Introduction
Depression, a mental disorder with a high incidence,
has been clinically characterized by mental retardation, anhedonia,
a lack of energy and numerous other physical symptoms (1). Depression brings severe stress and
places a burden on the patients themselves, their families and
society as a whole. The etiology and pathogenesis of depression
represent challenging issues in scientific and medical research.
Progress has been made in understanding the occurrence and
development of the disease, which involves several response
elements in a second messenger pathway, including G proteins,
adenylyl cyclase and protein kinase C (PKC) (2–4). In
particular, studies have shown that the expression and function of
PKC are altered in patients or animal models with depression
(5,6). Furthermore, the impairment of neurons
in the brain has been shown to contribute to the onset and
pathogenesis of depression. The modulation of the PKC-cAMP response
element binding protein (CREB) signaling pathway may improve the
plasticity and function of neurons, contributing to the protection
against depression (7,8).
Currently, antidepressant drugs are the major
treatment for depression in the clinic. Paroxetine, a type of
highly selective 5-hydroxytryptamine (5-HT)-reuptake inhibitor, can
inhibit 5-HT reuptake in synapses and increase the nerve conduction
rate of 5-HT, exerting antidepressant effects (9). Due to its rapid action and few adverse
side effects, paroxetine has been widely used in the clinical
treatment of depression; however, the effects of paroxetine on PKC
in depression have not been fully addressed.
In the present study, rat models of depression were
established by the application of chronic unpredictable mild stress
(CUMS), and the effects of paroxetine on the spatial memory and PKC
expression in these models were investigated. The findings provide
an in-depth understanding of the pathogenesis of depression and the
treatment of the disease with antidepressant drugs.
Materials and methods
Experimental animals and grouping
Thirty healthy adult male Sprague Dawley rats
(supplied by Qinglongshan Experimental Animal Breeding Farm,
Nanjing, China), weighing 200±20 g, were randomly divided into
control, model and paroxetine-treated groups (n=10 per group). No
significant differences in body weight, age and basal metabolic
values were found among the groups. The animal experiments were
conducted according to the ethical guidelines of Yan'an University
(Yan'an, China).
Animal modeling and drug
administration
A rat model of depression was established by CUMS,
as previously described (10). In
brief, the model rats were subjected to one of the following
treatments: Restraint through binding for 2 h, water deprivation
for 24 h, fasting for 24 h, reversed day/night cycle for 24 h,
forced swimming for 5 min, heat stress at 50°C for 5 min, ice
swimming at 4°C for 5 min, tail clip for 1 min and foot shock (1
mA) for 10 sec. The models experienced one stimulus on each day and
different stimuli on neighboring days. The procedures lasted for 4
weeks.
Rats in the control group were treated with saline
by gavage (5 ml/kg). The model group was also treated with saline
by gavage (5 ml/kg) prior to the modeling. In the
paroxetine-treated group, the rats were treated with paroxetine
(Yangzhou IL-YANG Pharmaceutical Co., Ltd., Yangzhou, China) by
gavage (10 mg/kg with the dose volume of 5 ml/kg) prior to
modeling.
Morris water maze (MWM)
The spatial learning and memory function of the rat
models were assessed by the MWM assay. A circular pool was divided
into four quadrants, and a circular black platform was placed in
the target quadrant, 2 cm beneath the water surface. Markers in
different shapes and colors were posted on the white curtain around
the pool for navigation. Each rat was allowed to swim for 90 sec to
find the hidden platform (11). The
swimming paths of these rats were recorded by a video capture
system (THDB-D5M, Terasic Technologies, Inc., Taiwan), and time
spent in the target quadrant and the number of times the rats
crossed the platform were calculated accordingly.
Western blot analysis
Following the behavioral test, the rats were
decapitated. The brains were removed and the hippocampi were
isolated and stored at −80°C. The samples were homogenized with
lysis buffer, containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40
and 0.5% Na-deoxycholate, and the supernatant was collected
following centrifugation at 20,000 × g at 4°C for 20 min. Total
protein concentrations were determined using BCA assay. The
expression levels of PKC were detected by western blotting, as
previously described (12–14). Mouse anti-rat monoclonal antibodies
against PKC (1:200; sc-17769) and β-actin (1:5,000; sc-8432) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Goat anti-mouse HRP-conjugated IgG (sc-47047; Santa Cruz
Biotechnology, Inc.) was used. The bound antibodies were determined
by the Pierce enhanced chemiluminescence system (Pierce
Biotechnology Inc., Rockford, IL, USA). The experiments were
performed >3 times. The mean optical density value of each
protein band relative to that of the β-actin band from the same
sample was calculated.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using SPSS software (version
17.0; SPSS Inc., Chicago, IL, USA). The t-test and χ2
test were used for data comparison. P<0.05 was considered to
indicate a statistically significant difference.
