Introduction
Parkinson's disease (PD) is a common degenerative
disease of the central nervous system in the elderly, which is
primarily associated with environmental and genetic factors. There
are a number of genes that have been established to be associated
with PD, which include LRRK2. Mutations of LRRK2 are considered to
be the most prevalent in the pathogenesis of PD; in studies of
North American and European PD patients, 5% had a mutation of LRRK2
and a family history of PD, while 1–2% had sporadic PD (1–4). The
LRRK2 gene is the causative gene of the autosomal dominant
hereditary type 8 PD (5,6). It is composed of five structural
domains, namely, the ankyrin repeat (ANK), leucine-rich repeat
(LRR), Ras of complex proteins (Roc) C-terminal of Roc (COR),
mitogen activated kinase kinase kinase (MAPKKK) and WD40 regions
(7–9). Major mutations of LRRK2 include R1441C,
R1441G, R1441H, R1514Q, Y1699C, G2019S, I2020T, I2012T and G2385R
(10–12). Mutations of LRRK2 have been
associated with a number of diseases, in particular with familial
PD and sporadic PD, and the G2019S mutation is one of the most
common mutations in PD (13). Clear
racial and regional differences exist in the incidence of PD.
Xinjiang is an autonomous region located in Central
Asia, which has two predominant populations with different genetic
backgrounds, namely, the Uyghur and Han populations. The current
case-control study selected PD patients and healthy individuals
from the Uyghur and Han populations of the Xinjiang region for the
analysis of LRRK2 gene mutations. To the best of our knowledge,
this is the first time that the association between the G2019S and
R1441C mutations of the LRRK2 gene and PD susceptibility has been
investigated in different ethnicities and regions.
Subjects and methods
Diagnostic criteria and study
subjects
From June 2010 to April 2013, 312 patients with PD
(all sporadic) visiting the specialist neurology clinic of the
First Affiliated Hospital of Xinjiang Medical University (Urumqi,
China) were enrolled in the study. The diagnosis was in line with
the UK Brain Bank diagnostic criteria for PD. Cerebrovascular
disease, encephalitis, trauma, drug-induced Parkinson's syndrome,
Parkinson's plus syndrome and other severe systemic diseases were
excluded. The control group consisted of 359 volunteers from the
same region, who had no family history or clinical manifestations
of PD. Gender, age and ethnicity were matched between the two
groups. The study was approved by the Ethics Committee of the First
Affiliated Hospital of Xinjiang Medical University, and all
subjects provided informed consent.
DNA extraction
A sample of blood (2 ml) was collected from each
subject, subsequent to the provision of informed consent. Following
EDTA anticoagulation, a non-centrifugal-type DNA Extraction kit
(Shanghai Tiangen Biotech Co., Ltd., Shanghai, China) was used to
extract the genomic DNA Tris-borate buffer (Sangon Biotech Co.,
Ltd., Shanghai, China) was added and the DNA was maintained at
−80°C.
Design of primers and amplification of
the gene
The primers used for G2019S were based on those used
in a previous study by Thaler et al (14) and the sequences were as follows:
upstream, 5′-CCTGTGCATTTTCTGGCAGATA-3′ and downstream,
5′-CCTCTGATGTTTTTATCCCCATTC-3′. According to the study by
Paisán-Rauíz et al (7), the
primer sequences for R1441C were as follows: upstream,
5′-TCAACAGGAATGTGAGCAGG-3′ and downstream
5′-CCCACAATTTTAAGTGAGTTGC-3′. DNA amplification was carried out as
follows: The total volume of the quantitative polymerase chain
reaction (qPCR) was 20 µl, including 100 ng/µl upstream and
downstream primers (0.5 µl), 2X Power Taqman Master Mix (10 µl;
Beijing Baitaike Biotechnology Co., Ltd., Beijing, China), 50 ng/µl
DNA (3.0 µl) and ddH2O (11 µl). Primers were synthesized
by Sangon Biotech Co., Ltd (Shanghai, China). A GeneAmp System 9700
thermal cycler (Applied Biosystems Corporation, Foster City, CA,
USA) was used. PCR was performed after the first denaturation at
95°C for 2 min; each cycle consisted of denaturation at 95°C for 20
sec, annealing at 62°C for 20 sec and extension at 72°C for 30 sec.
