Introduction
Rheumatoid arthritis (RA) is a chronic systemic
disease that leads to inflammation and tissue damage in joints and
is characterized by symmetrical joint alteration; however, the
etiology of the condition has yet to be fully elucidated. The
temporomandibular joint (TMJ) is known to be frequently affected by
RA (1,2), with reported frequencies of TMJ
involvement varying between 2 and 86% (2,3). TMJs
afflicted with RA may be associated with pain, swelling,
crepitation, stiffness on opening the mouth and limitation of
movement (4). The initial changes
that can be observed in the degenerating TMJ are proteoglycan
degradation and softening in the fibrocartilage of the condylar and
articular eminence (5). Severe
destruction of the cortical and subcortical bone can ultimately
lead to almost complete loss of the condyle. These changes are
followed by the exposure of subchondral bone and bone resorption by
osteoclasts (6).
Osteoclasts are multinucleated cells responsible for
bone resorption. The identification and quantification of
osteoclasts, as well as their development in osteoclastogenic
clusters and bone, require cell markers that are specific to this
type of cell; therefore, tartrate-resistant acid phosphatase
(TRAP), the vitronectin receptor (VNR) and the calcitonin receptor
(CTR) are frequently utilized in osteoclast identification
(7). The expression of TRAP and VNR,
however, can be observed prior to the expression of CTR and
indicates the presence of cells at an intermediate stage of
differentiation (8). Furthermore,
TRAP and VNR are expressed by macrophages, which can cause
complications in long-term bone marrow cultures in which both
osteoclasts and macrophage polykaryons are formed. As a result of
the issues associated with these phenotypic markers, the
determination of CTR expression is currently considered to be the
only approach suitable for providing both proof of the presence of
osteoclasts and the quantitative data associated with these
osteoclasts (9). Furthermore, the
identification of mononuclear osteoclasts is, at present, only
possible through the detection of cells expressing CTR (8). Although the CTR is known to play a role
in bone resorption in other joints (10), the importance of its role in the TMJ
arthritis model has not yet been elucidated.
Bone remodeling is one of the main metabolic
activities necessary for maintaining the normal structure and
function of bones. Osteoclasts play an important role in these
processes; osteoprotegerin (OPG), receptor activator of nuclear
factor-κB (RANK), and RANK ligand (RANKL) co-regulate the functions
of osteoclasts. Osteoclastic bone destruction is induced by RANKL,
while OPG acts as a decoy receptor and prevents RANKL binding to
RANK, its receptor, thus limiting bone destruction. The activity of
osteoclasts is likely to depend, at least partially, on the
relative balance existing between RANKL and OPG expression, and
studies have suggested the involvement of RANKL and OPG in the
pathogenesis of RA (11,12). An increase in the RANKL:OPG
expression ratio in the microenvironment of a joint with RA
suggests a major role of the RANKL-OPG pathway in RA pathogenesis,
as it has the potential to induce osteoclastogenesis (13).
The RANKL:OPG expression ratio in the local bone
microenvironment determines the direction of osteoclast
differentiation. Only CTR expression unambiguously identifies
osteoclasts and distinguishes them from macrophage polykaryons.
Several studies have described the involvement of the CTR and the
RANKL:OPG expression ratio in the pathogenesis of bone-destructive
RA in other joints (14–16), but the available data for their role
in the TMJ are limited. The aim of the present study, therefore,
was to investigate the involvement of osteoclasts at sites of bone
erosion using the osteoclast-specific marker CTR expression and to
determine the RANKL:OPG expression ratio in the TMJ.
Materials and methods
Animals
A total of 48 male Wistar rats (Laboratory Animal
Center of Jilin University, Changchun, China) aged ~5 weeks and
weighing 105–135 g, were included in this study. All experimental
procedures complied with the requirements of the Animal Care and
Use Committee of Laboratory Animal Center of Jilin University. All
rats were allowed to adapt to the facilities for one week and were
then randomly separated into two groups with 24 individuals: i)
Control group (healthy subjects), and ii) collagen-induced
arthritis (CIA) group.
Induction of CIA
The induction of arthritis was performed as
previously described (17). Briefly,
the CIA model was established by intradermally injecting the rats
at the base of the TMJ with 100 µl of an emulsion that contained
100 µg bovine type II collagen (2 mg/ml; Sigma-Aldrich, St. Louis,
MO, USA) dissolved in 0.05 M acetic acid and an equal volume of
complete Freund's adjuvant. Following confirmation of the
establishment of mononuclear cell infiltration, synovial lining and
villous hyperplasia and pannus formation, the unilateral
intra-articular injection of emulsion into the TMJ was performed.
Saline was injected into the contralateral joint as sham
induction.
Hematoxylin and eosin (HE)
staining
The animals were sacrificed using an overdose of
sodium pentobarbital (Nembutal®; Abbott Laboratories, Abbott Park,
IL, USA). Six rats were sacrificed at 0, 2, 4 and 6 weeks after the
induction of arthritis. Bilateral samples of the TMJ were
collected, fixed in 4% paraformaldehyde for 24 h, decalcified with
EDTA solution (10%) and embedded in paraffin. Sagittal sections
measuring 4 mm were cut in series, and HE was used to stain every
10th section. Adjacent sections to those used for HE staining were
used for immunohistochemical analysis. Endogenous peroxidase
activity was terminated using 3% H2O2 in
methanol, and the sections were then incubated overnight with
primary antibodies: 10 mg/ml goat anti-rabbit interleukin (IL)-1β
or 5 mg/ml sheep anti-rabbit IL-1 receptor antagonist at 4°C
(18). For the negative control,
normal rabbit IgG (CST Biological Reagents Company Ltd., Shanghai,
China) was used as the primary antibody. Following incubation with
the primary antibody, the sections were incubated for 30 min in 5
mg/ml biotinylated rabbit anti-goat or anti-sheep IgG (Vector
Laboratories, Burlingame, CA, USA) and then for a further 30 min
using an Elite ABC® kit (Vector Laboratories). Diaminobenzidine
(DAB Reagent Set; KPL, Inc., Gaithersburg, MD, USA) was used as a
chromogen, and the sections were counterstained with HE. In
addition, TRAP was detected in the paraffinized tissue sections
using a commercial acid phosphatase leukocyte kit
(Sigma-Aldrich).