Results
Paroxetine improves spatial learning
and memory function in a rat model of depression
To investigate the effects of paroxetine on the
spatial learning and memory function in depression models, an MWM
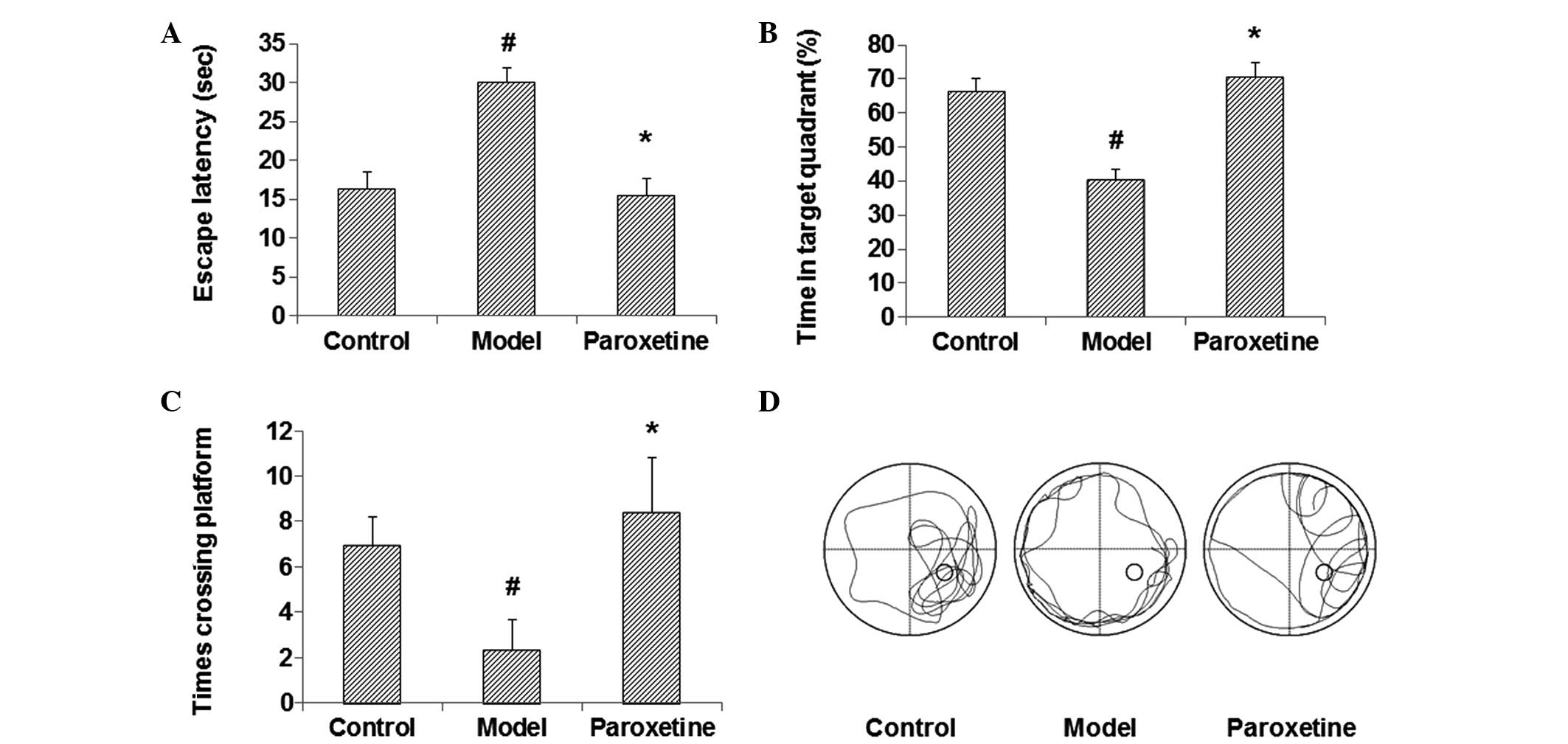
behavioral assay was performed. The results showed that, compared
with the control group, the escape latency was significantly
prolonged, and the percentage of time in the target quadrant and
the number of times the rats crossed the platform were notably
reduced in the model group, all with statistical significance
(P<0.05) (Fig. 1). Following
treatment with paroxetine, however, the escape latency was
evidently shortened and the percentage of time in the target
quadrant and the number of times the rats crossed the platform were
significantly elevated (P<0.05), with results comparable to
those of the control group (Fig. 1).