The number of total PCR cycles was 35.
qPCR product detection
Equal volumes of PCR products (7 µl) were taken,
sample buffer [2X bromophenol blue; Sangon Biotech (Shanghai) Co.,
Ltd.] was added and the solution was mixed. The sample was placed
on a 4% agarose gel for nucleic acid staining and 4 V/cm
electrophoresis was performed for one hour.
Enzyme digestion genotyping
PCR products (8 µl), 10X endonuclease buffer (2 µl;
New England Biolabs, Inc., Ipswich, MA, USA) and restriction
endonucleases ScfI and BstUl (5 units; New England
Biolabs Inc., Ipswich, MA, USA) were added to 20 µl sterile double
distilled water and incubated at 37°C overnight (16 h). The
digestion products (9 µl) were placed on a 4% agarose gel for
ethidium bromide staining, and underwent electrophoresis at 110 V
for 1.5 h. A Gel Doc 1000 gel imaging analysis system (Bio-Rad,
Hercules, CA, USA) was used to detect the electrophoretic bands
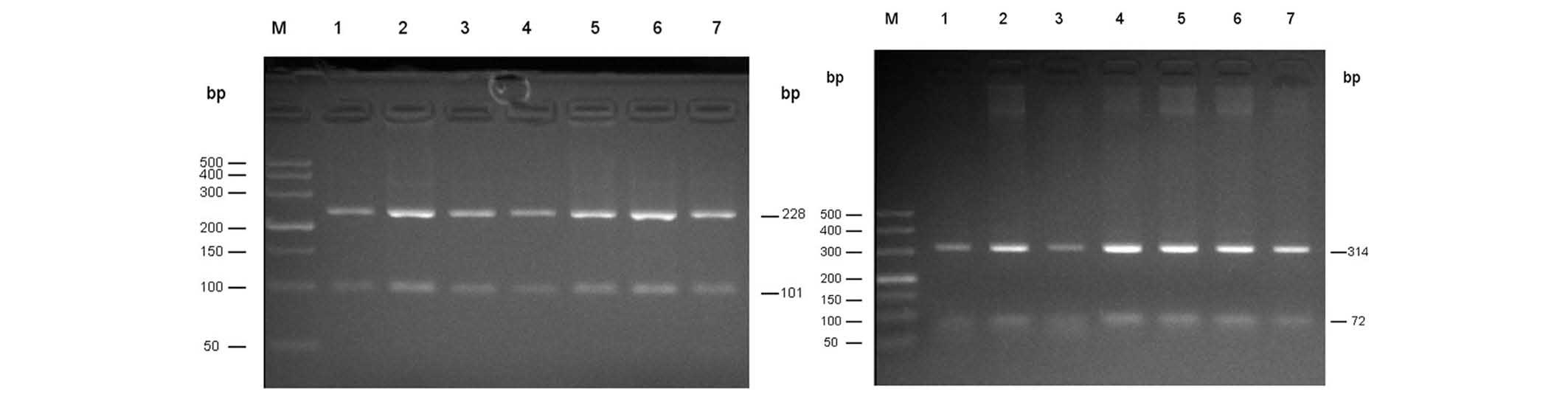
(Fig. 1).