Enzyme-linked immunosorbent assay
(ELISA)
Serum concentrations of tumor necrosis factor-α
(TNF-α) and IL-1β were measured by ELISA as previously described
(19). Blood samples were collected
by cardiac puncture at 0, 2, 4 and 6 weeks, and the serum
concentrations of TNF-α and IL-1β were measured using rat TNF-α and
IL-1β double-antibody sandwich ELISA kits (Endogen, Inc.,
Cambridge, MA, USA), in accordance with the manufacturer's
instructions. The mean of three assays was used in the
analysis.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from the synovial membrane of
the TMJ using TRIzol® reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). The RT-PCR was conducted in accordance with a
previously described method (20).
The primers used in this study are listed in
Table I. The PCR consisted of
denaturation at 94°C for 1 min (RANKL), 95°C for 40 sec (OPG and
β-actin) or 94°C for 40 sec (GAPDH and CTR), followed by annealing
at 58°C for 1 min (RANKL), 60°C for 1 min (OPG and GAPDH) or 60°C
for 1 min (CTR and β-actin) for 30, 28 and 30 cycles, respectively,
with a final extension step at 72°C for 10 min. The reproducibility
of the RT-PCR results was ensured by repeating each experiment
three times. A sample of PCR-amplified product (6 µl) was subjected
to 2% agarose gel electrophoresis for quantification, and the bands
were visualized using ethidium bromide. The RANKL:β-actin,
OPG:β-actin and CTR:GAPDH ratios were calculated for each lane and
used to compare the mRNA expression of RANKL, OPG and CTR at
different time-points.
 | Table I.Sequences and expected fragment sizes
of synthetic oligonucleotides used for the reverse
transcription-polymerase chain reaction. |
Table I.
Sequences and expected fragment sizes
of synthetic oligonucleotides used for the reverse
transcription-polymerase chain reaction.
| Gene | Primer sequence | Product (base
pairs) |
|---|
| RANKL | Sense:
5′-GAGACTACGGCAAGTA-3′ | 822 |
|
| Antisense:
5′-CCTCCAACGTTTATGG-3′ |
|
| OPG | Sense:
5′-TGGAGCTCGAATTCTGCTTG-3′ | 603 |
|
| Antisense:
5′-CATCAAGATGCGGAGCTGCT-3′ |
|
| β-actin | Sense:
5′-GAAATCGTGCGTGACATTAAG-3′ | 410 |
|
| Antisense:
5′-CTAGAAGCATTTGCGGTGCA-3′ |
|
| CTR | Sense:
5′-ACACCCTGACAGCAACCGAACCT-3′ | 447 |
|
| Antisense:
5′-GAACCCCCAGCCAAGTAAATAGTA-3′ |
|
| GAPDH | Sense:
5′-TATTGGGCGCCTGGTCACCA-3′ | 746 |
|
| Antisense:
