Introduction
Subclavian steal syndrome (SSS) is a condition
characterized by a steno-occlusive impairment of the proximal
subclavian artery (SA). SSS is most frequently observed in
Caucasians aged >50 years, due to the increased incidence of
atherosclerosis in this population. SSS causes reversal flow in the
ipsilateral vertebral artery (VA) away from the brainstem,
resulting in vertebrobasilar insufficiency (1). The primary cause of SSS is
atherosclerosis. The majority of patients with SSS are
asymptomatic, while symptomatic patients present with neurological
symptoms, including light-headedness, ataxia, vertigo, dizziness,
hearing loss, confusion, headache, nystagmus, visual disturbances,
focal seizures and presyncope (2–4).
Treatment of SSS remains controversial (5). Since the majority of patients with SSS
are asymptomatic, there has been debate as to the necessity of
conducting early treatment. Alcocer et al (6) recommended that patients presenting with
low symptomatology or non-life threatening symptoms be treated with
a conventional approach; however, Sharma et al (7) suggested that SSS-induced chronic
brainstem hypoperfusion could lead to a gradual slowing of speech
and gait in patients and that early diagnosis and treatment could
result in an improved quality of life. Notably, SSS is a risk
factor for cerebral ischemia, which may negatively affect cognitive
function (8); however, it remains
unknown whether SSS is able to cause progressive cognitive
impairment. Thus, the aim of the present study was to investigate
the potential effects of SSS on cognitive function in an
atherosclerotic rabbit model of SSS (9).
Materials and methods
Establishment of the SSS model in
atherosclerotic rabbits
All experimental procedures involving animals were
performed in accordance with the guidelines from the National
Institutes of Health and approved by the Animal Care and Use
Committee of Zhengzhou University Medical School (Zhengzhou,
China). A study population of 48 male New Zealand rabbits (mean
body weight, 3.60±0.4 kg) was purchased from the Shanghai
Laboratory Animal Center (Shanghai, China). The rabbits were
randomly divided into three groups: Control, sham and SSS (n=16 per
group). Rabbits in the sham and SSS groups received a high-lipid
diet (1% cholesterol, 5% yolk, 5% lard and 89% basic feedstuff) for
3 months. The disease model was established according to the method
described by Zhang et al (9).
Briefly, the rabbits were anesthetized with 3% pentobarbital sodium
(1 ml/kg), administered intravenously. The SA was exposed and
ligated proximal to the origin of the VA. Sham-operated rabbits
underwent all surgical procedures, with the exception of the SA
occlusion.
Eyeblink conditioning procedures
The eyeblink experiment was conducted 30 days after
the surgery. Rabbits (n=10 per group) were adapted to a Plexiglas
restrainer box (Sigma-Aldrich, St. Louis, MO, USA) for a single
20-min session 3 days prior to the initiation of the experiments.
The conditioned stimulus (CS) was a 1-kHz, 85-dB tone, and the
unconditioned stimulus (US) was a 6-psi corneal airpuff. In the
delay-conditioning paradigm, the duration of the tone was 500 msec,
followed 400 msec after its onset by a 100-msec airpuff US. The CS
and US co-terminated. The rabbits received 7 training sessions over
7 consecutive days. Each session consisted of 80 paired CS-US
trials, and the inter-trial interval was 20–30 sec. One week after
the last day of training, the rabbits received 15 trials in which
only the CS was presented.
5-Bromo-2′-deoxyuridine (BrdU)
labeling and tissue preparation
A further 18 rabbits (n=6 per group) were
administered BrdU (Sigma-Aldrich) at a dosage of 100 mg/kg body
weight (one intraperitoneal administration per day) for the last 3
days of the experimental period. The rabbits were sacrificed 2 h
after the last injection. The rabbits were intravenously
anesthetized with 3% pentobarbital sodium (1 ml/kg) and sacrificed.
The obtained tissues were transcardially perfused with ice-cold
0.9% NaCl solution, followed by a freshly prepared solution of 4%
paraformaldehyde in 0.1 M sodium phosphate buffer. The brains were
removed and postfixed overnight, cryostat-sectioned (30 µm) in
series and stored at −80°C.