Direct sequencing
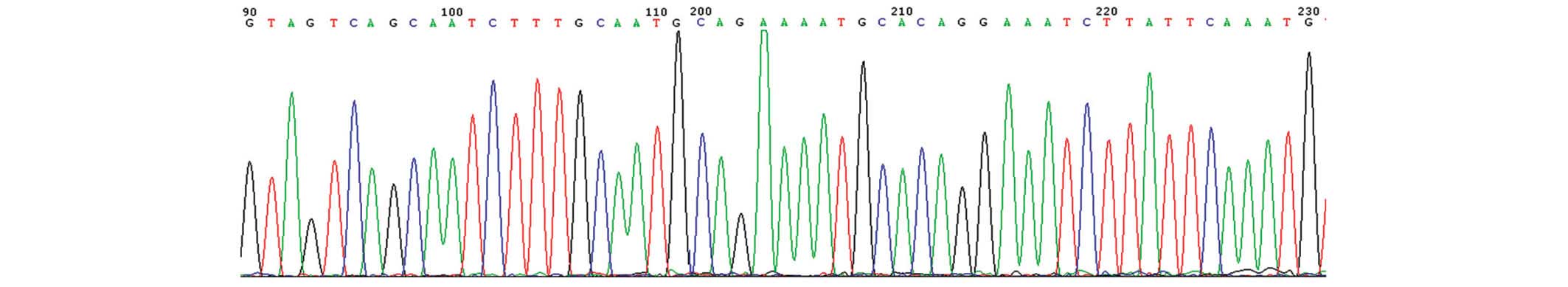
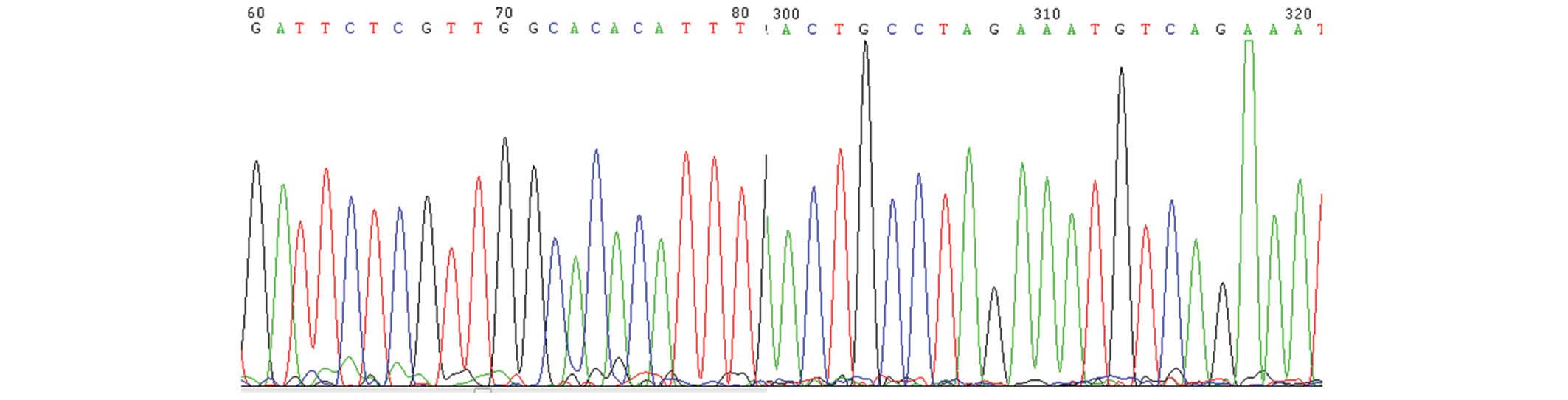
To determine the accuracy of the results, 10% of the
samples were randomly selected (31 from the patient group and 36
from the control group) for direct sequencing, which was performed
by Shanghai Invitrogen Biotechnology Co., Ltd. (Shanghai,
China).
Statistical methods
The age difference between the two groups was
compared using an independent samples t-test; the differences in
gender, allele and genotype frequencies between the two groups were
compared using a χ2 test. P<0.05 was considered to
indicate a statistically significant difference.
Results
qPCR
Using the template DNA, qPCR amplification products
of 329 bp were obtained from Uyghur (n=186) and Han (n=127)
patients with PD. All the products were digested with ScfI
overnight and 228 and 101 bp fragments were obtained as presented
in Fig. 1 (left). Following
amplification, PCR products of 386 bp were obtained, the resulting
products were digested overnight using BstU1, and two
fragments of 314 and 72 bp were obtained as presented in Fig. 1 (right).
Sequence analysis
Following random sequencing comparison, no G2019S
(Fig. 2) and R1441C (Fig. 3) mutations or novel heterozygotes
were found. For G2019S, only the GG genotype and no mutant
genotypes were identified in all subjects of the Han and Uygur
populations. For R1441C, only one genotype (CC) was identified in
the Han and Uygur populations. There were no differences between PD
patients and the control group in these two single nucleotide
polymorphisms (Tables I and II).
 | Table I.G2019S genotype and allele frequency
comparison/cases (%) of Parkinson's disease and control groups of
Uyghur and Han individuals from Xinjiang. |
Table I.
