Introduction
Gastrointestinal neuroendocrine neoplasms (GINENs),
originating from amine precursor uptake and decarboxylation cells
in the digestive tract, have the ability to undergo multiple
differentiation and secrete various active hormones, leading to
significant differences in biological behaviors and prognosis
(1). Previous studies have indicated
that the incidence rate of GINEN is low (2); however, an epidemiological
investigation in the USA showed that the incidence rate was
5.25/100,000 in 2004, with a 5-fold increase from that 30 years
earlier (3). The original
denomination and classification for GINEN was disorganized and
non-uniform, and knowledge concerning the neoplasms in clinical
practice was very limited. Therefore, the World Health Organization
(WHO) Classification of Tumors of the Digestive System (4) revised the denomination and
classification for GINEN, dividing it into neuroendocrine tumor
(NET), neuroendocrine carcinoma (NEC), mixed adenoneuroendocrine
carcinoma (MANEC), hyperplasia and pre-neoplasm (4). There are limited statistical data
regarding the incidence and pathology of GINENs in Chinese
patients.
In the present study, the clinicopathological data
from 74 patients with GINEN were retrospectively analyzed, with the
aim of summarizing and analyzing the clinicopathological
characteristics of GINEN and the factors affecting prognosis, and
thereby to help in improving the understanding of these
neoplasms.
Subjects and methods
Patients
Intact data of 74 cases with GINEN (from January
2012 to December 2013) confirmed by pathological examination from
the Affiliated Cancer Hospital of Tianjin Medical University
(Tianjin, China) were retrospectively reviewed. Among them, 39
cases were male and 35 were female, with a gender ratio of 1.15:1.
The ages of the patients ranged from 30 to 73 years, and the
average age was 56.9 years. In total, 32 of the neoplasms were
located in the rectum (43.2%), 29 in the stomach (39.2%), 6 in the
colon (8.1%), 2 in the small intestine (2.7%) and 5 in the appendix
(6.8%). This study was conducted in accordance with the Declaration
of Helsinki, and with the approval of the Ethics Committee of the
Affiliated Cancer Hospital of Tianjin Medical University. Written
informed consent was obtained from all the participants.
Symptoms
All but two of the patients manifested
non-functional symptoms of GINEN. These were mainly non-specific
digestive tract symptoms, for example, progressive dysphagia,
abdominal pain, abdominal distention, diarrhea, constipation,
abdominal mass, bloody stools or melena, anorexia and weight loss,
and 2 patients had carcinoid syndrome, for example, facial
flushing, diarrhea and excessive perspiration. The remaining 2
patients had gastrinoma-manifested functional symptoms, for
example, intractable peptic ulcer, upper abdominal pain and
diarrhea.
Histopathological examination
Following surgery, the tissues were sent for routine
histopathological examination. Two pathologists reviewed all the
pathological sections according to the WHO Classification of Tumors
of the Digestive System (4).
Immunopathological examination
Mouse anti-human pan-cytokeratin (PCK, AE1/AE3;
MAB-0049), low molecular weight cytokeratin (LCK, 35βH11; MAB-0051)
and Ki-67 (MIB-1; MAB-0129) monoclonal antibodies, and rabbit
anti-human synaptophysin (Syn, SP11; RMA-0537) and chromogranin A
(CgA, SP12; RMA-0548) for immunohistochemistry were purchased from
Maixin Biotechnology Inc. (Fuzhou, China). Polymeric horseradish
peroxidase conjugated anti-mouse/rabbit IgG (Maixin Biotechnology
Inc.) was used as the secondary antibody. The diagnostic basis for
GINEN was that at least one of the neuroendocrine markers was
diffusely positive or strongly positive; the partial expression of
several neuroendocrine markers was not considered to diagnose
GINEN.
Surgery
All 74 cases underwent surgery: 30 cases (40.5%)
where the maximum diameters of the neoplasms were <2 cm
underwent endoscopic mucosal dissection; 9 cases (12.2%) underwent
partial gastrectomy or partial enterectomy; and 35 cases (47.3%)
underwent radical excision.