These findings suggest that paroxetine can improve the spatial
learning and memory function in rat models of depression, almost to
a level comparable with that of the normal control animals.
Paroxetine upregulates the PKC
expression level in the hippocampus in rat models of
depression
PKC and the associated signaling pathways have been
closely linked with the mental processes in the brain and the
pathogenesis of mental disorders, including depression. To
investigate the mechanism through which paroxetine improves spatial
learning and memory function in these depression models, the
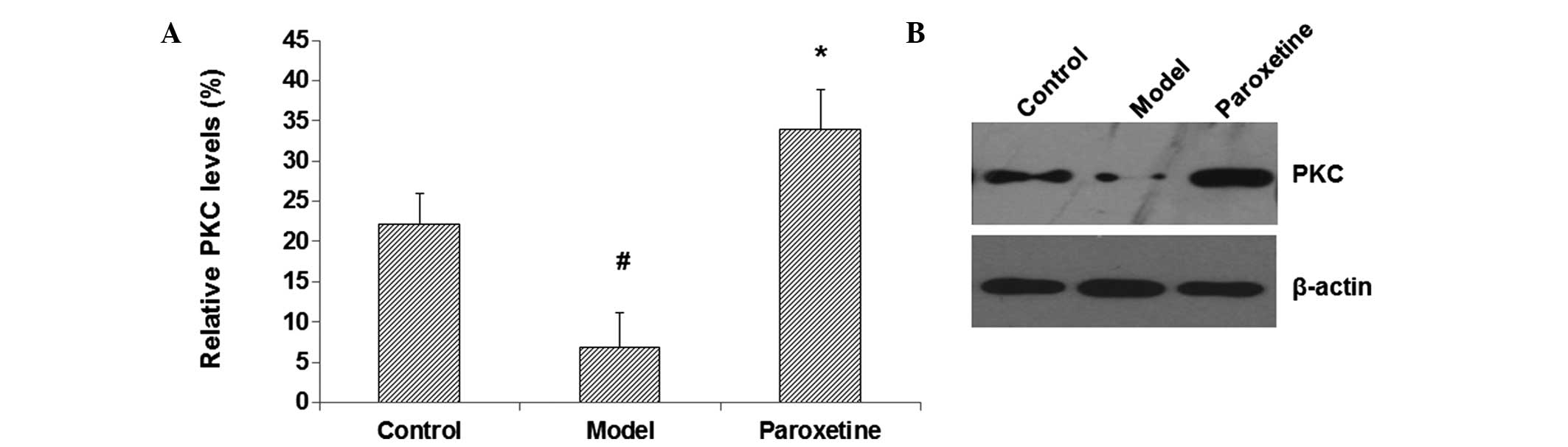
expression levels of PKC in the hippocampus were assessed by
western blot analysis. The results showed that, compared with the
control group, the hippocampal PKC content in the model group was
notably decreased (P<0.05) (Fig. 2A
and B). By contrast, the PKC expression level in the
paroxetine-treated group was significantly increased compared with
that in the model group (P<0.05) (Fig. 2A and 2B). These results suggest that
paroxetine can elevate the expression levels of PKC in the
hippocampus in rat models of depression, which may contribute to
the improvements in the spatial learning and memory function in
these model rats.
Discussion
In the present study, a rat model of depression was
established by CUMS, effectively simulating the major symptoms of
depression in human patients: Anhedonia, reduced autonomous
behavior, declined social communication abilities, and impaired
learning and memory function. A number of studies have shown that
abnormalities in the hippocampus are closely associated with the
occurrence and development of depression (15–18).
Based on these results, the effects of paroxetine on spatial
learning and memory function and the expression levels of PKC in
the hippocampus were investigated in a rat model of depression in
the present study.