G2019S genotype and allele frequency
comparison/cases (%) of Parkinson's disease and control groups of
Uyghur and Han individuals from Xinjiang.
|
|
| Genotype
frequency | Allele frequency |
| Control group | Allele frequency |
|---|
|
|
|
|
|
|
|
|
|---|
| Ethnic group | PD group | GG | GA | AA | G | A | Control group | GG | GA | AA | G | A |
|---|
| Uyghur | 130 | 130 (100) | 0 (0) | 0 (0) | 260 (100) | 0 (0) | 179 | 179 (100) | 0 (0) | 0 (0) | 358 (100) | 0 (0) |
| Male | 76 | 76
(100) | 0 (0) | 0 (0) | 152 (100) | 0 (0) | 104 | 104 (100) | 0 (0) | 0 (0) | 208 (100) | 0 (0) |
|
Female | 54 | 54 (100) | 0 (0) | 0 (0) | 108 (100) | 0 (0) | 75 | 75
(100) | 0 (0) | 0 (0) | 150 (100) | 0 (0) |
| Han | 182 | 182 (100) | 0 (0) | 0 (0) | 364 (100) | 0 (0) | 181 | 181 (100) | 0 (0) | 0 (0) | 362 (100) | 0 (0) |
|
Male | 109 | 109 (100) | 0 (0) | 0 (0) | 218 (100) | 0 (0) | 109 | 109 (100) | 0 (0) | 0 (0) | 218 (100) | 0 (0) |
|
Female | 73 | 73
(100) | 0 (0) | 0 (0) | 146 (100) | 0 (0) | 72 | 72
(100) | 0 (0) | 0 (0) | 144 (100) | 0 (0) |
 | Table II.R441C genotype and allele frequency
comparison/cases (%) of Parkinson's disease and control groups of
Uyghur and Han individuals from Xinjiang. |
Table II.
R441C genotype and allele frequency
comparison/cases (%) of Parkinson's disease and control groups of
Uyghur and Han individuals from Xinjiang.
|
|
| Genotype
frequency | Allele
frequency |
| Control group | Allele
frequency |
|---|
|
|
|
|
|
|
|
|
|---|
| Ethnic group | PD group | CC | CT | CG | GT | TT | GG | C | T | Control group | CC | CT | CG | GT | TT | GG | C | T |
|---|
| Uyghur | 130 | 130 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 260 (100) | 0 (0) | 179 | 179 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 358 (100) | 0 (0) |
|
Male | 76 | 76 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 152 (100) | 0 (0) | 104 | 104 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 208 (100) | 0 (0) |
|
Female | 54 | 54
(100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 108 (100) | 0 (0) | 75 | 75
(100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 150 (100) | 0 (0) |
| Han | 182 | 182 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 364 (100) | 0 (0) | 181 | 181 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 362 (100) | 0 (0) |
|
Male | 109 | 109 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 218 (100) | 0 (0) | 109 | 109 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 218 (100) | 0 (0) |
|
Female | 73 | 73
(100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 146 (100) | 0 (0) | 72 | 72
(100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 144 (100) | 0 (0) |
Discussion
In recent years, an increasing number of studies
have focused on PD, and in particular on the genes associated with
PD. By far, the LRRK2 gene is the most frequently mutated gene in
autosomal dominant hereditary PD patients, and its incidence
gradually increases with age (1,15–17). The
LRRK2 gene is located on chromosome 12p11.2-q13.1 and has a total
length of 1,441 nucleotides, containing 51 exons and encoding 2,527
amino acids, with a relative molecular weight of 286,000. The
product protein LRRK2/dardarin is rich in leucine (18). In the study of the molecular activity
of LRRK2, two regions associated with GTP enzymes and kinases have
been found. The most common LRRK2 mutations in the two regions
affect the enzymatic activity, suggesting that their functionality
is important (19). Different LRRK2
mutations have been reported in patients with familial and sporadic
PD. Genome-wide association studies (GWAS) have found that common
mutations in SNCA, LRRK2, MAPT and HLA are risk factors for PD, and
have demonstrated that a loss of chromosome 18 can significantly
increase the development of PD (20–33).
G2019S is the most common causative mutation of PD, and ~2% of PD
cases are caused by the G2019S mutation (34). G2019S is located in exon 41 of the
LRRK2 gene; it increases LRRK2 kinase activity and accelerates the
phosphorylation of ezrin/radixin/moesin protein (11); however, while it induces neuronal
apoptosis it has no effect on the GTP carrier or GTP activity.