Postoperative therapy
Of the 74 patients, 5 patients received
postoperative chemotherapy with cisplatin and etoposide, 4 patients
with oxaliplatin and 5-fluorouracil, and 3 patients with
oxaliplatin and capecitabine. Two patients received postoperative
biotherapy with octreotide, and 3 patients received postoperative
biotherapy and targeted therapy with octreotide and
bevacizumab.
Statistical analysis
SPSS software (version 13.0; SPSS Inc., Chicago, IL,
USA) was applied for statistical analysis. Comparisons of two or
multiple sample survival rates were performed using χ2
test, and the differences of survival rate among different groups
were performed using log-rank. P<0.05 was considered to indicate
a statistically significant difference.
Results
Histopathological examination
Two pathologists reviewed all the pathological
sections and provided a unified diagnosis. All 74 cases were
diagnosed with GINEN. Typing and grading: i) NET, 41 cases (55.4%),
among which 25 cases (33.8%) were at G1 and 16 cases (21.6%) were
at G2; ii) NEC, 21 cases, among which 8 cases were small cell
carcinoma and 13 cases were large cell carcinoma; iii) MANEC, 12
cases (16.2%), among which 5 cases were low-differentiation
adenocarcinoma with NEC, 1 case was middle-differentiation
adenocarcinoma with NET G2, 4 cases were mucinous adenocarcinoma
with NEC and 2 cases were signet-ring cell carcinoma with NEC as
shown in Fig. 1.
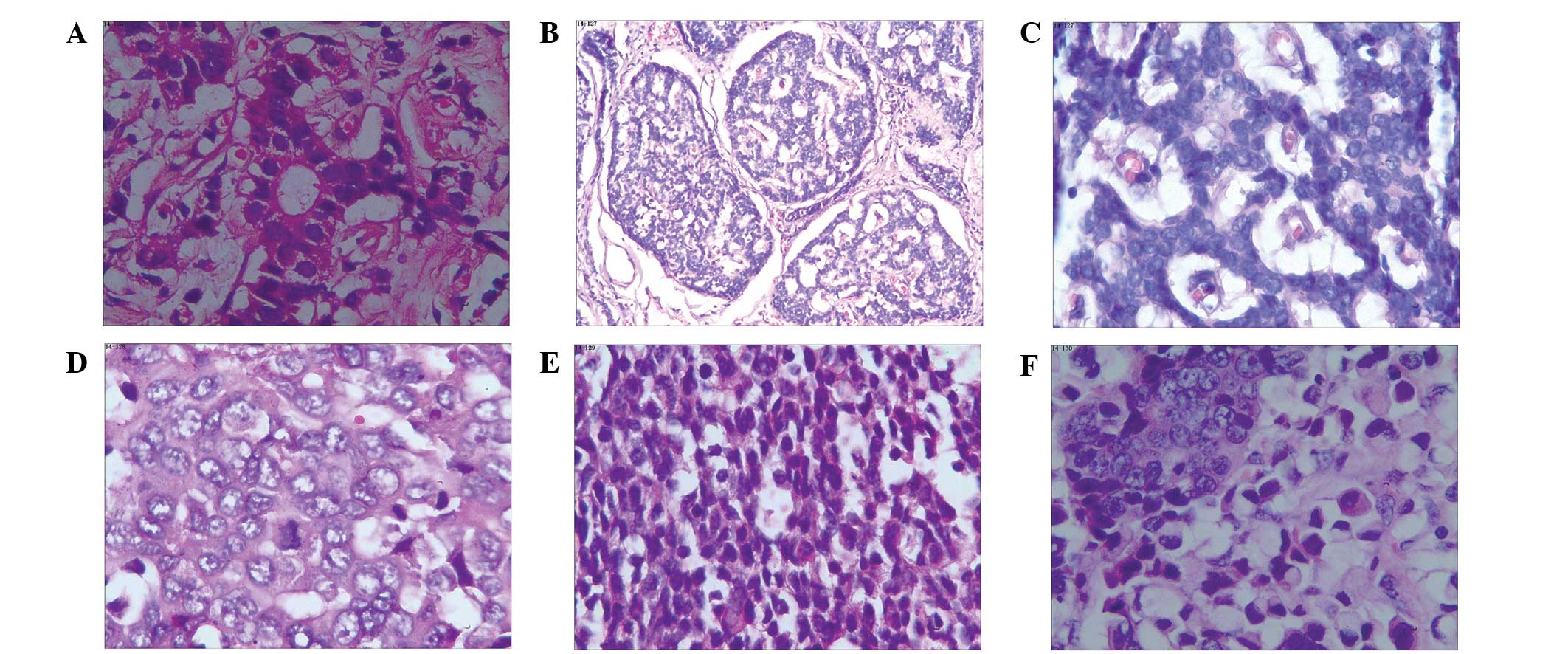 | Figure 1.Different types of gastrointestinal
neuroendocrine neoplasms with hematoxylin and eosin staining. (A)
Appendix, NET G1 (magnification, x200); (B) rectal, NET G2
(magnification, x40): (C) rectum, NET G2 (magnification, x200); (D)
stomach, NEC, large cell carcinoma, (magnification, x200); (E)
colon, NEC, small cell carcinoma (magnification, x200); (F)
stomach, mixed adenoneuroendocrine carcinoma (magnification, x200),