Immunohistology
For BrdU and Ki67 immunohistology, antigen retrieval
was performed by incubating the slides in 10 mM sodium citrate
buffer (pH 6.0) at 95–98°C for 15 min. After cooling for 1 h, the
sections were rinsed with 0.1 M phosphate-buffered saline (PBS) and
treated with blocking solution (3% bovine serum albumin, 5% goat
serum and 3% Triton in 0.1 M PBS; Sigma-Aldrich) for 2 h at room
temperature. The slides were incubated with monoclonal mouse
anti-rabbit antibodies against BrdU (1:5,000; NA61-100UGCN; EMD
Millipore, Billerica, MA, USA) and Ki67 (1:1,000; 12160; Cell
Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C.
After washing three times in 0.1 M PBS with Tween-20, the sections
were incubated with the Cyanine 3-conjugated secondary goat
anti-mouse Alexa Fluor 488 antibodies (1:1,000; A10547; Invitrogen
Life Technologies, Carlsbad, CA, USA) for 2 h at room temperature.
The sections were counterstained with DAPI and mounted with an
aqueous mounting medium.
Cerebellar dialysis and
high-performance liquid chromatography (HPLC) measurements
Extracellular concentrations of adenosine
triphosphate (ATP) and adenosine in the cerebellar cortex were
evaluated using microdialysis techniques. The animals were
positioned in a stereotactic frame. Prior to insertion, the probes
were perfused with artificial cerebrospinal fluid (CSF), containing
147 mM NaCl, 4 mM KCl, 2.3 mM CaCl2 and 0.9 mM
MgCl2 (pH 7.4), at a rate of 2 µl/min. In all rabbits, a
360-min stabilization period was implemented following the
insertion of the microdialysis probe. The dialysate was collected
every 15 min for later analysis. The concentrations of ATP and
adenosine were determined using an Agilent 1200 HPLC system with a