G2019S reduces the interaction of LRRK2 with the 14-3-3 proteins
and increases the aggregation of and interaction with FADD
(35). Healy et al (17) found that in 19,376 PD patients across
21 regions, the G2019S mutation was highest in North African
populations, and that it accounted for 39% of sporadic PD and 36%
of familial PD. In the Jewish population of Northern Europe, G2019S
accounted for 10% of cases of sporadic and 28% of cases of familial
PD. However, in Asia this locus mutation is very rare, only
accounting for 0.1% of LRRK2 mutations. In Japan, India, Singapore,
China, Taiwan and the mainland, Gly2019Ser and Arg1441Cys/Gly
mutations were not found in PD patients during LRRK2 gene
detection.
R1441C is another common mutation locus in LRRK2, it
is located in exon 31 of the LRRK2 gene, and it induces neuronal
apoptosis. It has no kinase activity, or at least the effect is
negligible; however, it stabilizes the LRRK2 dimer, reduces GTP
activity and participates in FADD aggregation (35). R1441G, found in northern Spain, has
the same codon as R1441H, which primarily occurs in the Caucasian
population (36). Of 304 patients
with PD in Belgium, 18.1% had familial PD, and the R1441C mutation
accounted for 10.7% (37). R1441C is
in the Roc functional domain, with GTP enzyme sequences to
participate in regulatory activities, including signal
transduction, cell differentiation and cell growth (38). R1441C impairs dopamine
neurotransmission and D2 receptor function, leading to degeneration
of the dopaminergic nervous system in patients with PD (38,39). To
the best of our knowledge, there has been no relevant report of the
mutation in Han PD patients.
In the current study, the G2019S and RL441C
mutations of the LRRK2 gene, or any novel variant of these, were
not found to be present in PD patients from the Han and Uyghur
populations. This is consistent with previous studies concerning
the Chinese population (40,41). These mutations may not be hot spot
mutations in PD; or the small sample size of this study may explain
why the mutations were not found. Future studies of these
populations may investigate recent-onset PD patients, larger sample
sizes and other mutations of the LRRK2 gene.
References
|
1
|
Kachergus J, Mata IF, Hulihan M, et al:
Identification of a novel LRRK2 mutation linked to autosomal
dominant parkinsonism: evidence of a common founder across European
populations. Am J Hum Genet. 76:672–680. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kay DM, Zabetian CP, Factor SA, et al:
Parkinson's disease and LRRK2: frequency of a common mutation in
U.S. movement disorder clinics. Mov Disord. 21:519–523. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aasly JO, Toft M, FernandezMata I, et al:
Clinical features of LRRK2-associated Parkinson's disease in
central Norway. Ann Neurol. 57:762–765. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gilks WP, AbouSleiman PM, Gandhi S, et al:
A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet.
365:415–416. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gatto EM, Parisi V, Converso DP, et al:
The LRRK2 G2019S mutation in a series of Argentinean patients with
Parkinson's disease: Clinical and demographic characteristic.
Neurosci Lett. 14:1–5. 2013. View Article : Google Scholar
|
|
6
|
Zhang ZX, Anderson DW, Huang JB, et al:
Prevalence of Parkinson's disease and related disorders in the
elderly population of greater Beijing, China. Mov Disord.
7:764–772. 2003. View Article : Google Scholar
|
|
7
|
Paisán-Ruíz C, Jain S, Evans EW, et al:
Cloning of the gene containing mutations that cause PARK8-linked
Parkinson's disease. Neuron. 44:595–600. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zimprich A, Biskup S, Leitner P, Lichtner
P, et al: Mutations in LRRK2 cause autosomal-dominant parkinsonism
with pleomorpific pathology. Neuron. 44:601–607. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Funayama M, Hasegawa K, Kowa H, et al: A
new locus for Parkinson's disease (PARK8) maps to chromosome 12p11.
