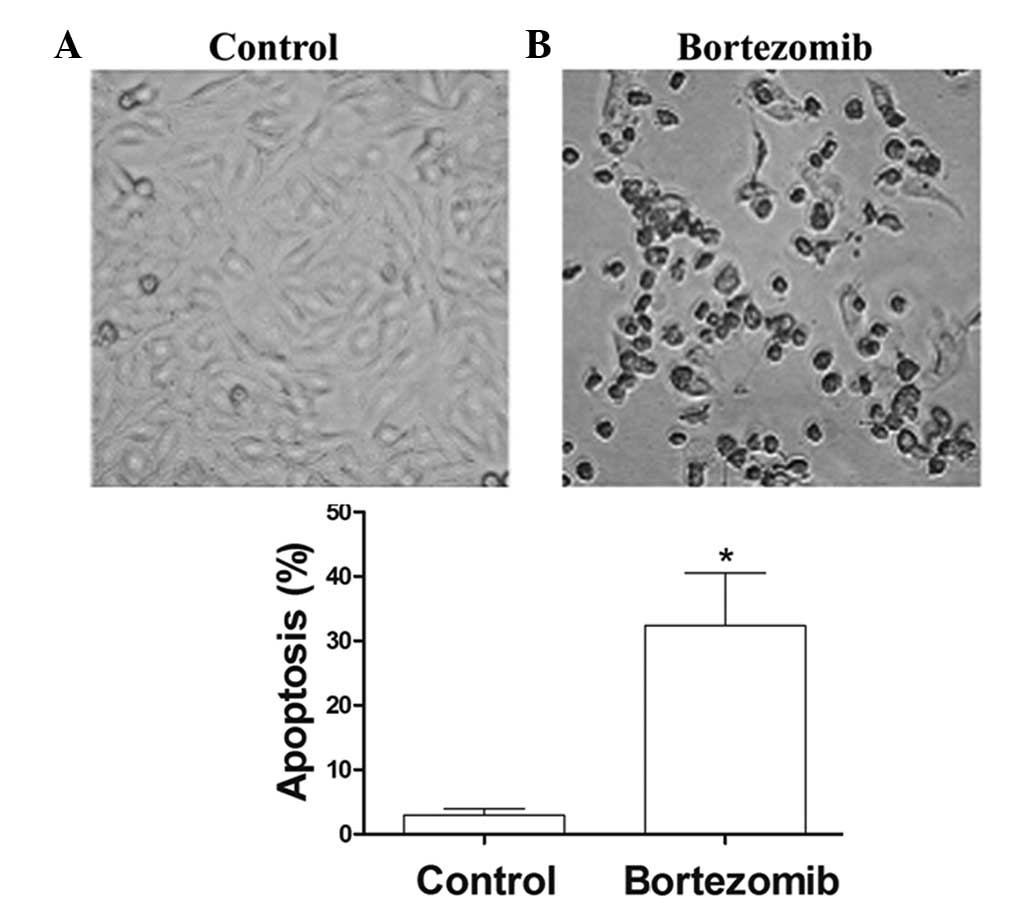
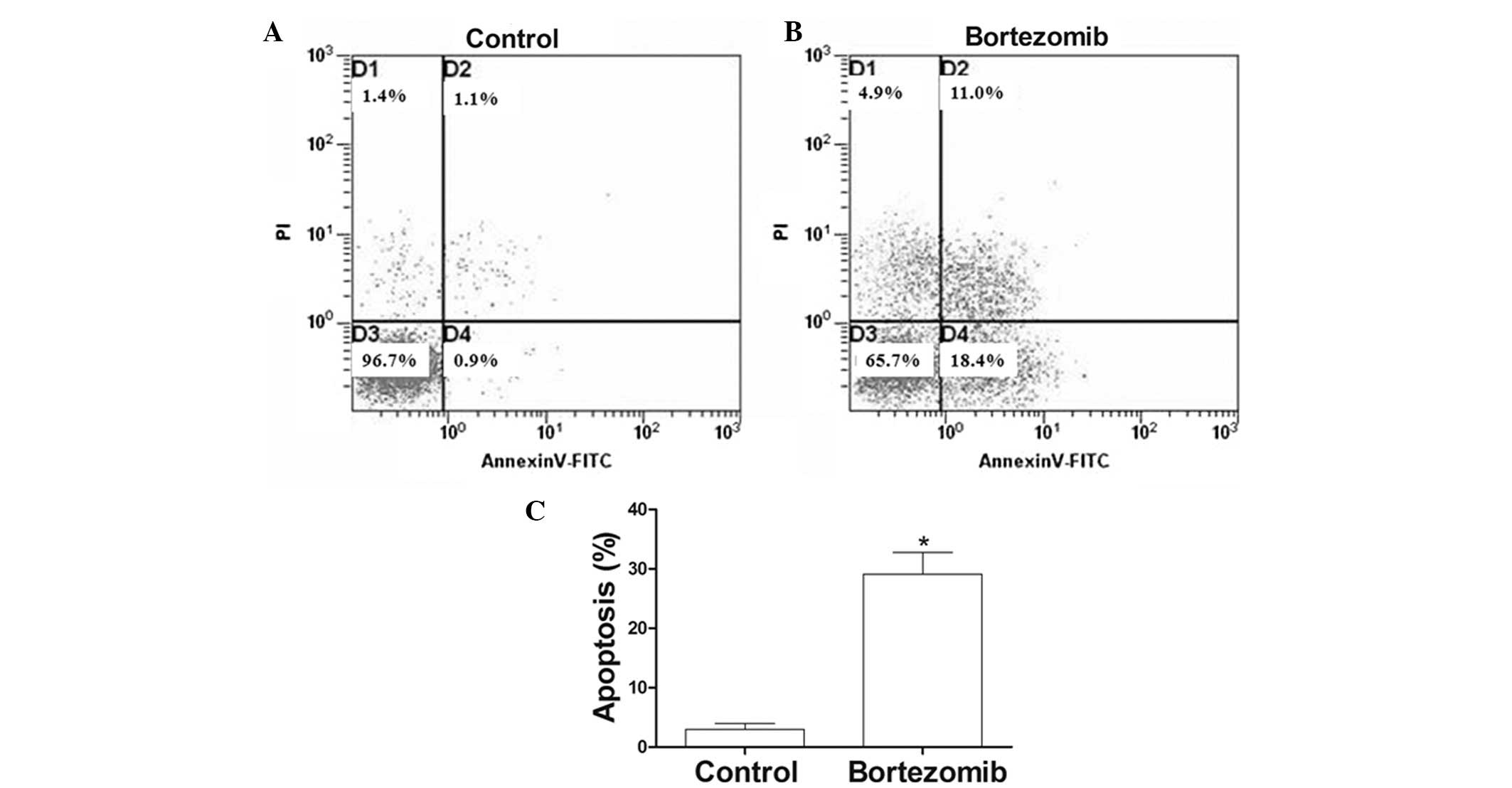
Bortezomib triggers apoptotic
morphological changes in DU145 cells
After 24 h of treatment with 1.6 µg/ml bortezomib,
the adherent cells showed morphological changes of apoptosis,
including condensed chromatin, nuclear condensation, nuclear
marginalized, nuclear fragmentation and apoptotic body formation.
These adherent cells then became crimpled, grew round and at last
detached (Fig. 2). FCM showed that,
at 24 h, the apoptosis rate of the 1.6 µmol/l bortezomib-induced
DU145 cells reached 30.1%, which was significantly higher than that
of the control group (Fig. 3A and
B).
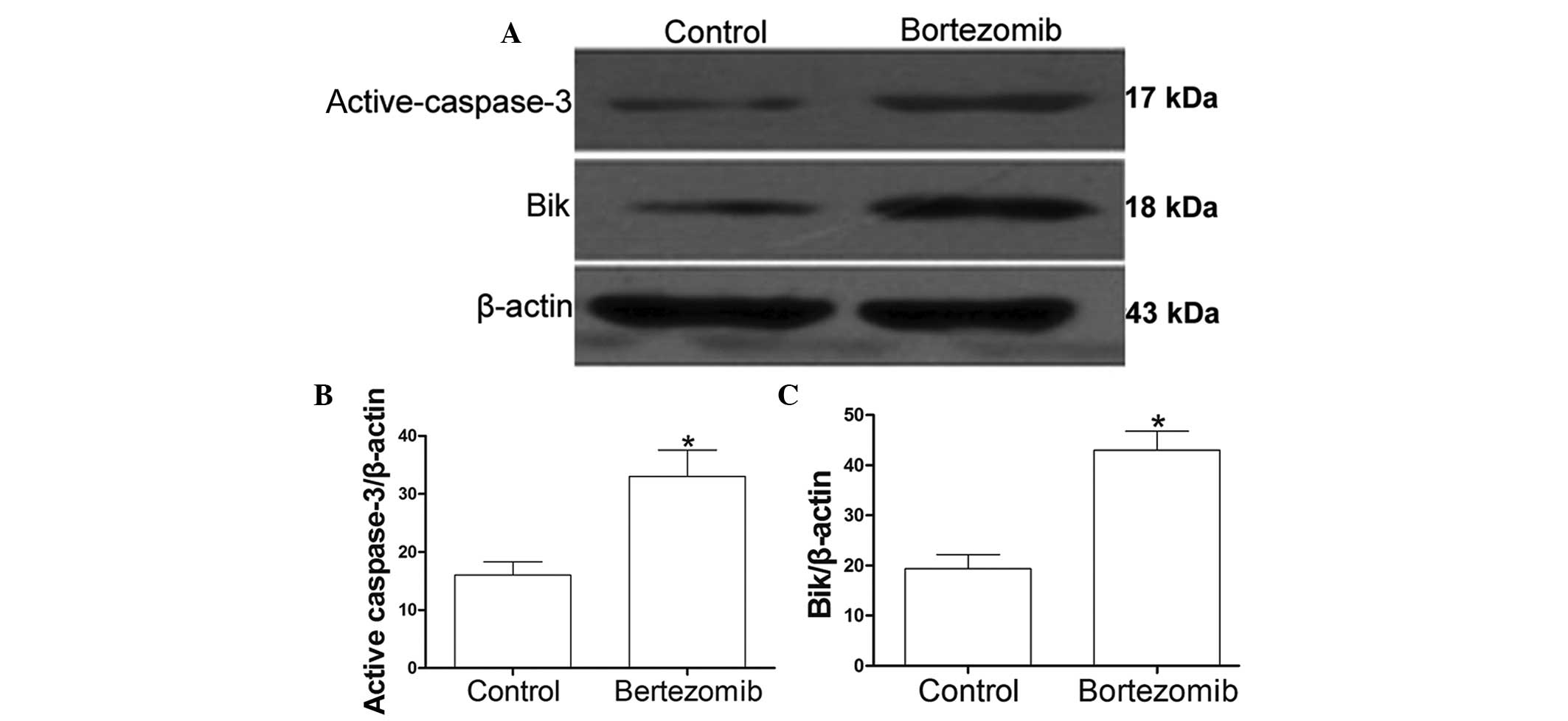
Bortezomib increases the levels of Bik
and active-caspase-3 in DU145 cells
The DU145 cells were incubated in the presence or
absence of 1.6 µmol/l bortezomib for 24 h. Western blot analysis
showed that the levels of active-caspase-3 (Fig. 4A and B) and Bik (Fig. 4A and C) in the DU145 cells were
significantly increased following treatment with bortezomib when
compared with the levels in the control group (P<0.05).