NET, neuroendocrine tumor; NEC, neuroendocrine carcinoma. |
Clinicopathological
characteristics
The maximum diameters of the tumors varied between
0.5 and 12 cm, with an average of 4.1 cm. The depth of the tumor
invasion was the mucous or sub-mucous layer in 33 cases (44.6%) and
the muscular layer and serosa in 41 cases (55.4%). Lymph node
metastasis occurred in 19 cases (25.7%).
Immunopathological examination
All 74 cases of GINEN underwent routine
immunohistochemical examination. The proportions of cases with
positive expression of PCK and LCK were 83.8 and 87.8%,
respectively. All of the GINENs (100%) were positive for least one
of PCK and LCK. The expression rates of Syn and CgA were 93.2 and
59.5%, respectively, and Ki-67 showed different expression levels
depending on the tumor type (Table I
and Fig. 2).
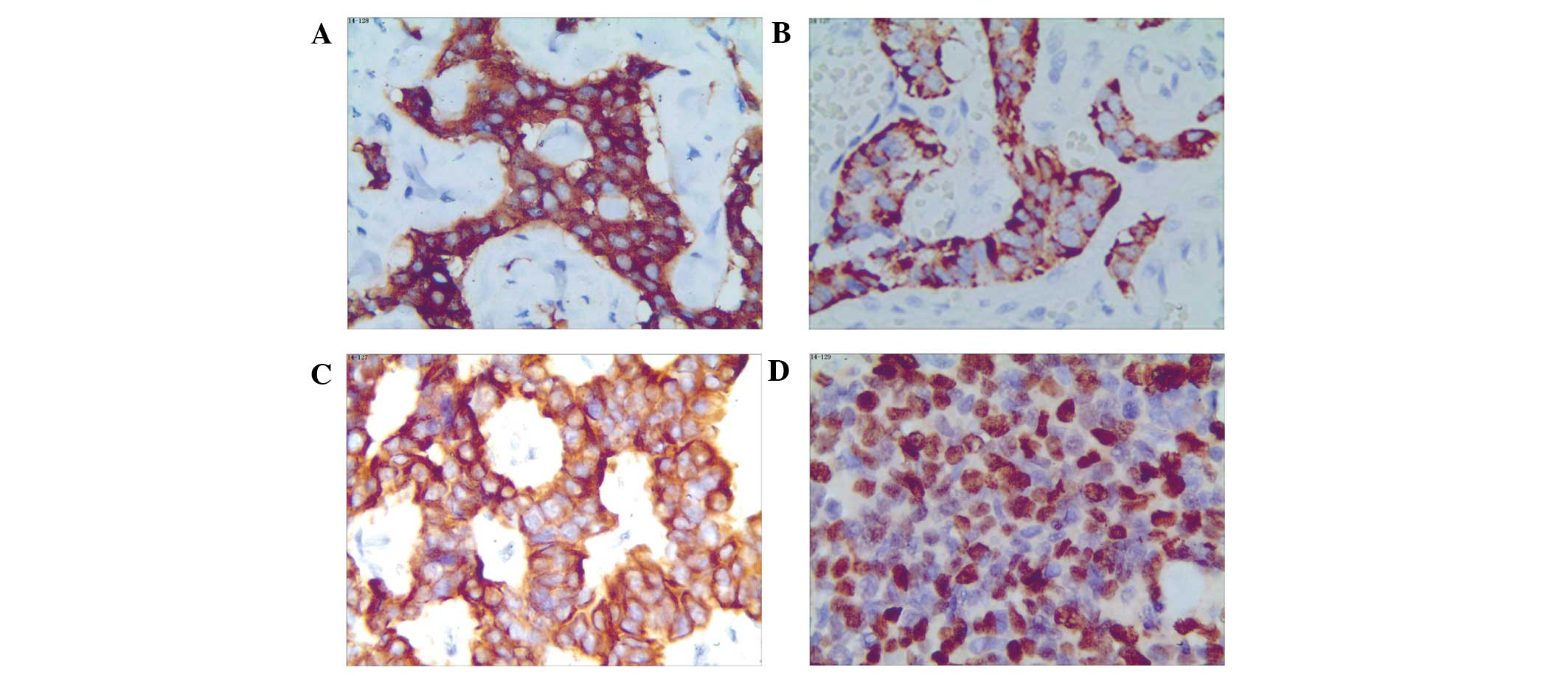 | Figure 2.Immunohistochemical staining in
different types of gastrointestinal neuroendocrine neoplasms. (A)
Appendix, NET G1, Syn; (B) small intestine, NET G2, CgA; (C)
Rectum, NET G2, PCK; (D) Rectum, NEC, small cell carcinoma, Ki-67
(all magnification, x200). NET, neuroendocrine tumor; NEC,
neuroendocrine carcinoma; Syn, synaptophysin; CgA, chromogranin A;
PCK, pan-cytokeratin. |
 | Table I.Expression of neuroendocrine markers
and Ki-67 in different types of gastrointestinal neuroendocrine
neoplasms. |
Table I.
Expression of neuroendocrine markers
and Ki-67 in different types of gastrointestinal neuroendocrine
neoplasms.
|
|
| Neuroendocrine
markers, n (%) | Ki-67 expression
levels, n (%) |
|---|
|
|
|
|
|
|---|
| Group | Cases | Syn+ | CgA+ | Syn+ or
CgA+ | Syn+
CgA+ | ≤2% | 3–20% | >20% |
|---|
| NET G1 | 25 | 22 (88.0) | 18 (72.0) | 25 (100) | 16 (64.0) | 25 (100.0) | 0 | 0 |
| NET G2 | 16 | 14 (87.5) | 10 (62.5) | 16 (100) | 9
(56.3) | 1 (6.2) | 15 (93.8) | 0 |
| NEC | 21 | 21 (100) | 10 (47.6) | 21 (100) | 10 (47.6) | 0 | 0 | 21 (100) |