2-q13.1. Ann Neurol. 51:296–301. 2002. View Article : Google Scholar
|
|
10
|
Toft M, Mata IF, Ross OA, Kachergus J, et
al: Pathogenicity of the Lrrk2 R1514Q substitution in Parkinson's
disease. Mov Disord. 22:389–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Punia S, Behari M, Govindappa ST,
Swaminath PV, Jayaram S, Goyal V, Muthane UB, Juyal RC and Thelma
BK: Absence/rarity of commonly reported LRRK2 mutations in Indian
Parkinson's disease patients. Neurosci Lett. 409:83–88. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dächsel JC and Farrer MJ: LRRK2 and
Parkinson Disease. Arch Neurol. 67:542–547. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thaler A, Ash E, GanOr Z, OrrUrtreger A
and Giladi N: The LRRK2 G2019S mutation as the cause of Parkinson's
disease in Ashkenazi Jews. J Neural Transm. 116:1473–1482. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thaler A, Mirelman A, Gurevich T, et al:
Lower cognitive performance in healthy G2019S LRRK2 mutation
carriers. Neurology. 79:1027–1032. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldwurm S, Zini M, Mariani L, Tesei S,
Miceli R, Sironi F, Clementi M, Bonifati V and Pezzoli G:
Evaluation of LRRK2 G2019S penetrance: relevance for genetic
counselling in Parkinson disease. Neurology. 68:1141–1143. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haugarvoll K, Rademakers R, Kachergus JM,
et al: LrrK2 R1441C parkinsonism is clinically similar to sporadic
Parkinson disease. Neurology. 70:1456–1460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Healy DG, Falchi M, O'Sullivan SS,
Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP,
Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR,
Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T,
Bhatia KP, Gasser T, Lees AJ and Wood NW: International LRRK2
Consortium: Phenotype, genotype, and worldwide genetic penetrance
of LRRK2-associated Parkinson's disease: a case-control study.
Lancet Neurol. 7:583–590. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paisán-Ruíz C, Lang AE, Kawari T, et al:
LRRK2 gene in Parkinson disease: Mutation analysis and case control
association study. Neurology. 65:696–700. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taymans JM: The GTPase function of LRRK2.
Biochem Soc Trans. 40:1063–1069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Simón-Sánchez J, Schulte C, Bras JM,
Sharma M, et al: Genome-wide association study reveals genetic risk
underlying Parkinson's disease. Nat Genet. 41:1308–1312. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Satake W, Nakabayashi Y, Mizuta I, Hirota
Y, Ito C, Kubo M, Kawaguchi T, Tsunoda T, Watanabe M, Takeda A,
Tomiyama H, Nakashima K, Hasegawa K, Obata F, Yoshikawa T, Kawakami
H, Sakoda S, Yamamoto M, Hattori N, Murata M, Nakamura Y and Toda
T: Genome-wide association study identifies common variants at four
loci as genetic risk factors for Parkinson's disease. Nat Genet.
1303–1307. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pankratz N, Beecham GW, DeStefano AL,
Dawson TM, Doheny KF, Factor SA, Hamza TH, Hung AY, Hyman BT,
Ivinson AJ, Krainc D, Latourelle JC, Clark LN, Marder K, Martin ER,
Mayeux R, Ross OA, Scherzer CR, Simon DK, Tanner C, Vance JM,
Wszolek ZK, Zabetian CP, Myers RH, Payami H, Scott WK and Foroud T:
Meta-analysis of Parkinson's disease: identification of a novel
locus, RIT2. Ann Neurol. 71:370–384. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pankratz N, Wilk JB, Latourelle JC,
DeStefano AL, Halter C, Pugh EW, Doheny KF, Gusella JF, Nichols WC,
Foroud T and Myers RH: PSG-PROGENI, Gene PD and Investigators,
Coordinators and Molecular Generic Laboratries: Genome wide
association study for susceptibility genes contributing to familial
Parkinson disease. Hum Genet. 124:593–605. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edwards TL, Scott WK, Almonte C, Burt A,
Powell EH, Beecham GW, Wang L, Züchner S, Konidari I, Wang G,
Singer C, Nahab F, Scott B, Stajich JM, Pericak-Vance M, Haines J,
Vance JM and Martin ER: Genome-wide association study confirms SNPs
in SNCA and the MAPT region as common risk factors for Parkinson
disease. Ann Hum Genet. 74:97–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hamza TH, Zabetian CP, Tenesa A, Laederach
A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E,
Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt
J, Factor SA and Payami H: Common genetic variation in the HLA
region is associated with late-onset sporadic Parkinson's disease.
