Introduction
Pancreatic cancer is one of the most fatal
malignances, with a poor overall 5-year survival rate of <5%
(1–3). In most patients, the tumors already
have local or distal metastasis at diagnosis and therefore are
unresectable. The tumorigenesis and metastasis of pancreatic cancer
have been extensively studied (4),
with most studies being performed in vitro using tumor cell
lines. Although tumor cell lines represent a useful model for
studying the biochemical and molecular changes of this malignancy,
they lack an orthotopic environment, which is crucial for analyses
of tumorigenesis, metastasis and response to treatments. In
vivo animal models represent a more desirable approach for the
study of this malignancy and cancer diseases as a whole.
A number of in vivo animal models have been
used to study pancreatic cancer. The most classical model is the
subcutaneous injection of human tumor cells into an
immunocompromised mouse, such as the severely compromised
immunodeficient mouse (5). This
model has certain advantages, including the simplicity of the
procedure, its less invasive nature and the ease of observations of
tumor growth and response to treatment; however, it still lacks an
orthotopic environment for pancreatic tumor formation. As an
improvement of the subcutaneous injection, the orthotopic injection
of tumor cells into the pancreas of the mouse produces a xenograft
model, which mimics the environment for cancer cells to grow and
migrate; however, the cell injection method can generate certain
problems, such as the leakage of cells into surrounding tissues. An
alternative method to the orthotopic cell injection model is to mix
tumor cells with Matrigel™ before the orthotopic injection
(6). Matrigel is a mixture of
extracellular matrix proteins secreted by mouse sarcoma cells and
has been used extensively for in vitro cell culture due to
its resemblance to the complex extracellular environment found in
numerous tissues (7,8). Mixing tumor cells with Matrigel could
potentially reduce the leakage of tumor cells.
In order to establish appropriate mouse xenograft
models for the study of tumorigenesis and evaluations of novel
therapeutics for pancreatic cancer, two orthotopic xenograft mouse
models were developed in the present study by directly implanting a
tumor mass or Matrigel-tumor cell block into the pancreas of a nude
mouse. The results were analyzed.
Materials and methods
Preparation of pancreatic cells stably
expressing red fluorescent protein (RFP)
AsPC-1 human pancreatic cancer cells were purchased
from the Cell Bank of the Chinese Academy of Sciences (Wuhan,
China). AsPC-1 cells were cultured in RPMI-1640 medium (Hyclone
Laboratories, Inc., Logan, UT, USA) containing 10% heat-inactivated
fetal bovine serum (FBS) (Hyclone Laboratories, Inc.), penicillin
(100 U/ml) and streptomycin (100 U/ml). 293T cells (The Cell Bank
of the Chinese Academy of Sciences, Wuhan, China) used for
producing lentiviral particles were cultured in Dulbecco's modified
Eagle's medium (Hyclone Laboratories, Inc.) containing 10%
heat-inactivated FBS, penicillin (100 U/ml) and streptomycin (100
U/ml). All cells were cultured in a humidified incubator at 37°C
with 5% CO2 in the atmosphere. A lentiviral system
(pLenti-DsRed-Monomer) expressing RFP was purchased from Shanghai
Invitrogen Biotechnology Co., Ltd. (Shanghai, China). AsPC-1 cells
in the logarithmic growth phase were trypsinized and seeded into
six-well plates at 4.5×105 cells/well. The
RFP-expressing lentiviral vectors were added to the cells slowly.
After 48 h, the expression of RFP was detected using fluorescence
microscopy. The cells with the highest levels of RFP expression
were chosen for continued culture in a medium containing antibiotic
Blasticidin (0.3 µg/ml; Shanghai Invitrogen Biotechnology Co.,
Ltd.) for the selection of RFP-positive cells. Selected cells were
referred to as AsPC-1-dsRed cells and maintained in culture in the
presence of Blasticidin (0.2 µg/ml).