| MANEC | 12 | 12 (100) | 6
(50.0) | 12 (100) | 6 (50.0) | 0 | 0 | 12 (100) |
| Total | 74 | 69 (93.2) | 44 (59.5) | 74 (100) | 41 (55.4) | 26 (35.1) | 15 (20.3) | 33 (44.6) |
Follow-up examination
Among the 74 patients with GINEN, 71 cases were
followed-up for 10–34 months. During follow-up, 15 cases had
distant metastasis and 24 patients succumbed. The survival rate
after 1 and 2 years was 87.8 and 74.3%, respectively. The results
showed that gender, age or neoplasm site did not have a significant
effect on survival time; however, the survival rate after 1 and 2
years for patients with a neoplasm diameter of ≤2 cm was
significantly higher than that of patients with larger neoplasms
(P=0.002 and P<0.001, respectively). The survival rate after 1
and 2 years for patients with neoplasms involving mucous or
sub-mucous layers was significantly higher than that for neoplasms
involving the muscular layer or serosa (P=0.004 and P<0.001,
respectively). The survival rate after 1 and 2 years for patients
with lymph node metastasis was significantly lower than without
(P<0.001 for both). The survival rate after 1 and 2 years for
patients with distant metastasis was significantly lower than
patients without distant metastasis (P=0.002 and P<0.001,
respectively), and the survival rate after 1 and 2 years for
patients with CgA expression was significantly higher than that for
patients without CgA expression (P=0.026 and P=0.006,
respectively). The difference of survival rate after 1 and 2 years
between patients with NET G1 and NET G2 was not statistically
significant (P=0.07 and P=0.146, respectively); nor was that for
NEC and MANEC (P=0.825 and P=0.895, respectively). However, the
survival rate after 1 and 2 years for patients with NET (including
G1 and G2) was significantly higher than that for patients with NEC
and MANEC (both P<0.001; Table
II).