5′-ACTACTGTAGTTCTTCCACC-3′ |
|
Statistical analysis
Statistical analysis was conducted using SPSS
software for Windows (version 11.5; SPSS, Inc., Chicago, IL, USA).
All data are presented as the mean ± standard error of the mean.
Differences between groups were analyzed using two-way analysis of
variance, and P<0.05 was considered to indicate a statistically
significant difference.
Results
Histological examination of joints
with CIA
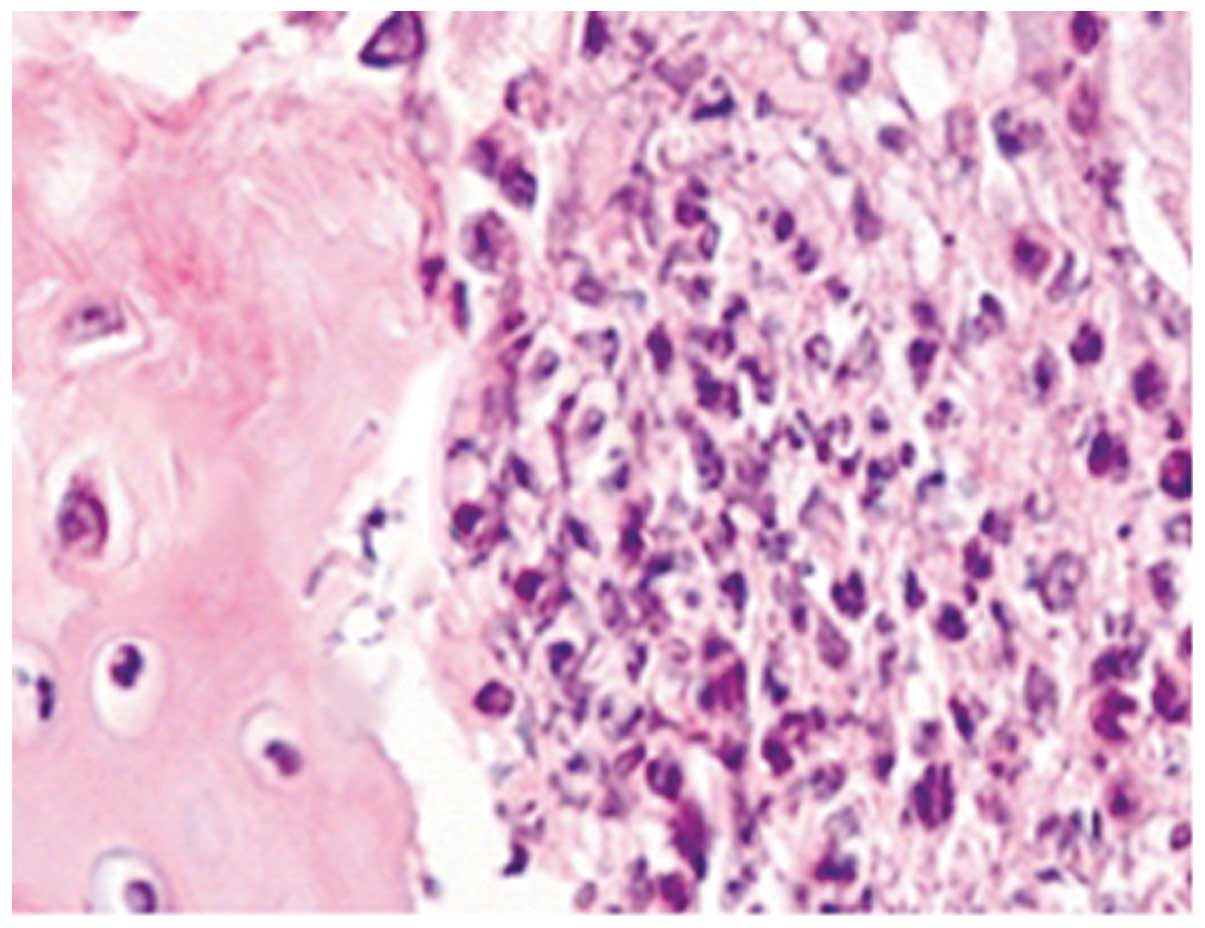
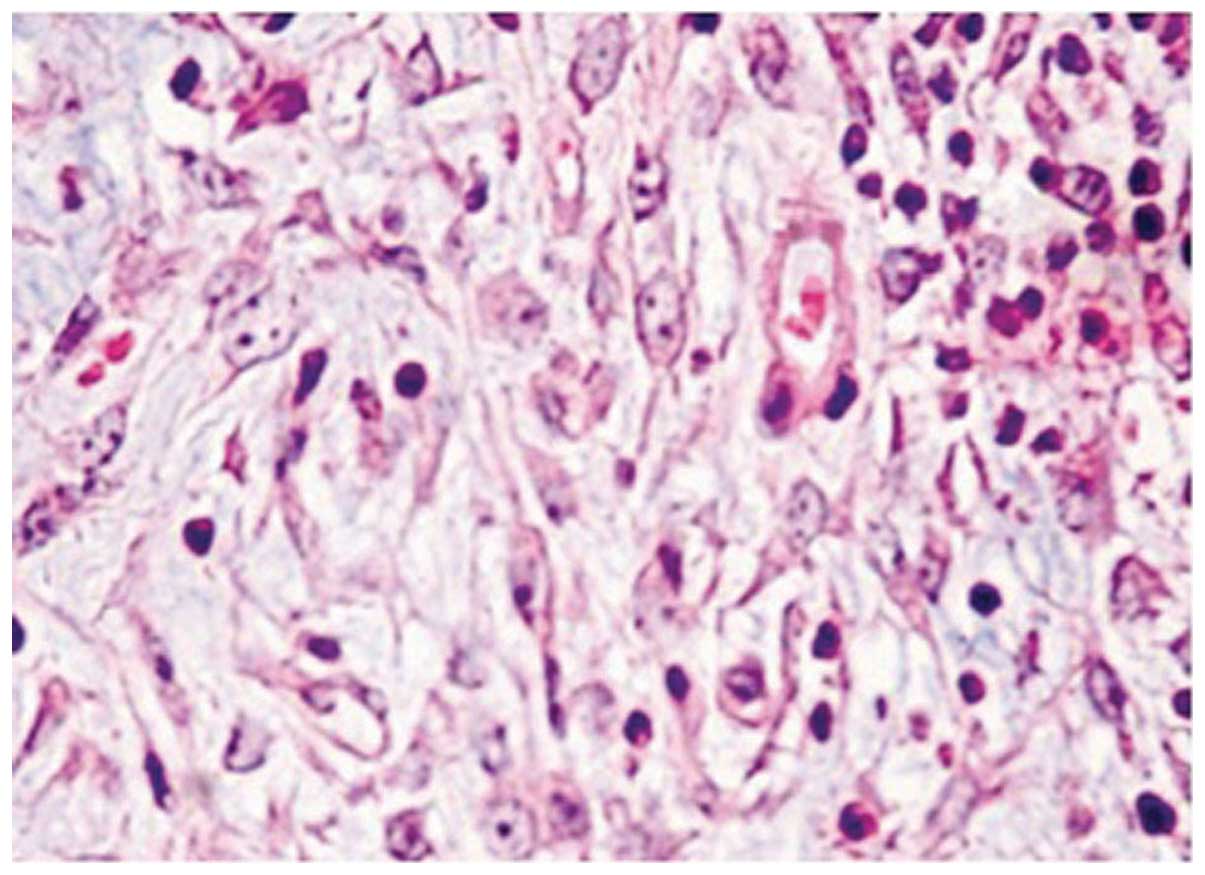
HE staining of TMJs in the CIA group showed severe
synovitis, including destruction of the cartilage and bone by
pannus tissue, which consisted of a hyperplastic synovium with
fibroblast-like cells, multiple polymorphonuclear leucocytes and a
limited number of mononuclear lymphocytes. Areas of severe
synovitis exhibited lining cell hypertrophy and the invasion of
spindle-shaped synovial cells into the stromal sublining.
Furthermore, the proliferation of spindle-shaped fibroblast-like
cells and inflammatory cell infiltration were noted in the pannus
tissue on the articular surface, and multiple TRAP-positive,
mature, multinucleated osteoclasts were observed, predominantly on
the eroded surface of the bone (Figs.
1–3).
Analysis of TNF-α and IL-1β
As shown in Table
II, the serum levels of TNF-α in the collagen-induced joints
were significantly increased compared with those in the control
joints. The 2-week period after the induction of arthritis is
considered to be the acute stage of the response. Table III shows that the serum levels of
IL-1β in the collagen-induced joints were significantly increased
compared with those in the control joints. The serum levels of
IL-1β and TNF-α peaked 4 weeks after the induction of
arthritis.
 | Table II.Serum levels of tumor necrosis
factor-α (pg/ml) in condylar cartilage of control and
collagen-induced joints. |
Table II.
Serum levels of tumor necrosis
factor-α (pg/ml) in condylar cartilage of control and
collagen-induced joints.
| Group | Week 0 | Week 2 | Week 4 | Week 6 |
|---|
| Healthy control | 25.68±1.28 | 26.86±2.68 | 27.38±4.20 | 27.16±2.78 |
| Collagen-induced
joints | 26.12±0.36 | 29.78±1.18 |
37.91±2.33a |
12.16±2.13a |
 | Table III.Serum levels of interleukin-1β (pg/ml)
in condylar cartilage of control and collagen-induced joints. |
Table III.