Preparation of animal models
BALB/C (nu/nu) nude mice of both genders aged 4–6
weeks and weighing 16–22 g were purchased from Shanghai SLAC
Laboratory Animal Co, Ltd. (Shanghai, China). The mice were housed
in a pathogen-free environment at a temperature of 25°C and
relative air humidity between 45 and 50%. All surgeries were
performed in a sterile environment. Sixty mice were randomly
divided into three groups of 20: Orthotopic tumor mass, orthotopic
Matrigel-cell block and subcutaneous tumor cell injection.
In order to establish an orthotopic tumor block
xenograft model, subcutaneous xenograft tumors were generated by
injecting AsPC-1-dsRed cells subcutaneously into the dorsal flank
of the mice. When the tumors grew to a size of ~1 cm3,
the mice were sacrificed by cervical dislocation and the tumors
were isolated and cut into 40-mm3 blocks. The mice
(n=20) were anesthetized by an intraperitoneal injection of 2%
sodium pentobarbital solution. A 1-cm longitudinal skin incision
was made on the left upper axillary region of the abdomen of the
mouse, the peritoneum was opened and the pancreas was well exposed.
The pancreatic capsule was cut open and a piece of
40-mm3 tumor block was implanted into the pancreas using
the purse-string suture surgical method with an 8-0 absorbable
suture. The pancreas was put back into the abdominal cavity gently
and the surgical opening was closed using a 6-0 absorbable surgical
suture (9).
In order to establish an orthotopic Matrigel block
xenograft model, AsPC-1-dsRed cells in single suspension were
prepared and mixed with Matrigel (BD Biosciences, Bedford, MA, USA)
at a ratio of 2.5×107 cells/ml Matrigel to form a
1-cm3 Matrigel-tumor cell block at room temperature.
Following solidification, the block was cut into smaller blocks of
~40 mm3 each. The mice (n=20) were anesthetized and
operated on in the same way as those for the orthotopic tumor block
xenograft model. Following the implantation of the Matrigel block,
the pancreas was put back into the abdominal cavity gently and the
surgical opening was closed.
In order to establish a subcutaneous xenograft
model, an AsPC-1-dsRed single cell suspension was prepared at a
concentration of 5×107 cells/ml. Cells (1×107
cells in 0.2 ml culture medium) were injected subcutaneously in the
dorsal flank region of mice (n=20). The animal use protocol was
approved by the Animal Care Committee of Ningbo University (Ningbo,
China).
Histological examination
The tumor size in the subcutaneous xenograft model
was measured every 6 days using a vernier caliper, while in the two
orthotopic xenograft models the size of the tumor was measured
using an in vivo animal fluorescence imager (Carestream
Health, Rochester, NY, USA) (excitation wavelength, 530 nm;
emission wavelength, 600 nm). The average tumor volume (V) was
calculated using the following equation: V = A × B2 ×
0.5 (A, long diameter; B, short diameter) (10). The tumor growth rate (U) was
calculated using the equation U = V (mm3)/tumor-bearing
time (days). Tumor metastasis was also monitored using the in
vivo animal fluorescence imager (Carestream Health). Ten weeks
after implantation, the mice were sacrificed and dissected. The
tumor volume was measured and the invasion and metastasis were
examined. The primary and metastatic tumors were collected, fixed
in 10% neutral formalin and embedded in paraffin. Serial sections
of 4-µm thickness were cut for hematoxylin and eosin staining.
Western blot analysis
Cultured cells were lysed using
radioimunoprecipitation assay buffer containing 50 mM Tris-HCl (pH
7.4), 150 mM NaCl, 1% NP-40 and 0.25% sodium deoxycholate, plus 1
mM phenylmethylsulfonyl fluoride and 1X Roche cOmplete Mini
Protease Inhibitor (Roche Diagnostics Corporation, Indianapolis,
IN, USA). Protein samples were loaded onto a 12% SDS-PAGE, and run
at a constant current. Following electrophoresis, proteins were
transferred to a nitrocellulose membrane. The membrane was blocked
with 4% fat-free milk powder in phosphate-buffered saline and
incubated with rabbit polyclonal anti-RFP antibody (R10367; Life
Technologies, Grand Island, NY, USA) and subsequently with
anti-rabbit IgG secondary antibody (1:5,000; sc-2004; Santa Cruz
Biotechnology, Inc. CA, USA). Protein signals were detected using
enhanced chemiluminescence reagents (Pierce Biotechnology, Inc.,
Rockford, IL, USA).