 | Table II.Factors affecting the survival rates
of patients with gastrointestinal neuroendocrine neoplasms. |
Table II.
Factors affecting the survival rates
of patients with gastrointestinal neuroendocrine neoplasms.
|
| Survival rate
(%) |
|---|
|
|
|
|---|
| Factors | 1 year | 2 year |
|---|
| Neoplasm size,
cm |
|
|
| ≤2 | 33/33 (100) | 33/33 (100) |
|
2.1–5 | 17/19 (89.5) | 13/19 (68.4) |
|
>5 | 15/22 (68.2) | 9/22
(40.9) |
| Depth of
invasion |
|
|
| Mucous or
sub-mucous layer | 33/33 (100) | 32/33 (97.0) |
| Muscular
layer or serosa | 32/41 (78.0) | 23/41 (56.1) |
| Lymph node
metastasis |
|
|
|
Without | 53/55 (96.4) | 49/55 (89.1) |
|
With | 12/19 (63.2) | 6/19
(31.6) |
| Distant
metastasis |
|
|
|
Without | 56/59 (94.9) | 53/59 (89.8) |
|
With | 9/15
(60.0) | 2/15
(13.3) |
| Histopathological
types |
|
|
| NET
G1 | 25/25 (100) | 25/25 (100) |
| NET
G2 | 16/16 (100) | 14/16 (87.5) |
|
NEC | 15/21 (71.4) | 10/21 (47.6) |
|
MANEC | 9/12
(75.0) | 6/12
(50.0) |
| CgA expression |
|
|
|
Positive | 42/44 (95.5) | 38/44 (86.4) |
|
Negative | 23/30 (76.7) | 17/30 (56.7) |
Discussion
The reported incidence rate of GINEN is
significantly lower than that of gastrointestinal adenocarcinoma,
accounting for 0.4–1.8% of gastrointestinal malignant tumors
(5). With the popularization of
gastrointestinal endoscopy and the development of immunopathology,
the overall incidence rate for GINEN has been rising in the last
few years (6–8). GINEN is the most common type of
neuroendocrine neoplasm (NEN), and 67.5% of NEN originates from the
gastrointestinal tract (9).
Previously, it was considered that GINEN mainly affected the
appendix. but Maggard et al (10) analyzed 11,427 cases of GINEN and
showed that the small intestine was the most common site (44.7%),
followed by the rectum (19.6%), appendix (16.7%), colon (10.6%) and
stomach (7.2%). In 2008, data from the USA showed that the most
common sites of GINEN were the rectum, jejunum and stomach
(3). In 2010 (11), data from the National Registration
Center of Spain showed that the jejunum-ileum was the most common
site of GINEN. In the present study, the most common sites were the
rectum and stomach, which is significantly different from other
reports (3,10,11),
possibly as a result of differences in nationalities or sample
sizes.
Since the differentiation of GINEN from
gastrointestinal adenocarcinoma on the basis of clinical symptoms
and endoscopic and ultrasonic morphologies is challenging, the
diagnosis of GINEN principally depends on pathological examination.