photodiode array detector (Agilent Technologies, Santa Clara, CA,
USA), and separation was performed using a Hypersil
base-deactivated silica C-18 reverse-phase column (column length,
300 mm; column ID, 4.6 mm; particle size, 10 µm; Thermo Scientific,
Inc., Waltham, MA, USA). The mobile phase consisted of 0.1 M
phosphate buffer (pH 7.0) and methanol (1%). The flow rate was 1
ml/min and the column temperature was 25°C. Ultraviolet (UV)
detection was conducted at 254 nm using an Ultraviolet Detector
(GD-3; Puyang Medical Instrument Co. Ltd., Nanjing, China). The
concentrations of ATP and adenosine were quantified based on linear
calibration.
Cytokine and enzyme activity
assays
The levels of interleukin (IL)-1β, IL-6,
malondialdehyde (MDA) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) and
the activities of CuZn-superoxide dismutase (CuZn-SOD) and catalase
(CAT) were determined using commercial kits (Nanjing Jiancheng
Bioengineering Research Institute, Nanjing, China).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. The differences in cell-counting results were analyzed
using the non-parametric Mann-Whitney test. Repeated measures
analysis of variance (ANOVA) and Fisher's protected least
significant difference (LSD) multiple comparison test were used to
determine differences in ATP and adenosine concentrations among the
groups. All other results were compared using one-way ANOVA,
followed by the LSD post hoc test for intergroup comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Behavioral experiments
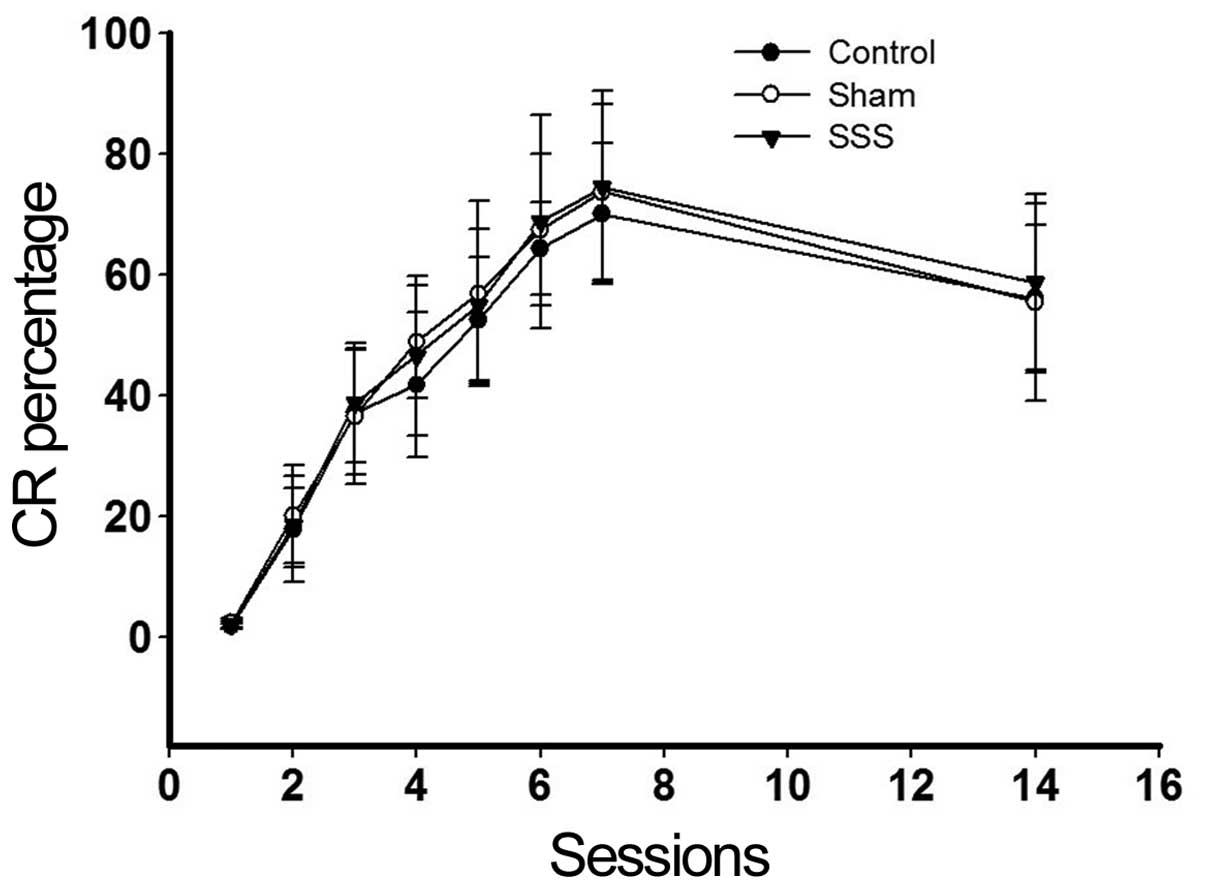
To observe the effects of SSS on the cognition of
the rabbits, eyeblink experiments were conducted. No significant
differences were found among the results from three groups
(Fig. 1; P>0.05).