Statistical analysis
SPSS software, version 18.0 (SPSS, Inc., Chicago,
IL, USA) was used to perform statistical analysis. Tumor volumes
and average growth rates are expressed as the mean ± standard
deviation. Analysis of variance was used to detect any
statistically significant differences in the tumor volumes and
growth rates among the three models 36 days after implantation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Tumor formation and growth rate
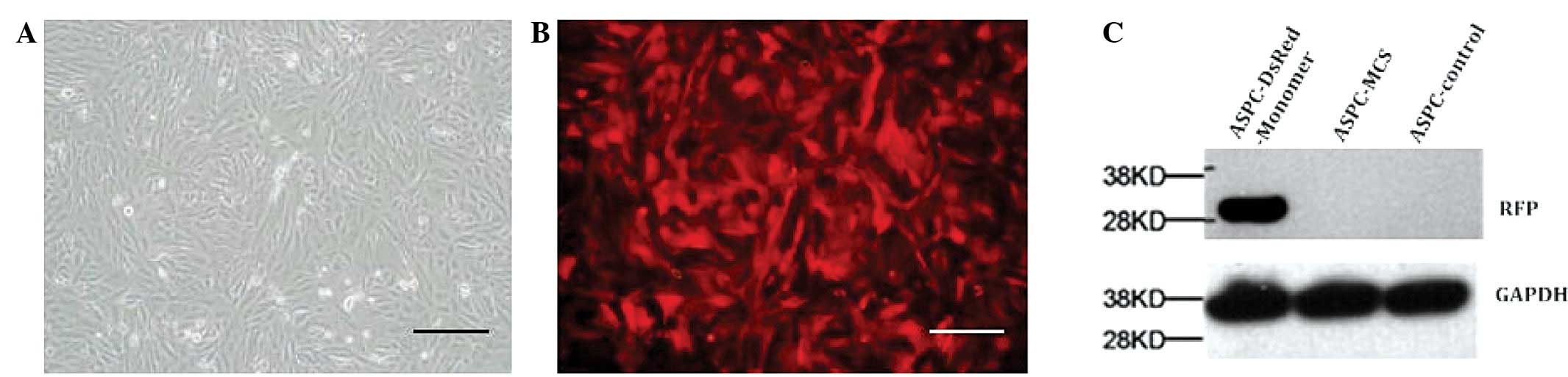
Using a lentiviral system, a stable AsPC-1 line
highly expressing RFP was obtained. The high expression of RFP in
AsPC-1 cells was confirmed using western blotting (Fig. 1).
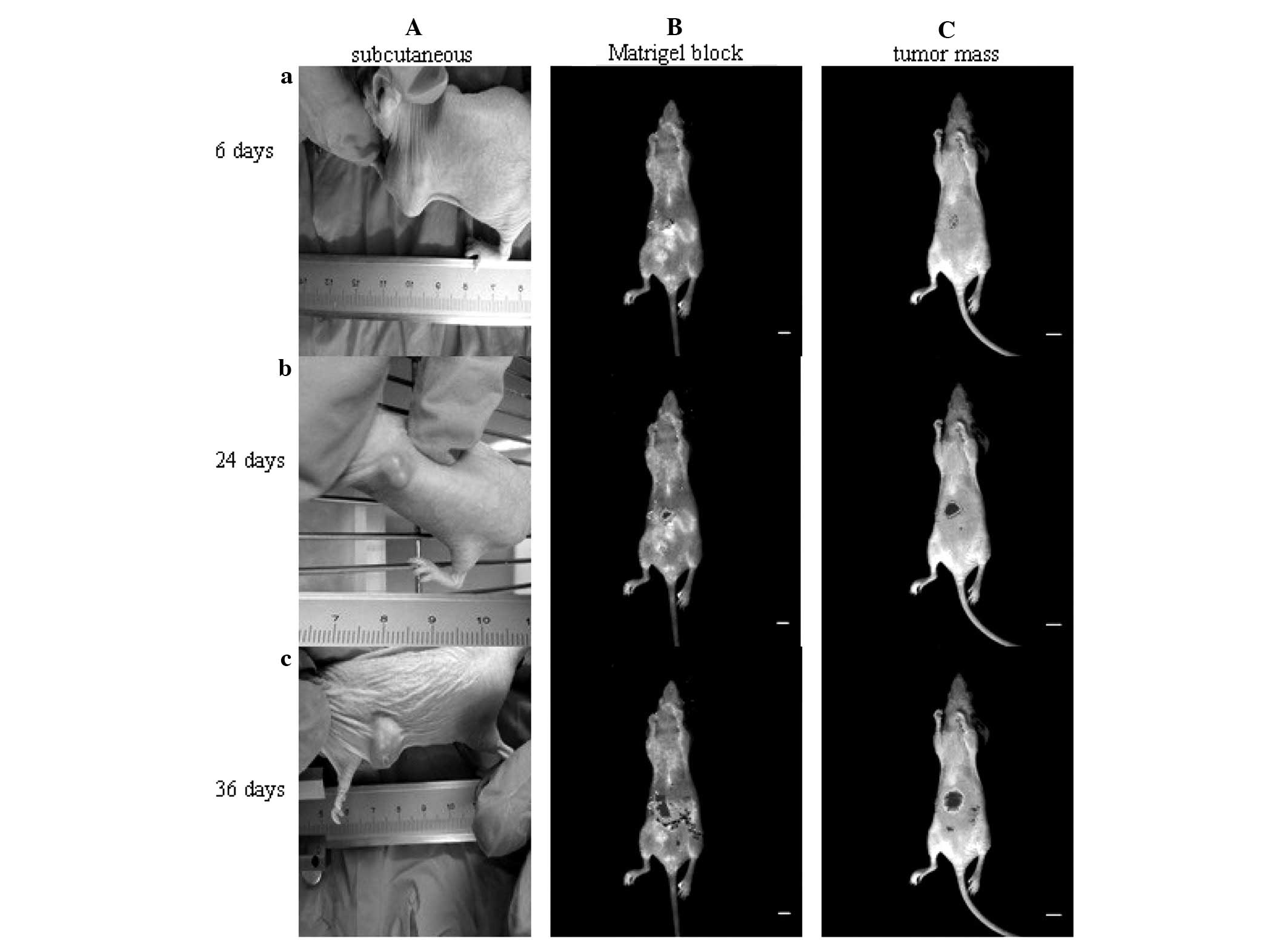
Thirty-six days after implantation, the tumor
formation rate was 100% (20/20) for the mice that received
orthotopic tumor mass implantation, 100% (20/20) for those that
received orthotopic Matrigel-tumor cell block implantation and 55%
(11/20) for the mice that received subcutaneous injection of AsPC-1
cells (Fig. 2 and Table I). All tumor-bearing mice exhibited