However, GINEN has complex and various histomorphological
manifestations, and its pathological diagnosis criteria,
denomination and classification have experienced some revision. In
1907, Oberndorfer (12) proposed the
term ‘carcinoid’ for GINEN, which was regarded as a benign tumor
similar to carcinoma. Subsequent studies, however, showed that
GINEN may be malignant and metastasize. In 1963, based on its
embryological origin, ‘carcinoid’ was simply divided into neoplasms
of the anterior intestines (lung, stomach, duodenum, proximal
jejunum and pancreas), middle intestines (distal jejunum, ileum,
appendix and caecum) and posterior intestines (colon and rectum)
(13). In 1980, the WHO
classification of Tumors of the Digestive System (2nd revision)
designated all NENs as ‘carcinoid.’ In 2000, the WHO classification
(3rd revision) divided digestive system NEN into 5 primary types:
Well-differentiated endocrine tumor, well-differentiated
neuroendocrine carcinoma, poorly differentiated endocrine
carcinoma, small cell carcinoma and tumor-like lesion. In 2010, the
WHO Classification was further improved to divide digestive system
NEN into 6 types: NET G1, NET G2, NEC, MANEC, hyperplasia and
pre-neoplasia (4). Typing and
grading-scale systems depend on pathological histology,
pathological mitosis and the Ki-67 index. When pathological mitosis
grading is not consistent with Ki-67 index grading, the highest
grading between the two is taken as their grading. In the present
study, one case had a Ki-67 index of ≤2% but a pathological mitosis
rate of 5/10 high-power fields; therefore, the pathological
diagnosis was NET G2.
With regard to immunohistochemistry, the present
study considered that at least one neuroendocrine marker being
diffusely positive or strongly positive was diagnostic for GINEN.
In this study, the proportions of cases with positive expression of
Syn, CgA and Syn + CgA were 93.2, 59.5 and 55.4%, respectively. In
addition, 28 cases were Syn+ CgA−, and 3
cases were CgA+ Syn−, which suggested that
the expression spectrum of Syn and CgA did not entirely overlap
with each other, and any single indicator was not perfect; hence,
two or more neuroendocrine markers should be combined to improve
the accuracy of GINEN diagnosis. The above-mentioned results were
in line with the report of Hirabayashi et al (14).
Furthermore, several factors can affect the
prognosis of patients with GINEN. During follow-up (10–34 months),
the survival rate after 1 or 2 years was 87.8 and 74.3%,
respectively. Through analysis of follow-up data, it was found that
gender, age or neoplasm site did not significantly influence the
survival time of the patients. Certain studies, however, have shown
the survival rate after 5 years of patients with GINEN in the
rectum or appendix to be significantly higher than that of patients
with GINEN in the stomach or colon (3,15,16).
This differs from what the present study showed, which may be due
to a small sample size and shorter duration of follow-up in the
present study. Patients with a small neoplasm size, shallow
invasion, no lymph node metastasis, a low pathological grading and
no expression of CgA have a better prognosis, which is in line with
previous reports (17–21).
Currently, surgery is the preferred treatment for
patients with GINEN (22–24). Regardless of whether or not
metastasis has occurred, surgery dependent upon neoplasm size, site
and depth of invasion is required to resect the primary site of the
neoplasm, any sites of metastasis and lymph nodes in order to
improve survival rates (23–26). Other treatments for GINEN include
chemotherapy, biotherapy and molecular targeted therapy (27,28).
Chemotherapy is mainly used to treat patients with NEC or MANEC,
while NETs have low sensitivity to chemotherapy (29,30).
Biotherapy and molecular targeted therapy have good prospects in
the treatment of patients with progressive NET (31). In the present study, all 74 cases of
GINEN underwent surgery with different surgical scope; 12 cases
underwent postoperative chemotherapy; and 5 cases underwent
postoperative biotherapy and molecular targeted therapy. Due to the
relatively short duration of follow-up, it was challenging to
adequately evaluate therapeutic schemes; however, GINEN, with a
superior prognosis, has slower progression than gastrointestinal
adenocarcinoma.
Future studies should expand the sample size and
follow-up time period in order to more accurately summarize the
pathological and immunohistochemical features of GINEN, promote
pathologists' understanding of the neoplasm and improve the
diagnostic accuracy of GINEN and its subtypes. Furthermore, the
factors affecting the prognosis of patients with GINEN may be more
fully explored and therapeutic schemes compared, in order to raise
awareness of GINEN and bring the benefit of comprehensive treatment
for GINEN to clinical practice.