Serum levels of interleukin-1β (pg/ml)
in condylar cartilage of control and collagen-induced joints.
| Group | Week 0 | Week 2 | Week 4 | Week 6 |
|---|
| Healthy control | 3.69±0.46 | 4.65±1.13 | 4.26±0.52 | 4.19±2.23 |
| Collagen-induced
joints | 3.76±0.34 |
10.25±1.54a |
16.18±1.20a |
12.16±2.13a |
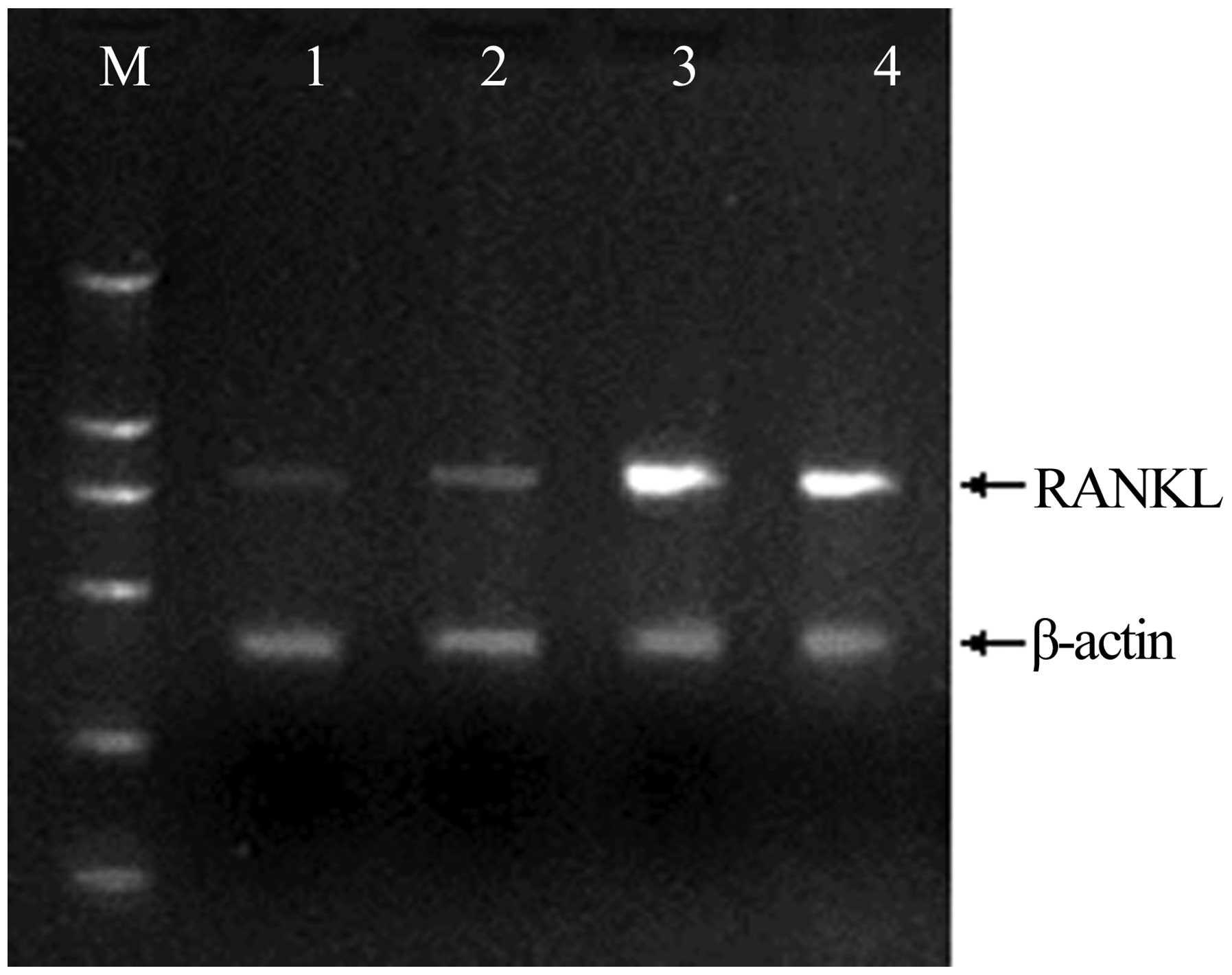
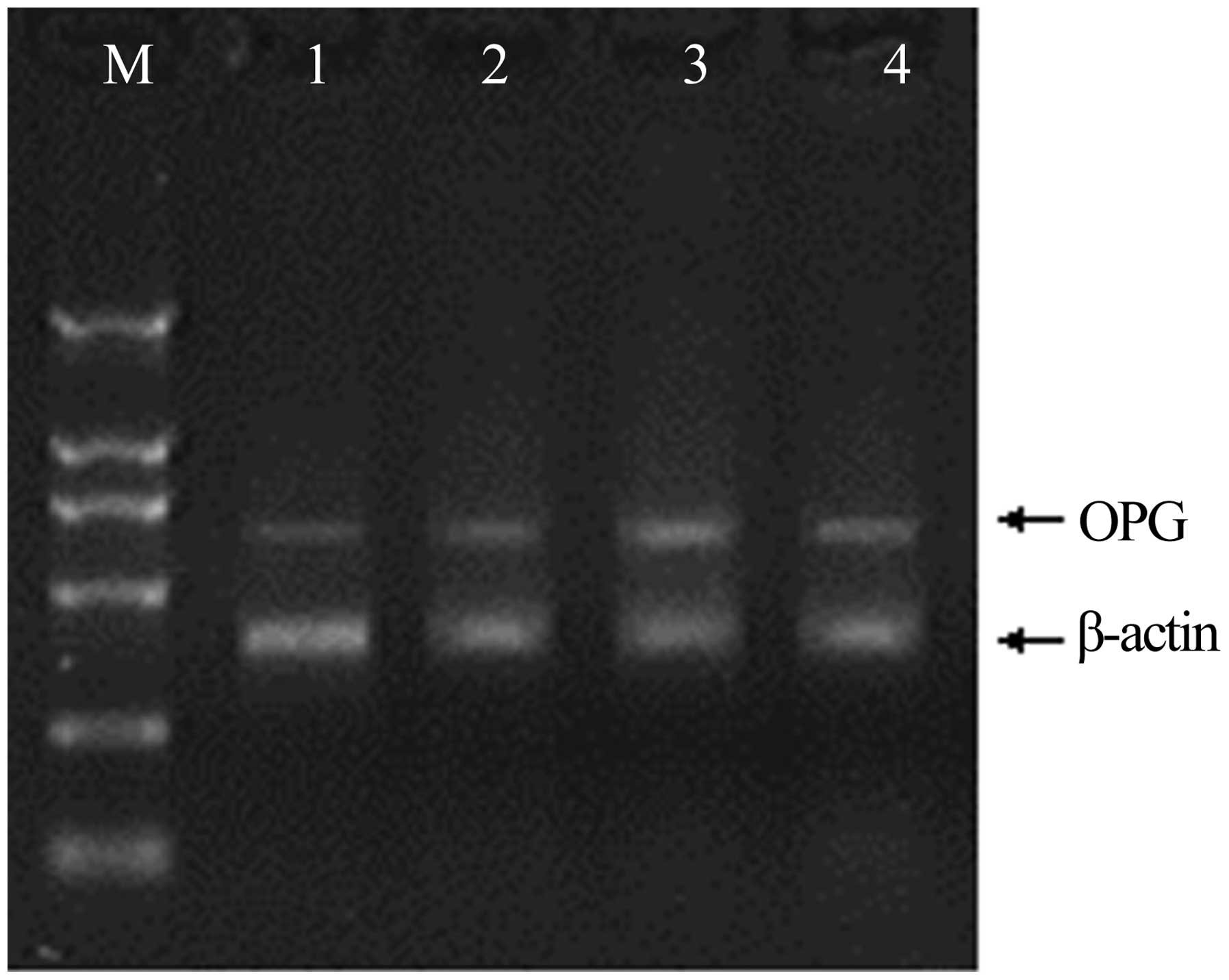
Expression of RANKL, OPG, and CTR mRNA
in rheumatoid pannus tissue
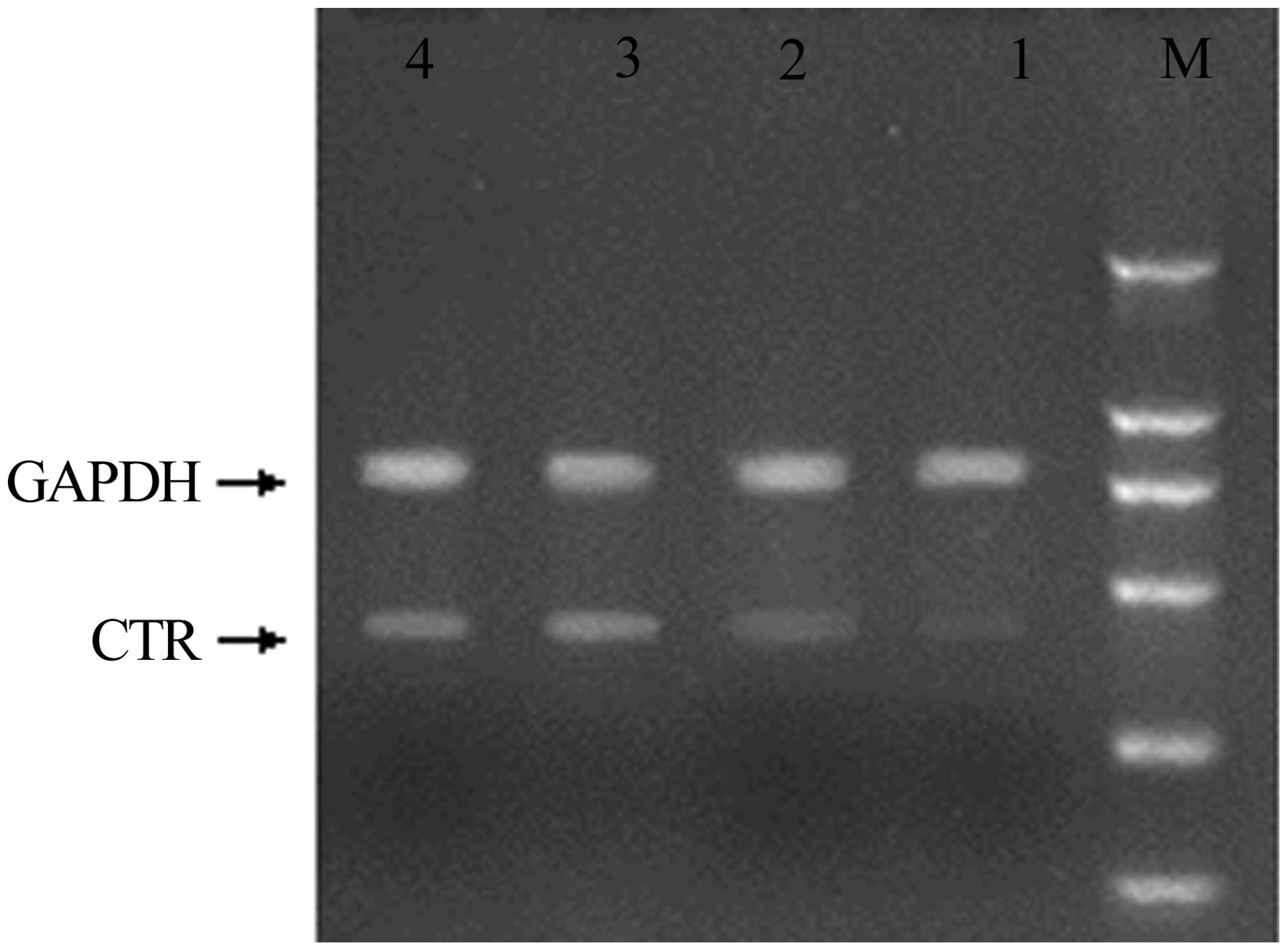
The mRNA expression of genes associated with
osteoclastogenesis in the synovium was investigated using RT-PCR;
the expression of the genes was confirmed by the presence of
specific single bands. The results demonstrated that RANKL and OPG
mRNA expression in the rat CIA model was significantly increased
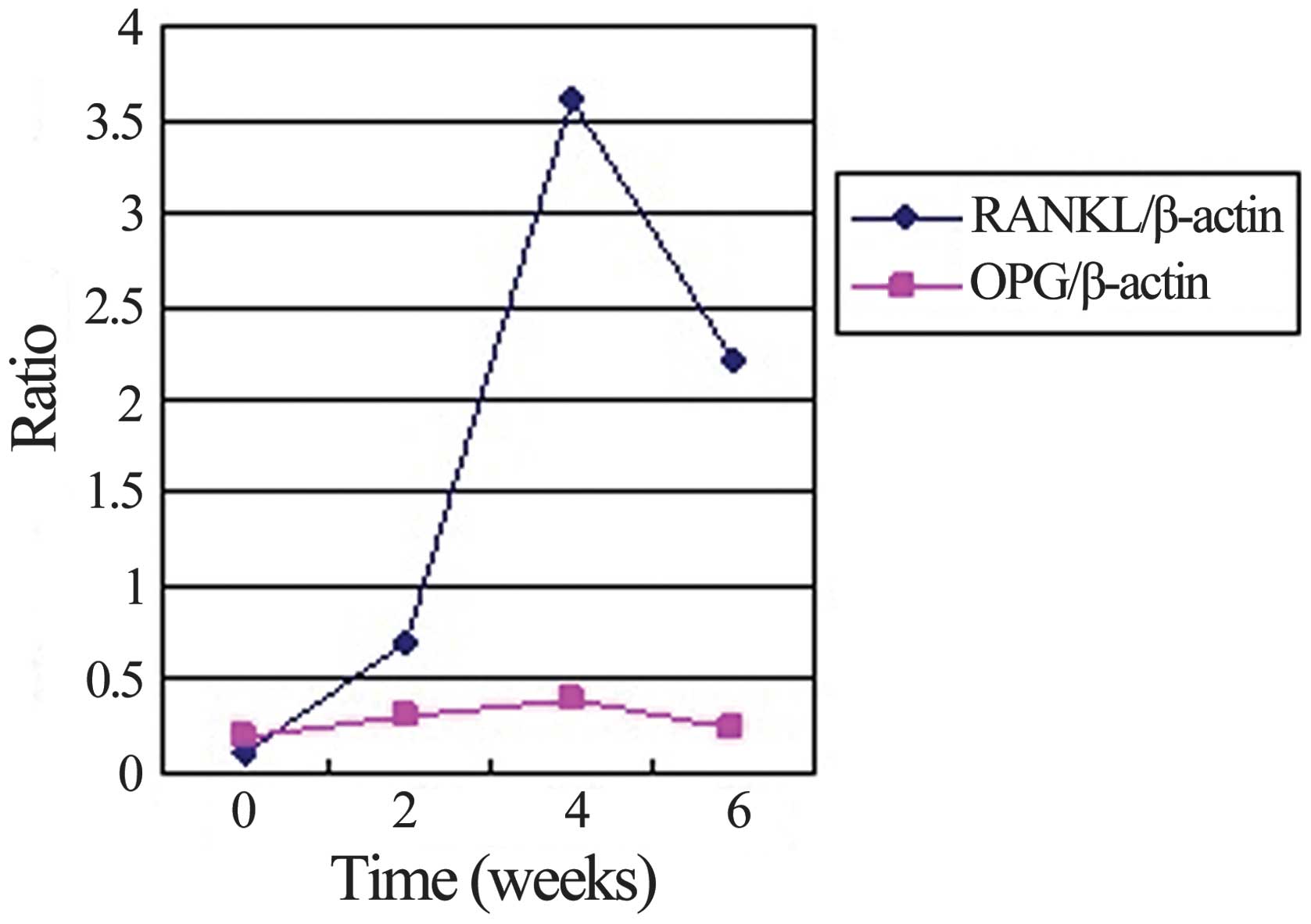
compared with that in the non-immunized rats (Figs. 4 and 5). The mRNA expression levels of RANKL
peaked 4 weeks after immunization, and at weeks 2 and 6 were
significantly higher than levels in the synovium of non-immunized
rats. Mirroring the increase in the number of osteoclasts, the
RANKL:OPG expression ratio on the trabecular bone surface increased
to 9.0:1 and 6.4:1 in the CIA rats at weeks 4 and 6, respectively,
while the RANKL:OPG expression ratio in the control group was 1.0:2
(Fig. 6).
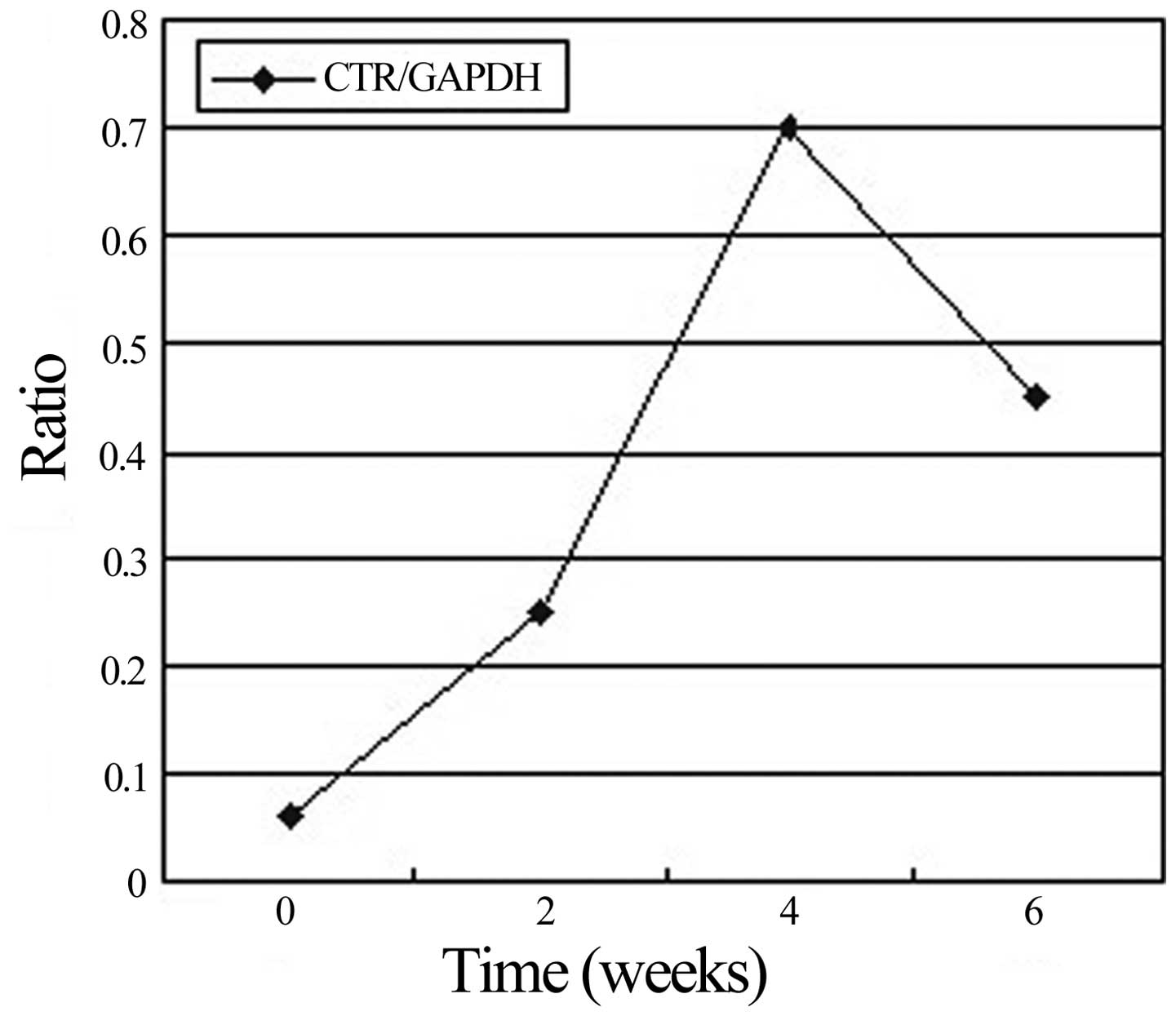
The mRNA expression levels of CTR peaked 4 weeks
after immunization, and at weeks 2 and 6 they were significantly
higher than the levels in the synovium of non-immunized rats
(Fig. 7). Mirroring the increase in