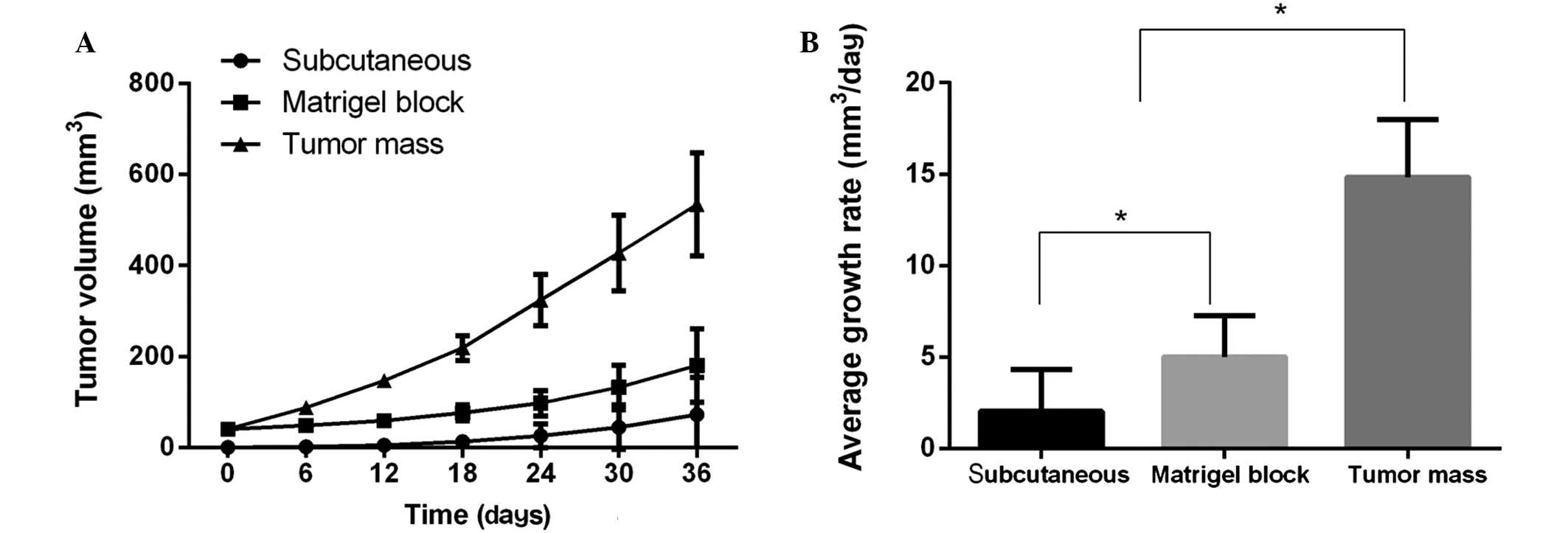
gradual weight loss and reduced activity. Thirty-six days after
implantation, the growth rates among the three xenograft models
were significantly different (Fig.
3). The tumors of the mice that received the orthotopic tumor
mass implantation exhibited the highest growth rate, followed by
the tumors of those that received orthotopic Matrigel tumor block
implantation and the tumors of the subcutaneous xenograft mice
(P<0.01) (Fig. 3). The average
tumor volume of the mice that received orthotopic tumor mass
implantation was approximately ~3 fold that of the mice that
received orthotopic Matrigel block transplantation and ~7.4 fold
that of the mice that received the subcutaneous injection of tumor
cells (Table I).
 | Table I.Comparison of the three xenograft
mouse models of human pancreatic cancer. |
Table I.
Comparison of the three xenograft
mouse models of human pancreatic cancer.
|
|
| Volume of tumor
(mm3) |
| Metastasis, n
(%) |
|---|
|
|
|
|
|
|
|---|
| Model | Tumor formation rate
(%) | Day 0 | Day 36 | Average growth rate
(mm3/day) | Liver | Peritoneal | Mesenteric | Total |
|---|
| Orthotopic tumor mass
implantation | 100 | 40 |
534.40±112.58a |
14.84±3.13a | 4 (20) | 15 (75) | 5 (25) | 16 (80) |
| Orthotopic Matrigel™
block implantation | 100 | 40 |
180.24±80.46a |
5.01±2.24a | 3 (15) | 16 (80) | 6 (30) | 16 (80) |
| Subcutaneous | 55 | 0 |
72.61±81.94a |
2.02±2.28a | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
Tumor metastasis
Using an in vivo imaging system, the local
invasion and metastasis of the tumors were observed in the
orthotopic tumor mass implantation and Matrigel block implantation
models, but not in the subcutaneous xenograft mice (Fig. 2). At day 36, the tumor-bearing mice
were sacrificed and examined for metastatic tumors. In the
orthotopic tumor mass xenograft model, 80% of the mice exhibited
tumor metastasis, with the majority exhibiting peritoneal
metastasis. Similarly, in the orthotopic Matrigel block xenograft
model, 80% of the mice exhibited tumor metastasis, but the
metastatic sites were slightly different. No tumor metastasis was
identified in the subcutaneous xenograft model (Table I).