References
|
1
|
Li CC, Xu B, Hirokawa M, Qian Z, Yoshimoto
K, Horiguchi H, Tashiro T and Sano T: Alterations of E-cadherin,
alpha-catenin and beta-catenin expression in neuroendocrine tumors
of the gastrointestinal tract. Virchows Arch. 440:145–154. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Scherübl H, Streller B, Stabenow R, Herbst
H, Höpfner M, Schwertner C, Steinberg J, Eick J, Ring W, Tiwari K
and Zappe SM: Clinically detected gastroenteropancreatic
neuroendocrine tumors are on the rise: Epidemiological changes in
Germany. World J Gastroenterol. 19:9012–9019. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
United States. J Clin Oncol. 26:3063–3072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: World Health Organization Classification of Tumours of
the Digestive System. 3. 4th. IARC Press; Lyon: 2010
|
|
5
|
Shebani KO, Souba WW, Finkelstein DM,
Stark PC, Elgadi KM, Tanabe KK and Ott MJ: Prognosis and survival
in patients with gastrointestinal tract carcinoid tumors. Ann Surg.
229:815–821. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buitrago D, Trencheva K, Zarnegar R,
Finnerty B, Aldailami H, Lee SW, Sonoda T, Milsom JW and Fahey TJ
III: The impact of incidental identification on the stage at
presentation of lower gastrointestinal carcinoids. J Am Coll Surg.
213:652–656. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Federspiel BH, Hansen CP, Vainer B,
Hasselby JP, Bardram L and Knigge U: Incidence, pathology and
clinical course and symptoms of neuroendocrine gastrointestinal
tumours. Ugeskr Laeger. 172:2946–2950. 2010.(In Danish). PubMed/NCBI
|
|
8
|
Lawrence B, Gustafsson BI, Chan A, Svejda
B, Kidd M and Modlin IM: The epidemiology of gastroenteropancreatic
neuroendocrine tumors. Endocrinol Metab Clin North Am. 40:1–18.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Salyers WJ, Vega KJ, Munoz JC, Trotman BW
and Tanev SS: Neuroendocrine tumors of the gastrointestinal tract:
Case reports and literature review. World J Gastrointest Oncol.
6:301–310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maggard MA, O'Connell JB and Ko CY:
Updated population-based review of carcinoid tumors. Ann Surg.
240:117–122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garcia-Carbonero R, Capdevila J,
Crespo-Herrero G, DíazPérez JA, Martínez Del Prado MP, Alonso
Orduña V, Sevilla-García I, Villabona-Artero C, Beguiristain-Gómez
A, Llanos-Muñoz M, et al: Incidence, patterns of care and
prognostic factors for outcome of gastroenteropancreatic
neuroendocrine tumors (GEP-NETs): Results from the National Cancer
Registry of Spain (RGETNE). Ann Oncol. 21:1794–1803. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oberndorfer S: Carcinoid tumors of the
duodenum. Frankfurter Zeitschrift für Pathologie. 1:426–432.
1907.(In German).
|
|
13
|
Williams ED and Sandler M: The
classification of carcinoid tumours. Lancet. 1:238–239. 1963.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirabayashi K, Zamboni G, Nishi T, Tanaka
A, Kajiwara H and Nakamura N: Histopathology of gastrointestinal
neuroendocrine neoplasms. Front Oncol. 3:22013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weinstock B, Ward SC, Harpaz N, Warner RR,
Itzkowitz S and Kim MK: Clinical and prognostic features of rectal
neuroendocrine tumors. Neuroendocrinology. 98:180–187. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Landry CS, Brock G, Scoggins CR, McMasters
KM and Martin RC II: Proposed staging system for colon carcinoid
tumors based on an analysis of 2,459 patients. J Am Coll Surg.