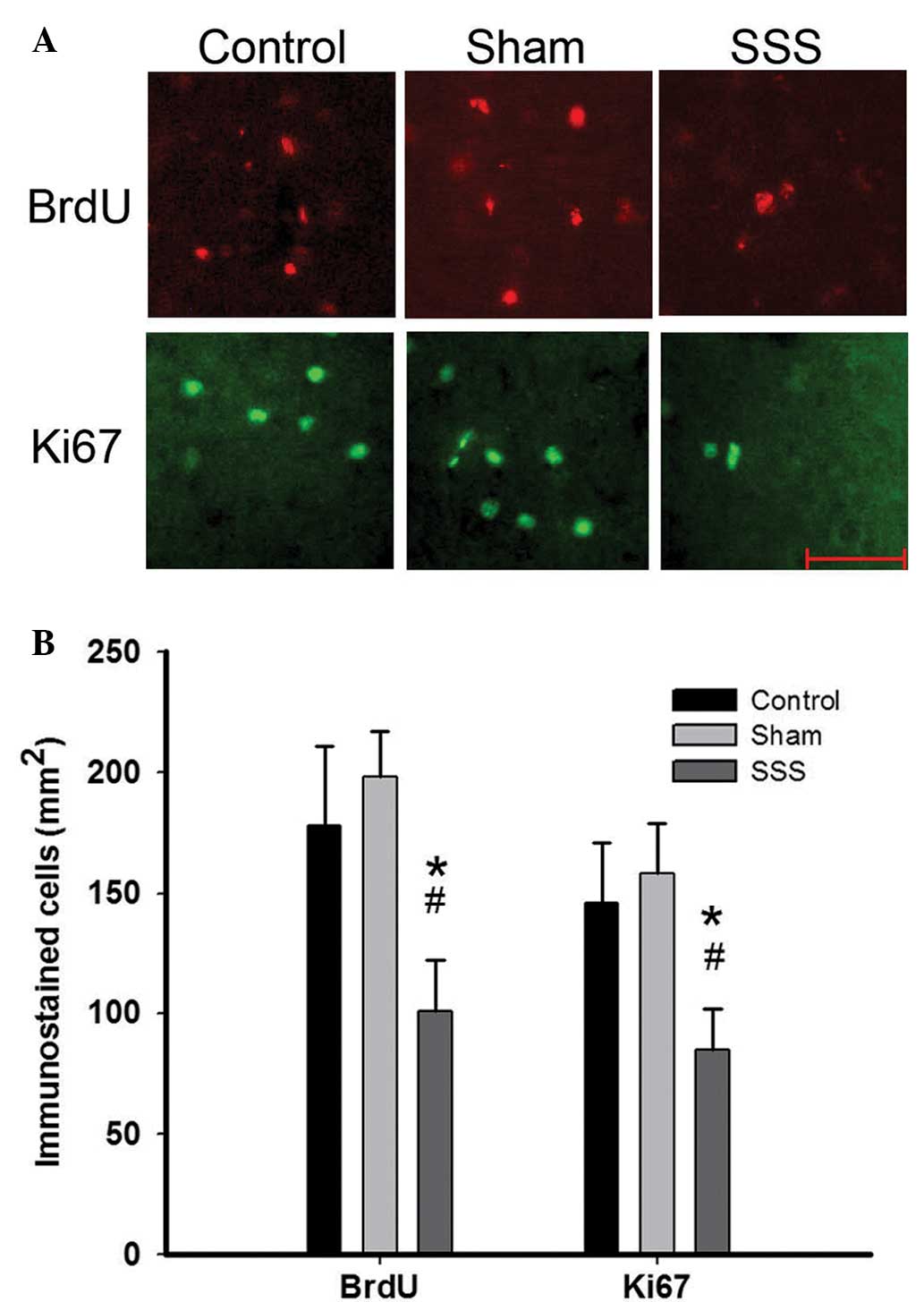
Effects of SSS on newly generated
cells in the rabbit brains
Neurogenesis in the rabbit brains was examined.
Quantitative assessment revealed no significant difference in the
number of BrdU- or Ki67-labeled cells in the subventricular or
subgranular zones among the three groups (data not shown). Notably,
a reduced number of BrdU- and Ki67-positive cells were observed in
the cerebellar cortex of rabbits in the SSS group (101±21 and 85±17
cells/mm2, respectively) compared with the control
(178±33 and 146±25 cells/mm2, respectively) and sham
(198±19 and 158±21 cells/mm2, respectively) groups
(Fig. 2; P<0.05), suggesting that
SSS exerted a negative impact on neurogenesis in the cerebellar
cortex.