the number of osteoclasts, the level of CTR mRNA in the
CTR-positive osteoclasts on the trabecular bone surface was 10.9-
and 7.8-fold higher in rats with CIA at weeks 4 and 6 after
challenge, respectively, than that in the control rats (Fig. 8).
Discussion
This study is the first, to the best of our
knowledge, to demonstrate the changes in CTR-positive osteoclastic
cells that develop in the mandibular cartilage of the TMJ during
the progression of autoimmune inflammatory joint disease in the rat
CIA model. The fact that the multinucleated cells found within
erosive pits are osteoclasts can be confirmed by their expression
of CTR. This finding in CIA is consistent with the findings in
human RA (21), which suggests that
CTR expression can be consistently found in rheumatoid tissues;
osteoclasts readily form within these tissues, and this indicates
that the expression of CTR is associated with the later stages of
osteoclast differentiation. Bone erosion has been demonstrated to
be correlated with CTR expression in long-term cultures of bone
marrow, and CTR expression is considered to be a reliable marker
in vitro and in vivo for the osteoclast phenotype
(8).
Among the models of RA, the CIA model, which
exhibits numerous morphological similarities to human RA, including
pannus formation, patterns of synovitis and cartilage and bone
erosion, has been the most widely studied; however, external
clinical signs of erythema and induration in TMJ arthritis are
generally not evident. These findings are in contrast to those of