207:874–881. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Medrano-Guzmán R, Alvarado-Cabrero I,
González-Rodríguez D, López-García SC and Páez-Agraz F: Surgical
prognostic factors of gastroenteropancreatic neuroendocrine tumors
(GEP NET). Rev Med Inst Mex Seguro Soc. 50:243–248. 2012.(In
Spanish). PubMed/NCBI
|
|
18
|
Lim T, Lee J, Kim JJ, Lee JK, Lee KT, Kim
YH, Kim KW, Kim S, Sohn TS, Choi DW, et al: Gastroenteropancreatic
neuroendocrine tumors: Incidence and treatment outcome in a single
institution in Korea. Asia Pac J Clin Oncol. 7:293–299. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Curran T, Tulin-Silver S, Patel K, Ward S,
Schneiderman M, Harpaz N, Schwartz M, Itzkowitz S, Warner RR and
Kim MK: Prognostic clinicopathologic factors in longitudinally
followed patients with metastatic small bowel carcinoid tumors.
Pancreas. 40:1253–1257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chou WC, Hung YS, Hsu JT, Chen JS, Lu CH,
Hwang TL, Rau KM, Yeh KY, Chen TC and Sun CF: Chromogranin A is a
reliable biomarker for gastroenteropancreatic neuroendocrine tumors
in an Asian population of patients. Neuroendocrinology. 95:344–350.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chou WC, Chen JS, Hung YS, Hsu JT, Chen
TC, Sun CF, Lu CH and Hwang TL: Plasma chromogranin A levels
predict survival and tumor response in patients with advanced
gastroenteropancreatic neuroendocrine tumors. Anticancer Res.
34:5661–5669. 2014.PubMed/NCBI
|
|
22
|
Knigge U and Hansen CP: Surgery for
GEP-NETs. Best Pract Res Clin Gastroenterol. 26:819–831. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fendrich V and Bartsch DK: Surgical
treatment of gastrointestinal neuroendocrine tumors. Langenbecks
Arch Surg. 396:299–311. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang LC, Poultsides GA and Norton JA:
Surgical management of neuroendocrine tumors of the
gastrointestinal tract. Oncology (Williston Park). 25:794–803.
2011.PubMed/NCBI
|
|
25
|
Ohtsuka T, Takahata S, Ueda J, Ueki T,
Nagai E, Mizumoto K, Shimizu S and Tanaka M: Surgical treatment of
gastroentero-pancreatic neuroendocrine tumor. Gan To Kagaku Ryoho.
40:843–846. 2013.(In Japanese). PubMed/NCBI
|
|
26
|
Gaujoux S, Sauvanet A and Belghiti J:
Place of surgical resection in the treatment strategy of
gastrointestinal neuroendocrine tumors. Target Oncol. 7:153–159.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scherübl H, Jensen RT, Cadiot G, Stölzel U
and Klöppel G: Management of early gastrointestinal neuroendocrine
neoplasms. World J Gastrointest Endosc. 3:133–139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pavel ME, Hainsworth JD, Baudin E, Peeters
M, Hörsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM,
et al: RADIANT-2 Study Group: Everolimus plus octreotide
long-acting repeatable for the treatment of advanced neuroendocrine
tumours associated with carcinoid syndrome (RADIANT-2): A
randomised, placebo-controlled, phase 3 study. Lancet.
378:2005–2012. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reidy DL, Tang LH and Saltz LB: Treatment
of advanced disease in patients with well-differentiated
neuroendocrine tumors. Nat Clin Pract Oncol. 6:143–152. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khasraw M, Yap SY and Ananda S:
Neuroendocrine neoplasms of the GI tract: The role of cytotoxic
chemotherapy. Expert Rev Anticancer Ther. 13:451–459. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Castellano D, Bajetta E, Panneerselvam A,
Saletan S, Kocha W, O'Dorisio T, Anthony LB and Hobday T: RADIANT-2
Study Group: Everolimus plus octreotide long-acting repeatable in
patients with colorectal neuroendocrine tumors: A subgroup analysis
of the phase III RADIANT-2 study. Oncologist. 18:46–53. 2013.
View Article : Google Scholar : PubMed/NCBI
|
