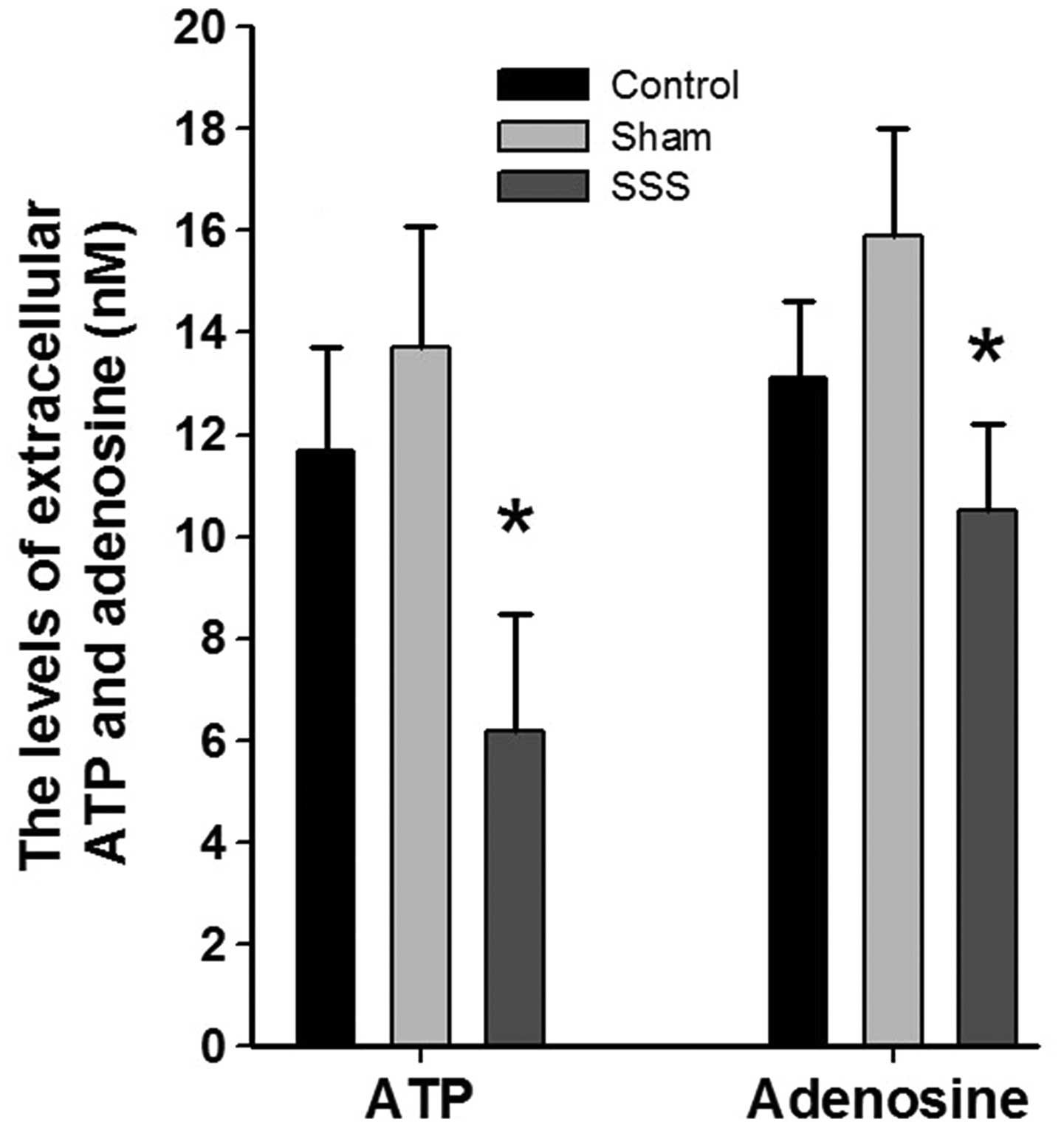
Extracellular concentrations of ATP
and adenosine in the cerebellar cortex
ATP and adenosine levels in the CSF of the
cerebellar cortex were measured in vivo using microdialysis. The
CSF samples were processed using HPLC, and the levels of ATP and
adenosine were determined with a UV detector. Immediately following
probe insertion, the ATP and adenosine levels were elevated due to
the tissue damage caused by probe insertion. The initially elevated
levels of ATP and adenosine declined to plateau levels within 6 h.
At 6 h after probe insertion, the extracellular concentrations of
ATP and adenosine in the SSS group rabbits (n=5) were 6.2±2.3 and
10.5±1.7 nM compared with the control group, respectively (Fig. 3; P<0.05). By comparison, the
concentrations of ATP and adenosine in the control and sham group
rabbits (n=5 per group) were 11.7±2.0 and 13.1±1.5 nM, and 13.7±2.4
and 15.9±2.1 nM, respectively (Fig.