CIA of the knee joint, where swelling and erythema are frequently
observed and used to grade the severity of arthritis (22,23). The
fact that TMJ swelling is rarely observed, in contrast to swelling
of the knee joint, is likely due to the fact that the inflammatory
exudate is able to be distributed into anatomical spaces medial,
posterior and anterior to the TMJ, thus causing less external
swelling.
From the observation of animal models, it has been
suggested that RANKL plays a pivotal role in osteoclast generation
and in the subsequent bone erosion in inflammatory arthritis
(24). The present study clearly
demonstrated a significant increase in RANKL levels and an increase
in OPG levels in the TMJ of the CIA rats. It was found that the
RANKL:OPG ratio was 9.0:1 and 6.4:1 in the CIA rats at weeks 4 and
6, respectively, which was considerably higher than the ratio in
the control rats (1.0:2). These data therefore suggest a
correlation between the RANKL:OPG expression ratio and the severity
of RA in the TMJ. This finding is in agreement with the data of
Geusens et al (15), which
demonstrated that the radiographic progression of the bone
component of joint destruction was dependent on osteoclast
activation (i.e., the OPG:RANKL ratio) in animal models of
arthritis. The present data demonstrated that CIA is associated
with an imbalance in the RANKL:OPG system that favors RANKL; this
imbalance occurs both locally, in foci of bone metabolism, and in
the systemic circulation. The RANKL:OPG ratio is significantly
higher in more severe cases of RA. Such an imbalance has already