As a selective 5-HT reuptake inhibitor, paroxetine
can rapidly increase the level of 5-HT in the brain, suggesting
that paroxetine may target the post-receptor cell signal
transduction pathway (19,20). PKC, a main regulator of the
phospholipase C/Ca2+ system, plays an important role in
cell signal transduction (21).
Through the PKC/CREB signaling pathway, PKC regulates the
excitability of nerve cells and affects the synthesis and release
of monoamine neurotransmitters, with a relatively high content in
the hippocampus (22). Autopsy has
shown that there are lower levels of CREB in the brains of patients
with depression, and CREB levels are elevated in those patients who
have been using antidepressants prior to mortality, indicating that
antidepressants may act on the PKC/CREB signaling pathway (23,24). Liu
and Xu (25) have suggested that the
expression levels of PKCβII on cell membranes in the cerebral
cortex are significantly decreased in rat models of depression, and
that the changes in PKC concentration and/or activity are
associated with the etiology of the disease. In the present study,
the PKC content in the model group was evidently decreased compared
with that in the control group. Furthermore, compared with the
model group, the expression levels of PKC in the paroxetine-treated
group were significantly increased. These results indicate that the
changes in PKC expression levels may be associated with the
pathogenesis of depression. Paroxetine is able to reverse the
changes in PKC levels in the hippocampus, and exert antidepressant
effects, most likely through the PKC/CREB signaling pathway. Zheng
et al (26) have suggested
that the long-term use of paroxetine may restore the
phosphorylation of CREB to normal levels in the hippocampus in rat
models of depression, and increase the concentration of
phosphorylated CREB. The longer the duration of the treatment, the
more reversal effects due to paroxetine would be observed.
In conclusion, the present results showed that
paroxetine improved the spatial learning and memory function in rat
models of depression, and the antidepressant effect may have been
associated with the PKC/CREB signaling pathway. These findings not
only enhance the understanding of the pathogenesis of depression
but also provide experimental evidence for the treatment of
depression with such antidepressant drugs as paroxetine.
Acknowledgements
This study was supported by the High-Level
University Construction Project of Shaanxi, China (grant no.
2013SXTS02).
References
|
1
|
Burton C, McKinstry B, Szentagotai Tătar
A, SerranoBlanco A, Pagliari C and Wolters M: Activity monitoring
in patients with depression: A systematic review. Affect Disord.
145:21–28. 2013. View Article : Google Scholar
|
|
2
|
Wu F, Kong LT and Tang YQ: Effects of
fluoxetine and tianeptine on protein kinase C expression in
hippocampus in rat models of chronic stress. Zhong Guo Quan Ke Yi
Xue. 15:1375–1377. 2012.(In Chinese).
|
|
3
|
Vorhees CV, Morford LR, Graham DL, Skelton
MR and Williams MT: Effects of periadolescent fluoxetine and
paroxetine on elevated plus-maze, acoustic startle, and swimming
immobility in rats while on and off-drug. Behav Brain Funct.
7:412011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie Z, Li HF and Liu JM: Study on the
expression of central protein kinase C and adenylate cyclase in
depression rats with and without antidepressant treatment. Zhong
Guo Shen Jing Jing Shen Ji Bing Za Zhi. 36:225–228. 2010.(In
Chinese).
|
|
5
|
Hahn CG, Umapathy, Wang HY, et al: Lithium
and valproic acid treatments reduce PKC activation and receptor-G
protein coupling in platelets of bipolar manic patients. J
Psychiatr Res. 39:355–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu J and Liu J: Change of cyclic adenosine
monophosphate concentration and PKC expression in behavioral
deficit-induced depression rat brain. Zhong Hua Jing Shen Ke Za
Zhi. 35:173–176. 2002.(In Chinese).
|
|
7
|
Nestler EJ, Barrot M, DiLeone RJ, et al:
Neurobiology of depression. Neuron. 34:13–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wierda KD, Toonen RF, de Wit H, Brussaard
AB and Verhage M: Interdependence of PKC-dependent and
PKC-independent pathways for presynaptic plasticity. Neuron.
54:275–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Purgato M, Papola D, Gastaldon C, Trespidi
C, Magni LR, Rizzo C, Furukawa TA, Watanabe N, Cipriani A and
Barbui C: Paroxetine versus other anti-depressive agents for
depression. Cochrane Database Syst Rev. 4:CD0065312014.PubMed/NCBI
|
|
10
|
Bai YF, Wang Y and Liu XD: Changes of
glial fibrillary acidic protein and c-fos levels in rat models of
chronic stress depression. Zhong Guo Zhi Ye Yi Xue. 37:458–461.