3). Thus, reduced levels of ATP and decreased levels of
adenosine were found in the cerebellar cortex, and these
submicromolar differences in ATP concentration among the groups
were consistent with the results of the immunohistological
analyses.
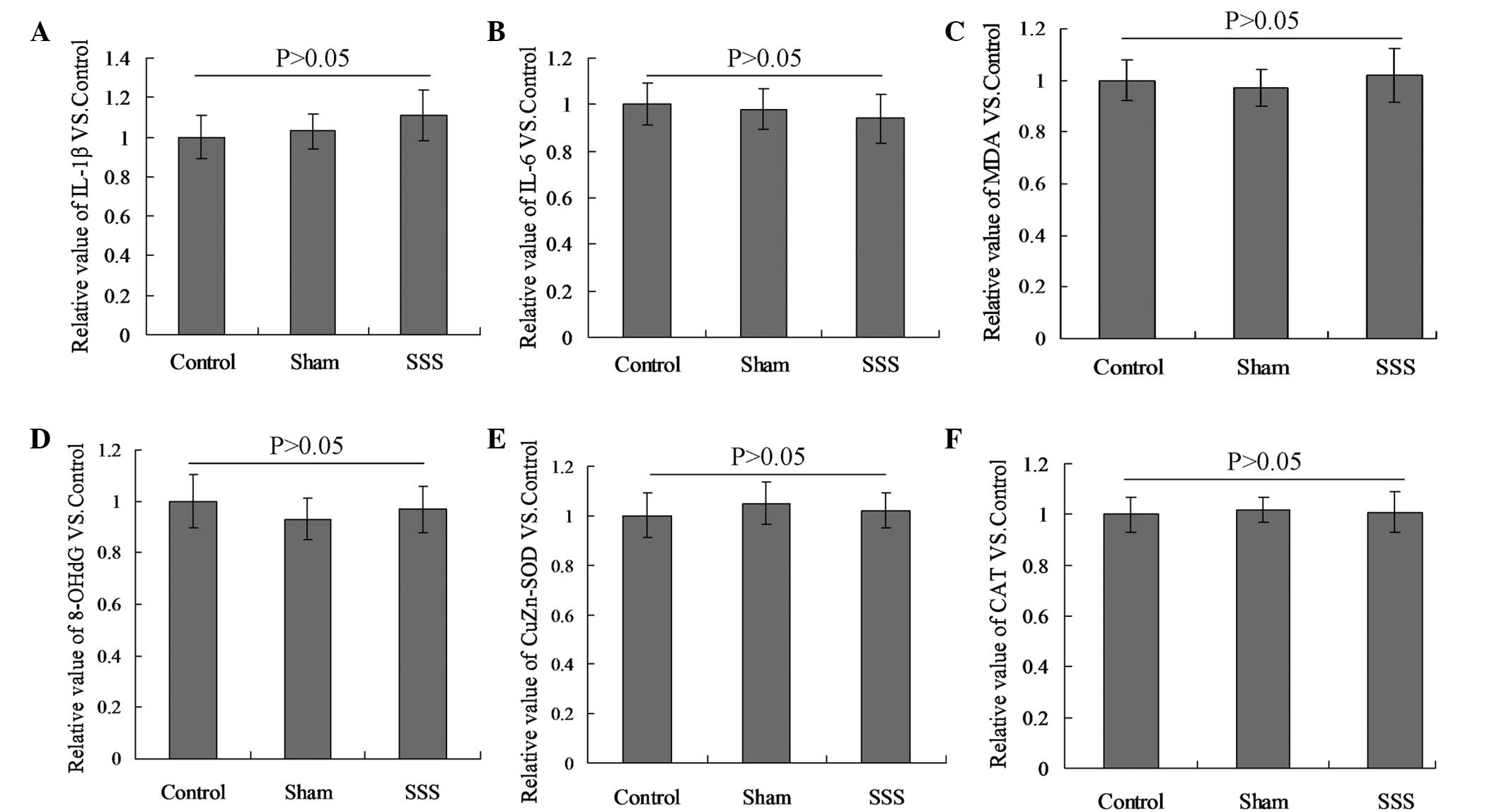
SSS does not affect cytokine
levels
No differences were detected in the levels of IL-1β,
IL-6, MDA, 8-OHdG, CuZn-SOD and CAT among the three groups
(Fig. 4; P>0.05).