been considered for the osteoclast cells that modify the human bone
marrow environment and induce osteoclastogenesis (20). With regard to the role of increased
OPG in the affected TMJ, the marked increase of OPG in the synovial
fluid may indicate that OPG contributes to the protection against
further cartilage degradation or it may be reflective of a
compensatory response by macrophages, chondrocytes or synovial
fibroblasts to the destabilization of the normal balance between
the degradation and synthesis of articular cartilage.
The RANKL:OPG expression ratio was significantly
increased in the CIA rats compared with that in the control rats;
as a result, there was an imbalance that favored RANKL and,
therefore, bone resorption. We cannot, however, exclude the
possibility of an alternative mechanism of osteoclast activation
involving the release of growth factors from osteoblast-like or
immune cells. In this way, the action of cytokines such as TNF-α
and IL-1β on bone cells would be independent of RANKL and would
result in the RANK- and OPG-independent modulation of osteoclast
activity (25). In the present
study, the serum levels of TNF-α and IL-1β during the acute phase
were found to be at increased levels; these cytokines have been
found to be associated with inflammatory diseases such as RA that
produce localized bone loss (18,26). A
positive correlation was noted between all the inflammatory
cytokines and RANKL, as well as CTR, the marker for osteoclasts,
which indicated that these cytokines were involved in the
differentiation of osteoclasts.