Anatomical and histological
examinations
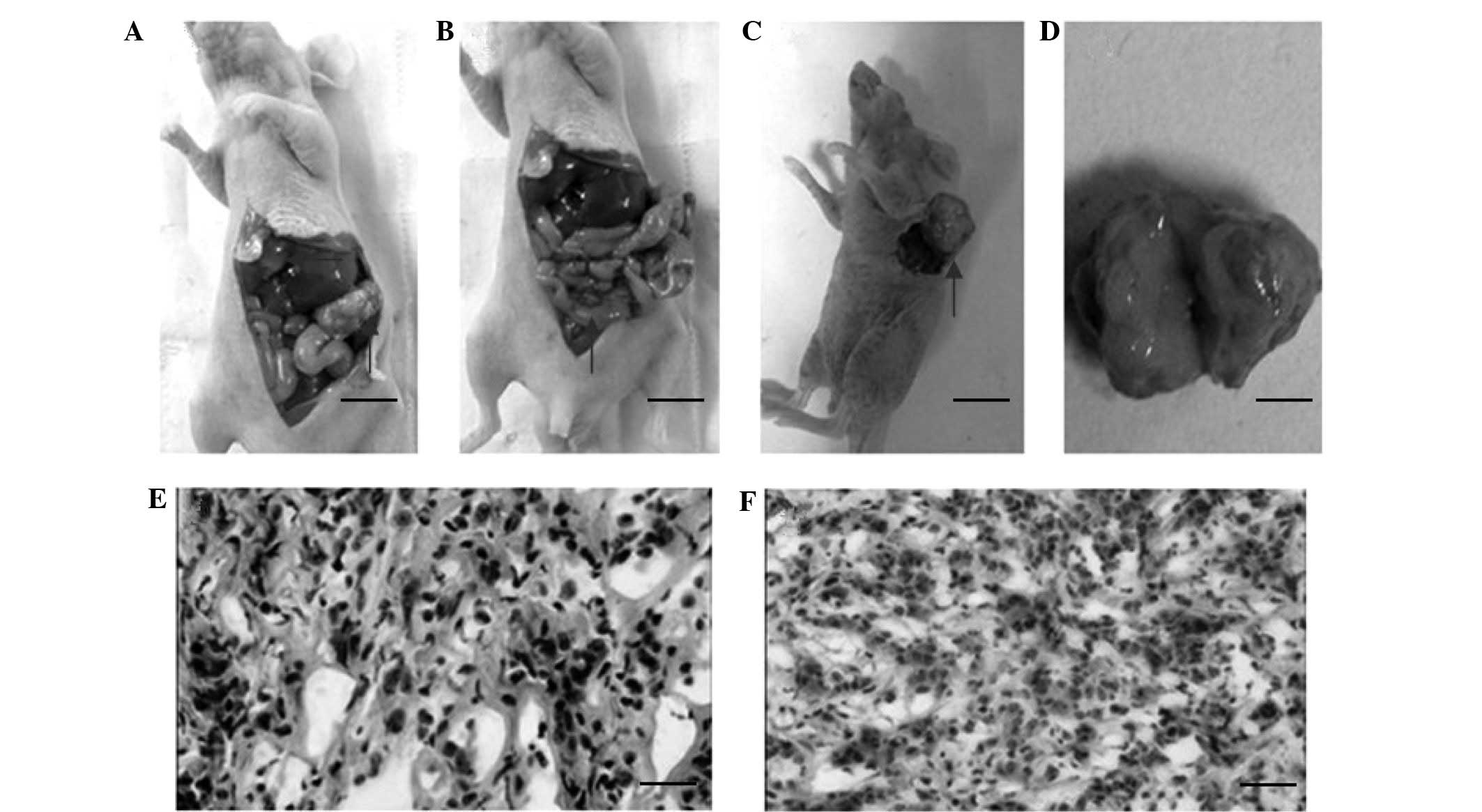
In the subcutaneous xenograft model, the tumors were
found to adhere to the skin tightly and the tumor sections had a
gray, fish-like appearance. No distant metastases were found in the
thoracic or abdominal cavities. In the orthotopic tumor mass
implantation model, the tumors in the pancreas were irregularly
shaped and were found to adhere to surrounding tissues, such as the
stomach, spleen and intestine. Metastases to the liver and
peritoneum were observed (Table I).