Discussion
Patients with SSS may present with a series of
neurological and vascular symptoms; however, the condition is most
commonly diagnosed as an incidental finding in asymptomatic
patients. For certain patients, the symptoms of cerebral ischemia
resulting from SSS may be aggravated by arm exercise. Reduced flow
resistance due to exercise-induced vasodilation in the upper
extremities may cause the diversion of cerebral blood flow to
threshold in certain patients, resulting in an increased risk of
ischemic stroke in the posterior circulation (8). As the brainstem and cerebellum are
primarily supplied by the basilar and vertebral arteries, ischemic
stroke in the posterior circulation can result in a broad range of
symptoms. These include classic manifestations of ischemia in the
posterior territory, including reduction in consciousness, visual
field defects, vertigo, ataxia and eye movement disorders, in
addition to non-localizing symptoms, such as confusion,
disorientation and memory loss (10).
The cerebellum plays a central role in movement
execution and motor control functions, such as maintaining balance
and muscle tension. Furthermore, it has been suggested that the
cerebellum is involved in learning and memory, cognition, speech,
perception and the generation of emotions (11). In the present study, the effects of
SSS on the cognition of rabbits were characterized using eyeblink
experiments. Although the results of the eyeblink experiments
indicated no significant difference among the three groups, SSS
appeared to exert a negative impact on neurogenesis in the rabbit
cerebellar cortex (Fig. 2;
P<0.05).
A previous study described the existence of a
secondary germinal matrix persisting after puberty in a subpial
position of the cerebellum, called the subpial layer (SPL), in the
New Zealand white rabbit (12). The
SPL originates from structural modifications of the external
granule layer and has the ability to generate neuronal precursors
on the cerebellar surface. Analyses of neurogenesis in the
cerebellum at different stages, by the detection of Ki67 and BrdU,
revealed marked cell proliferation occurring around puberty
(12). In addition, it was notable
that a number of newly born cells were detectable in fully adult
rabbits (1–2 years old) in the absence of a germinative SPL. The
function of these newly generated cells is not yet clear, although
it is possible that these new cells (such as interneurons) are
required in neuronal circuits formed by pre-existing cells
(13). The neural circuits in the
cerebellar cortex are simple, and the function of the cerebellar
cortex is closely associated with motor learning (14). Wang and Liu (15) investigated the motor learning process
in rabbits using an eyeblink experiment. The results showed that
the cerebellar cortex was able to execute the function of spatial
and temporal focusing in motor learning and control. On the basis
of these results and those of previous studies, it is possible that
the negative effect of SSS on neurogenesis observed in the present
study may potentially result in the damage of motor memory.
In order to further clarify the mechanisms
underlying the SSS-induced reduction in cell proliferation, the
energy metabolism (extracellular ATP and adenosine), immune
function (IL-1β and IL-6) and oxidative stress (CuZn-SOD, CAT, MDA
and 8-OHdG) statuses were detected. The results indicated that
energy metabolism in the cerebellar cortex was altered, while the
levels of extracellular ATP were decreased and the levels of
adenosine were also decreased (Fig.
3; P<0.05). It is established that brain tissues respire
glucose to produce energy under normal conditions. While under the
conditions of chronic ischemia, this glucose utilization in the
cerebellum is reduced. We hypothesized that chronic ischemia
induced by SSS were the cause of the observed changes.
Extracellular ATP is involved in the induction of neurogenesis
(16). Deficient ATP release from
astrocytes leads to dysfunction in neural stem cell proliferation,
and this can be normalized by the administration of exogenous ATP
(17). Furthermore, it has been
revealed that ATP functions as a mitogenic signal for progenitor
and neural stem cells, potentially in an autocrine manner (16). In addition, previous studies have
demonstrated that cognitive deficits induced by seizures in young
animals may be a consequence of the increased ATP hydrolysis
(18,19). In the present study, SSS appeared to
induce a reduction in extracellular ATP levels in rabbits, and we
hypothesize that this change may impair cognition in rabbits, via
direct and indirect mechanisms.