The present study has provided the first evidence,
to the best of our knowledge, that the focal bone destruction
occurring in experimental TMJ arthritis is attributable to cells
exhibiting defining characteristics of osteoclasts, including CTR
expression. The RANKL and OPG mRNA expression within the inflamed
synovium has given an insight into the mechanism underlying the
differentiation and function of osteoclasts at the sites of bone
erosion in arthritis. OPG and/or agents targeting RANKL should be
considered as novel therapeutic strategies to attenuate the bone
loss and internal derangement of the TMJ in RA.
References
|
1
|
Scolozzi P, Bosson G and Jaques B: Severe
isolated temporomandibular joint involvement in juvenile idiopathic
arthritis. J Oral Maxillofac Surg. 63:1368–1371. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin YC, Hsu ML, Yang JS, Liang TH, Chou SL
and Lin HY: Temporomandibular joint disorders in patients with
rheumatoid arthritis. J Chin Med Assoc. 70:527–534. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Helenius LM, Tervahartiala P, Helenius I,
et al: Clinical, radiographic and MRI findings of the
temporomandibular joint in patients with different rheumatic
diseases. Int J Oral Maxillofac Surg. 35:983–989. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Narváez JA, Narváez J, Roca Y and Aguilera
C: MR imaging assessment of clinical problems in rheumatoid
arthritis. Eur Radiol. 12:1819–1828. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quinn JH: Arthroscopic and histologic
evidence of chondromalacia in the temporomandibular joint. Oral
Surg Oral Med Oral Pathol. 70:387–392. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kapila S, Lee C, Tavakkoli Jou MR, Miller
AJ and Richards AW: Development and histologic characterizations of
an animal model of antigen-induced arthritis of the juvenile rabbit
temporomandibular joint. J Dent Res. 74:1870–1879. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Horton MA, Lewis D, McNulty K, Pringle JA
and Chambers TJ: Monoclonal antibodies to osteoclastomas (giant
cell bone tumors): Definition of osteoclast-specific cellular
antigens. Cancer Res. 45:5663–5669. 1985.PubMed/NCBI
|
|
8
|
Quinn JM, Morfis M, Lam MH, et al:
Calcitonin receptor antibodies in the identification of
osteoclasts. Bone. 25:1–8. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roodman GD: Advances in bone biology: The
osteoclast. Endocr Rev. 17:308–332. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Romas E, Bakharevski O, Hards DK,
Kartsogiannis V, Quinn JM, Ryan PF, Martin TJ and Gillespie MT:
Expression of osteoclast differentiation factor at sites of bone
erosion in collagen-induced arthritis. Arthritis Rheum. 43:821–826.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hofbauer LC and Heufelder AE: Role of
receptor activator of nuclear factor-kappaB ligand and
osteoprotegerin in bone cell biology. J Mol Med (Berl). 79:243–253.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohazama A, Courtney JM and Sharpe PT: Opg,
Rank and Rankl in tooth development: Co-ordination of odontogenesis
and osteogenesis. J Dent Res. 83:241–244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wakita T, Mogi M, Kurita K, Kuzushima M
and Togari A: Increase in RANKL: OPG ratio in synovia of patients
with temporomandibular joint disorder. J Dent Res. 85:627–632.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haynes DRI, Crotti TN, Loric M, Bain GI,
Atkins GJ and Findlay DM: Osteoprotegerin and receptor activator of
nuclear factor kappaB ligand (RANKL) regulate osteoclast formation
bycells in the human rheumatoid arthritic joint. Rheumatology
(Oxford). 40:623–630. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Geusens PP, Landewé RB, Garnero P, et al:
The ratio of circulating osteoprotegerin to RANKL in early
rheumatoid arthritis predicts later joint destruction. Arthritis
Rheum. 54:1772–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Martínez-Calatrava MJ, Prieto-Potín I,
Roman-Blas JA, Tardio L, Largo R and Herrero-Beaumont G: RANKL
synthesized by articular chondrocytes contributes to
juxta-articular bone loss in chronic arthritis. Arthritis Res Ther.
18:R1492012. View
Article : Google Scholar
|
|
17
|
Brand DD, Latham KA and Rosloniec EF:
Collagen-induced arthritis. Nat Protoc. 2:1269–1275. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirota Y, Habu M, Tominaga K, et al:
Relationship between TNF-alpha and TUNEL-positive chondrocytes in
antigen-induced arthritis of the rabbit temporomandibular joint. J
Oral Pathol Med. 35:91–98. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsukawa A, Ohkawara S, Maeda T, Takagi K
and Yoshinaga M: Production of IL-1 and IL-1 receptor antagonist
and the pathological significance in lipopolysaccharide-induced
arthritis in rabbits. Clin Exp Immunol. 93:206–211. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Grimaud E, Soubigou L, Couillaud S, et al:
Receptor activator of nuclear factor kappaB ligand
(RANKL)/osteoprotegerin (OPG) ratio is increased in severe
osteolysis. Am J Pathol. 163:2021–2031. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gravallese EM, Harada Y, Wang JT, Gorn AH,
Thornhill TS and Goldring SR: Identification of cell types
responsible for bone resorption in rheumatoid arthritis and
juvenile rheumatoid arthritis. Am J Pathol. 152:943–951.
1998.PubMed/NCBI
|
|
22
|
Kuruvilla AP, Shah R, Hochwald GM, Liggitt
HD, Palladino MA and Thorbecke GJ: Protective effect of
transforming growth factor beta 1 on experimental autoimmune
diseases in mice. Proc Natl Acad Sci USA. 88:2918–2921. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Holmdahl R, Jansson L, Andersson M and
Jonsson R: Genetic, hormonal and behavioural influence on
spontaneously developing arthritis in normal mice. Clin Exp
Immunol. 88:467–472. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwan Tat S, Padrines M, Théoleyre S,
Heymann D and Fortun Y: IL-6, RANKL, TNF-alpha/IL-1: Interrelations
in bone resorption pathophysiology. Cytokine Growth Factor Rev.
15:49–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tominaga K, Alstergren P, Kurita H,
Matsukawa A, Fukuda J and Kopp S: Interleukin-1beta in
antigen-induced arthritis of the rabbit temporomandibular joint.
Arch Oral Biol. 46:539–544. 2001. View Article : Google Scholar : PubMed/NCBI
|