Discussion
Prostate cancer occurs mainly in elderly male
patients. The treatment of prostate cancer is closely dependent on
clinical staging and mainly includes active surveillance, radical
surgery, radiotherapy and endocrine therapy. Due to the high risk
of bone metastases associated with prostate cancer, surgical
treatment has significant limitations (10). The majority of patients with prostate
cancer that are initially treated by androgen suppression
medication experience relapse or refractory disease due to the
evolution of the tumor from hormone-dependent to
hormone-independent (11). Few
treatments are effective against androgen-independent prostate
cancer (12).
In the present study, different concentrations of
bortezomib were used to treat the DU145 prostate cancer cell line.
In the DU145 cells treated for >12 h, cell growth was
significantly inhibited in a time- and dose-dependent manner. FCM
demonstrated the occurrence of G0/G1-phase cell cycle arrest in the
bortezomib-treated group of DU145 cells. The mechanisms responsible
for this bortezomib-induced apoptosis may involve the inhibition of
the 26S proteasome, an essential element in the degradation of
intracellular proteins, including p53, NF-κB inhibitor IB and
cyclin-dependent kinase inhibitors, and particularly the repression
of the ability of NF-κB to transcribe its target genes involved in
tumor growth, angiogenesis and metastasis, such as VEGF and IL-8
(13). Bortezomib therefore induces
cell cycle arrest and apoptosis by shifting the balance between
proapoptotic and antiapoptotic signals (14), accompanying the degradation of DNA in
the target cells (15). The
evolvement of androgen-independent tumors has been associated with
several different mechanisms, such as the perturbation of mediators
of prostate cancer cell growth and tumor suppression in the cell.
The most important mediators are PTEN and HER2/neu, which act
upstream of the PI3K/Akt signaling pathway; in turn, the critical
proapoptotic signaling by Bad and p27 is deactivated and the
prosurvival NF-κB signaling is activated (16). The presence of bortezomib may thus
prevent the transformation from androgen-dependence to
androgen-independence in prostate cancer cells.