2010.(In Chinese).
|
|
11
|
Keck ME, Sartori SB, Welt T, Müller MB,
Ohl F, Holsboer F, Landgraf R and Singewald N: Differences in
serotonergic neurotransmission between rats displaying high or low
anxiety/depression-like behaviour: Effects of chronic paroxetine
treatment. J Neurochem. 92:1170–1179. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tyeryar KR, Vongtau HO and Undieh AS:
Diverse antidepressants increase CDP-diacylglycerol production and
phosphatidylinositide resynthesis in depression-relevant regions of
the rat brain. BMC Neurosci. 9:122008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang X: Effect of cAMP/PKA/CREB signaling
pathway and regulatory proteins PDE-4 and ERK on learning and
memory function. Yi Xue Zong Shu. 17:2241–2243. 2011.(In
Chinese).
|
|
14
|
Zheng L and Wang YM: Changes in serum
neurotrophic factor 3 after paroxetine treatment in depression. Gui
Yang Yi Xue Yuan Xue Bao. 38:347–350. 2013.(In Chinese).
|
|
15
|
Hu Y, Yin WG, Lin R and Li W: Comparison
of brain-derived neurotrophic factor levels in hippocampus and
serum in two rat models of depression. Zhong Guo Lao Nian Xue Za
Zhi. 29:2188–2190. 2009.(In Chinese).
|
|
16
|
Wei KL, Cheng YM, Sang WH, et al:
Comparative study of duloxetine and paroxetine in treating
depression with different symptoms. Zhongguo Lin Chuang Yao Li Xue
Za Zhi. 27:252–254. 2011.(In Chinese).
|
|
17
|
Yang M, Wen SY and Wu MC: Improvement in
negative emotion and inflammatory factor levels in chronic heart
failure patients after paroxetine treatment. Zhong Guo Yao Fang.
24:3433–3435. 2013.(In Chinese).
|
|
18
|
Fei HZ, Wang H, Hu XY, et al: Improvement
in oxidative stress, HPA axis function, and hippocampal
brain-derived neurotrophic factor expression after paroxetine
treatment. Zhong Guo Lin Chuang Yao Li Xue Za Zhi. 17:1137–1142.
2012.(In Chinese).
|
|
19
|
Meng X: Effects of paroxetine combined
with mental intervention on post-stroke depression. Pract Prev Med.
18:491–493. 2011.
|
|
20
|
Ni GH, Shao B and Fan H: Effects of
restraint stress and paroxetine in post-stroke rats. Chongqing Yi
Xue Za Zhi. 391033–1035. (1038)2010.(In Chinese).
|
|
21
|
Abrial E, Lucas G, Scarna H, Haddjeri N
and Lambás-Señas L: A role for the PKC signaling system in the
pathophysiology and treatment of mood disorders: Involvement of a
functional imbalance? Mol Neurobiol. 44:407–419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Réus GZ, Stringari RB, Ribeiro KF, Ferraro
AK, Vitto MF, Cesconetto P, Souza CT and Quevedo J: Ketamine plus
imipramine treatment induces antidepressant-like behavior and
increases CREB and BDNF protein levels and PKA and PKC
phosphorylation in rat brain. Behav Brain Res. 221:166–171. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding G, Yu G, Wu Y, et al: Effects of
Jiawei Xiaoyao decoction on cAMP, PKA, and PKC levels in
hippocampus of depression rats. Zhong Guo Shi Yan Fang Ji Xue Za
Zhi. 18:162–164. 2012.(In Chinese).
|
|
24
|
Li Z: Role of brain-derived neurotrophic
factor in depression pathogenesis. Shanghai Jiao Tong Da Xue Xue
Bao (Yi Xue Ban). 30:651–655. 2010.(In Chinese).
|
|
25
|
Liu X and Xu J: Changes of protein kinase
CβII expression in subcellular fractions from stress-induced
depression rat brain. Zhong Guo Xing Wei Yi Xue Ke Xue Za Zhi.
14:678–680. 2005.(In Chinese).
|
|
26
|
Zheng H, Ma G and Fu X: Effects of
paroxetine on hippocampus-dependent learning and memory and
ERK-CREB signaling pathway in rat models of depression. Zhong Guo
Yao Xue Za Zhi. 43:1234–1238. 2008.(In Chinese).
|