The tumors were generally rich in blood vessels on the surface and
had necrosis at the center. In the orthotopic Matrigel tumor block
implantation model, ascites were observed in the abdominal cavities
of the mice, and metastases to the liver, peritoneum and spleen
were found.
Histological examination was performed for all
tumors isolated from the tumor-bearing mice. Various cell
morphologies were observed, with most cells having a polygonal or
spindle shape (Fig. 4). The tumors
were surrounded by a fibrous stroma. No glandular differentiated
cells were observed. Pathological mitotic figures were found in the
tumors, which were consistent with the poorly differentiated
adenocarcinoma (Fig. 4).
Discussion
Animal models are indispensable in the study of
biological mechanisms and the development of therapeutics for human
diseases. A number of mouse models of human pancreatic cancer have
been reported, including the injection of pancreatic tumor cells
subcutaneously or into the pancreas of the mouse (5,6). In the
present study, two orthotopic xenograft models were developed, in
which either a tumor mass or Matrigel-tumor cell mixture was
directly implanted into the pancreas of mice. The findings showed
that the orthotopic tumor mass implantation model had superior
performance results than the other models in terms of tumor volume
and metastasis.
Due to the easy protocol of the subcutaneous
xenograft model, it has been used extensively in cancer research
(11,12); however, the subcutaneous injection of
tumor cells often results in local growth but rarely distant
metastasis, and the tumor may even fail to develop. In addition,
the growth rate of a subcutaneous tumor is influenced by numerous
factors, such as cell type and number. Furthermore, in light of the
importance of the tumor environment to the growth and progression
of tumors (13), the lack of an
orthotopic environment makes the subcutaneous xenograft model less
attractive for cancer research.
With regard to the orthotopic xenograft model for
pancreatic cancer, the most commonly used method includes injecting
a tumor cell suspension into the pancreas (5,14). The
orthotopic injection method was attempted in the present study;
however, the results showed that there was a high risk of cell
leakage, which reduced the rate of tumor inoculation orthotopically
but increased the rate of peritoneal inoculation. In order to avoid
this leakage associated with the orthotopic injection model, the
Matrigel-tumor cell xenograft model was established. In this model,
the tumor cells were first mixed with Matrigel, and the solidified
block was then implanted into the pancreas. This model was 100%
successful and had a 0% mortality rate under well-controlled
anesthesia and with skilled surgical techniques. Metastasis is one
the most prominent pathological features of pancreatic cancer. A
successful animal model of pancreatic cancer should be able to
develop metastasis. In the present study, the two orthotopic
xenograft models successfully developed tumor metastasis. This
property makes these two models particularly useful in the study of
tumor metastasis mechanisms and intervention development.
In the models of the present study, an in
vivo imaging system was used to monitor tumor growth in a
real-time and non-invasive fashion. This proved very convenient for
evaluating the efficiency of the models and would be beneficial in
the monitoring of anti-cancer drug efficacy. The current method
involved the use of RFP, which may not have been the most suitable,
since its fluorescent intensity could be subject to interference by
layers of biological tissues, thus leading to a limitation of the
depth of fluorescence imaging. An infrared fluorescent protein with
a longer wavelength or a fluorescence imaging system with higher
photosensitivity would help overcome these challenges. In addition,
since Matrigel is a preparation of basement membrane, its quality
could vary, which would lead to numerous variations in the
xenograft model. The use of a new material that could substitute
Matrigel has been reported in a prostate cancer xenograft model
(15). Testing the material in the
pancreatic cancer xenograft model could prove beneficial.
In conclusion, two orthotopic xenograft mouse models
for human pancreatic cancer were successfully developed, which
could be useful in the study of tumorigenesis, tumor progression
and metastasis.