To explore the mechanisms associated with the effect
of bortezomib, the expression of the proapoptotic proteins Bik and
active-caspase-3 was examined in the present study. Enhanced Bik
protein expression can block the pathway of the antiapoptotic
proteins B-cell lymphoma 2 (Bcl-2) and Bcl-XL, and therefore
activate the mitochondrial pathway of apoptosis, which involves
cytochrome c release, and lead to apoptosis (17). Proteasome inhibitors have been
confirmed to induce the rapid aggregation of the Bik/NBK
proapoptotic proteins in the DLD-l, LoVo, SW620 and HCTI116 human
colon cancer cell lines and then promote apoptosis (7). Similarly, the Bik/NBK proteins are
activated by bortezomib in DU145 prostate cancer cells. The caspase
family plays an important role in the regulation of apoptosis.
Caspase-3 has an indispensable function in the caspase cascade
reaction and is a key enzyme and initiator of apoptosis (18). The inhibition of caspase-3 activity
significantly blocks apoptosis in vitro and in vivo
(19). In the present study it was
found that the treatment of DU145 cells with bortezomib caused an
increase in the levels of Bik and active-caspase-3, which was
responsible for the bortezomib-induced apoptosis.
In conclusion, the present study has shown that
bortezomib can inhibit prostate cancer cell growth and induce
apoptosis. The underlying mechanism may be associated with the
upregulation of Bik and active-caspase-3 expression. This finding
suggests the potential promise of bortezomib in the treatment of
prostate cancer.
Acknowledgements
This study was supported by a grant from National
Natural Science Foundation (no. 81460067).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Sankila R, Ferlay J and Parkin DM:
Estimates of cancer incidence and mortality in Europe in 1995. Eur
J Cancer. 38:99–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mateos MV, Hernandez JM, Hernandez MT, et
al: Bortezomib plus melphalan and prednisone in elderly untreated
patients with multiple myeloma: Results of a multicenter phase 1/2
study. Blood. 108:2165–2172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ryan DP, O'Neil BH, Supko JG, et al: A
Phase I study of Bortezomib plus irinotecan in patients with
advanced solid tumors. Cancer. 107:2688–2697. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Voorhees PM and Orlowski RZ: The
proteasome and proteasome inhibitors in cancer therapy. Annu Rev
Pharmacol Toxicol. 46:189–213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu H, Zhang L, Dong F, et al: Bik/NBK
accumulation correlates with apoptosis-induction by Bortezomib
(PS-341, Velcade) and other proteasome inhibitors. Oncogene.
24:4993–4999. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsapakidis K, Vlachostergios PJ,
Voutsadakis IA, et al: Bortezomib reverses the proliferative and
antiapoptotic effect of neuropeptides on prostate cancer cells. Int
J Urol. 19:565–574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morris MJ, Kelly WK, Slovin S, et al: A
phase II trial of Bortezomib and prednisone for castration
resistant metastatic prostate cancer. J Urol. 178:2378–2383. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ulmer JB, Wahren B and Liu MA: DNA
vaccines: Recent technological and clinical advances. Discov Med.
6:109–112. 2006.PubMed/NCBI
|
|
12
|
Nasu Y and Kumon H: Prostate cancer gene
therapy. Nihon Rinsho. 63:485–490. 2005.(In Japanese). PubMed/NCBI
|
|
13
|
Chen Z, Hagler J, Palombella VJ, et al:
Signal-induced site-specific phosphorylation targets I kappa B
alpha to the ubiquitin-proteasome pathway. Genes Dev. 9:1586–1597.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adams J: The development of proteasome
inhibitors as anticancer drugs. Cancer Cell. 5:417–421. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fan XM, Wong BC, Wang WP, et al:
Inhibition of proteasome function induced apoptosis in gastric
cancer. Int J Cancer. 93:481–488. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Papandreou CN and Logothetis CJ:
Bortezomib as a potential treatment for prostate cancer. Cancer
Res. 64:5036–5043. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang DC and Strasser A: BH3-only proteins
- essential initiators of apoptotic cell death. Cell. 103:839–842.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumar S: Caspase function in programmed
cell death. Cell Death Differ. 14:32–43. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de Oca J, Azuara D, Sanchez-Santos R, et
al: Caspase-3 activity, response to chemotherapy and clinical
outcome in patients with colon cancer. Int J Colorectal Dis.
23:21–27. 2008. View Article : Google Scholar : PubMed/NCBI
|