Nat Genet. 42:781–785. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saad M, Lesage S, SaintPierre A, Corvol
JC, Zelenika D, Lambert JC, Vidailhet M, Mellick GD, Lohmann E,
Durif F, Pollak P, Damier P, Tison F, Silburn PA, Tzourio C,
Forlani S, Loriot MA, Giroud M, Helmer C, Portet F, Amouyel P,
Lathrop M, Elbaz A, Durr A, Martinez M and Brice A: French
Parkinson's Disease Genetics Study Group: Genome-wide association
study confirms BST1 and suggests a locus on 12q24 as the risk loci
for Parkinson's disease in the European population. Hum Mol Genet.
20:615–627. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Spencer CC, Plagnol V, Strange A, et al:
Dissection of the genetics of Parkinson's disease identifies an
additional association 5′ of SNCA and multiple associated
haplotypes at 17q21. Hum Mol Genet. 20:345–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nalls MA, Plagnol V, Hernandez DG, Sharma
M, Sheerin UM, Saad M, Simón-Sánchez J, Schulte C, Lesage S,
Sveinbjörnsdóttir S, Stefánsson K, Martinez M, Hardy J, Heutink P,
Brice A, Gasser T, Singleton AB and Wood NW: Imputation of sequence
variants for identification of genetic risks for Parkinson's
disease: a meta-analysis of genome-wide association studies.
Lancet. 377:641–649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Plagnol V, Nalls MA, Bras JM, et al: A
two-stage meta-analysis identifies several new loci for Parkinson's
disease. PLoS Genet. 7:e10021422011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Do CB, Tung JY, Dorfman E, Kiefer AK,
Drabant EM, Francke U, Mountain JL, Goldman SM, Tanner CM, Langston
JW, Wojcicki A and Eriksson N: Web-based genome-wide association
study identifies two novel loci and a substantial genetic component
for Parkinson's disease. PLoS Genet. 7:e10021412011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu X, Cheng R, Verbitsky M, Kisselev S,
Browne A, MejiaSanatana H, Louis ED, Cote LJ, Andrews H, Waters C,
Ford B, Frucht S, Fahn S, Marder K, Clark LN and Lee JH:
Genome-wide association study identifies candidate genes for
Parkinson's disease in an Ashkenazi Jewish population. BMC Med.
12:1042011.
|
|
32
|
Pihlstrøm L, Axelsson G, Bjørnarå KA,
Dizdar N, Fardell C, Forsgren L, Holmberg B, Larsen JP, Linder J,
Nissbrandt H, Tysnes OB, Ohman E, Dietrichs E and Toft M:
Supportive evidence for 11 loci from genome-wide association
studies in Parkinson's disease. Neurobiol Aging.
34:1708.e7–1708.e13. 2012. View Article : Google Scholar
|
|
33
|
Sharma M, Ioannidis JP, Aasly JO, et al:
Large-scale replication and heterogeneity in Parkinson disease
genetic loci. Neurology. 79:659–667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martin I, Dawson VL and Dawson TM: The
impact of genetic research on our understanding of Parkinson's
disease. Prog. Brain Res. 183:21–41. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rideout HJ and Stefanis L: The
neurobiology of LRRK2 and its role in the pathogenesis of
Parkinson's disease. Neurochem Res. 39:576–592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rideout HJ and Stefanis L: The
neurobiology of LRRK2 and its role in the pathogenesis of
Parkinson's disease. Neurochem Res. 39:576–592. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mata IF, Wedemeyer WJ, Farrer MJ, Taylor
JP and Gallo KA: LRRK2 in Parkinson's disease: Protein domains and
functional insights. Trends Neurosci. 29:286–293. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Paisán-Ruíz C, Jain S, Evans EW, et al:
Cloning of the gene containing mutations that cause PARK8-linked
Parkinson's disease. Neuron. 44:595–600. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tong Y, Pisani A, Martella G, Karouani M,
Yamaguchi H, Pothos EN and Shen J: R1441C mutation in LRRK2 impairs
dopaminergic neurotransmission in mice. Proc Natl Acad Sci USA.
106:14622–14627. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tan EK, Peng R, Teo YY, et al: Multiple
LRRK2 variants modulate risk of Parkinson disease: a Chinese
multicenter study. Hum Mutat. 31:561–568. 2010.PubMed/NCBI
|
|
41
|
Fung HC, Chen CM, Hardy J, et al: Lack of
G2019S LRRK2 mutation in a cohort of Taiwanese with sporadic
Parkinson's disease. Mov Disord. 21:880–881. 2006. View Article : Google Scholar : PubMed/NCBI
|