Acknowledgements
This study was supported by the Scientific
Innovation Team Project of Ningbo (grant no. 2013B82010), the Key
Project for Social Development of Ningbo (grant no. 2011C51005) and
the K.C. Wong Magna Fund in Ningbo University (Ningbo, China).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brennan MF: Adjuvant therapy following
resection for pancreatic adenocarcinoma. Surg Oncol Clin N Am.
13:555–566. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stojadinovic A, Hoos A, Brennan MF and
Conlon KC: Randomized clinical trials in pancreatic cancer. Surg
Oncol Clin N Am. 11:207–229. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamura K, Kasuya H, Sahin TT, Tan G,
Hotta Y, Tsurumaru N, Fukuda S, Kanda M, Kobayashi D, Tanaka C, et
al: Combination treatment of human pancreatic cancer xenograft
models with the epidermal growth factor receptor tyrosine kinase
inhibitor erlotinib and oncolytic herpes simplex virus HF10. Ann
Surg Oncol. 21:691–698. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nikfarjam M, Yeo D, He H, Baldwin G, Fifis
T, Costa P, Tan B, Yang E, Wen S and Christophi C: Comparison of
two syngeneic orthotopic murine models of pancreatic
adenocarcinoma. J Invest Surg. 26:352–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Troiani T, Schettino C, Martinelli E,
Morgillo F, Tortora G and Ciardiello F: The use of xenograft models
for the selection of cancer treatments with the EGFR as an example.
Crit Rev Oncol Hematol. 65:200–211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bimonte S, Barbieri A, Palma G, Luciano A,
Rea D and Arra C: Curcumin inhibits tumor growth and angiogenesis
in an orthotopic mouse model of human pancreatic cancer. BioMed Res
Int. 2013:8104232013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nangami GN, Watson K, Parker Johnson K,
Okereke KO, Sakwe A, Thompson P, Frimpong N and Ochieng J: Fetuin-A
(α2HS-glycoprotein) is a serum chemo-attractant that also promotes
invasion of tumor cells through Matrigel. Biochem Biophys Res
Commun. 438:660–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Büchler P, Reber HA, Roth MM, Shiroishi M,
Friess H and Hines OJ: Target therapy using a small molecule
inhibitor against angiogenic receptors in pancreatic cancer.
Neoplasia. 9:119–127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hakkarainen T, Särkioja M, Lehenkari P,
Miettinen S, Ylikomi T, Suuronen R, Desmond RA, Kanerva A and
Hemminki A: Human mesenchymal stem cells lack tumor tropism but
enhance the antitumor activity of oncolytic adenoviruses in
orthotopic lung and breast tumors. Hum Gene Ther. 18:627–641. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poplin E, Feng Y, Berlin J, Rothenberg ML,
Hochster H, Mitchell E, Alberts S, O'Dwyer P, Haller D and Catalano
P: Phase III, randomized study of gemcitabine and oxaliplatin
versus gemcitabine (fixed-dose rate infusion) compared with
gemcitabine (30-minute infusion) in patients with pancreatic
carcinoma E6201: A trial of the Eastern Cooperative Oncology Group.
J Clin Oncol. 27:3778–3785. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McAllister SS and Weinberg RA: Tumor-host
interactions: A far-reaching relationship. J Clin Oncol.
28:4022–4028. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Colucci G, Labianca R, Di Costanzo F,
Gebbia V, Cartenì G, Massidda B, Dapretto E, Manzione L, Piazza E,
Sannicolò M, et al: Gruppo Oncologico Italia Meridionale (GOIM);
Gruppo Italiano per lo Studio dei Carcinomi dell'Apparato Digerente
(GISCAD); Gruppo Oncologico Italiano di Ricerca Clinica (GOIRC):
Randomized phase III trial of gemcitabine plus cisplatin compared
with single-agent gemcitabine as first-line treatment of patients
with advanced pancreatic cancer: The GIP-1 study. J Clin Oncol.
28:1645–1651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cui L, Chen P, Tan Z, Li W and Dong Z:
Hemostatic gelatin sponge is a superior matrix to matrigel for
establishment of LNCaP human prostate cancer in nude mice.
Prostate. 72:1669–1677. 2012. View Article : Google Scholar : PubMed/NCBI
|