In conclusion, the results of the present study
suggest that SSS may inhibit neurogenesis in the cerebellar cortex
of rabbits. Furthermore, the findings indicate that the
extracellular ATP reduction may be closely associated with reduced
cell proliferation. Collectively, the results of the present study
suggest that SSS may impair cognitive function in rabbits. The
early diagnosis and treatment of SSS may, therefore, mitigate or
prevent cognitive impairment in the future.
Acknowledgements
This study was supported by the National Key
Clinical Unit Project (2011).
References
|
1
|
Reivich M, Holling HE, Roberts B and Toole
JF: Reversal of blood flow through the vertebral artery and its
effect on cerebral circulation. N Engl J Med. 265:878–885. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aribas BK, Arda K, Aribas O, Ciledag N,
Yologlu Z, Aktas E, Seber T, Kavak S, Cosar Y, Kaygusuz H and Tekin
E: Comparison of subcutaneous central venous port via jugular and
subclavian access in 347 patients at a single center. Exp Ther Med.
4:675–680. 2012.PubMed/NCBI
|
|
3
|
Fields WS: Reflections on ‘the subclavian
steal’. Stroke. 1:320–324. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith JM, Koury HI, Hafner CD and Welling
RE: Subclavian steal syndrome. A review of 59 consecutive cases. J
Cardiovasc Surg (Torino). 35:11–14. 1994.PubMed/NCBI
|
|
5
|
de Souza JM, Espinosa G, Santos Machado M
and Soares PJ: Bilateral occlusion associated to steal phenomenon
of internal carotid and left subclavian arteries: treatment by
angioplasty and stenting. Surg Neurol. 67:298–302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alcocer F, David M, Goodman R, Jain SK and
David S: A forgotten vascular disease with important clinical
implications. Subclavian steal syndrome. Am J Case Rep. 14:58–62.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharma VK, Chuah B, Teoh HL, Chan BP,
Sinha AK and Robless PA: Chronic brainstem ischemia in subclavian
steal syndrome. J Clin Neurosci. 17:1339–1341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yonas H, Smith HA, Durham SR, Pentheny SL
and Johnson DW: Increased stroke risk predicted by compromised
cerebral blood flow reactivity. J Neurosurg. 79:483–489. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang GY, Chen ZQ, Ling F, Li YJ, Wang Y
and Gu BX: Establishment of a novel hemodynamic cerebral ischemia
model of atherosclerotic rabbit. Neurol India. 58:191–194. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Markus HS, van der Worp HB and Rothwell
PM: Posterior circulation ischaemic stroke and transient ischaemic
attack: Diagnosis, investigation and secondary prevention. Lancet
Neurol. 12:989–998. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buckner RL: The cerebellum and cognitive
function: 25 years of insight from anatomy and neuroimaging.
Neuron. 80:807–815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ponti G, Peretto P and Bonfanti L: A
subpial, transitory germinal zone forms chains of neuronal
precursors in the rabbit cerebellum. Dev Biol. 294:168–180. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ponti G, Crociara P, Armentano M and
Bonfanti L: Adult neurogenesis without germinal layers: The
‘atypical’ cerebellum of rabbits. Arch Ital Biol. 148:147–158.
2010.PubMed/NCBI
|
|
14
|
Yoshizawa K, Emoto Y, Kinoshita Y, Yuri T
and Tsubura A: N-methyl-N-induced cerebellar hypoplasia in rats:
Effect of arachidonic acid supplementation during the gestational,
lactational and post-weaning periods. Exp Ther Med. 6:627–634.
2013.PubMed/NCBI
|
|
15
|
Wang L and Liu SQ: Neural circuit and its
functional roles in cerebellar cortex. Neurosci Bull. 27:173–184.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin JH, Takano T, Arcuino G, Wang X, Hu F,
Darzynkiewicz Z, Nunes M, Goldman SA and Nedergaard M: Purinergic
signaling regulates neural progenitor cell expansion and
neurogenesis. Dev Biol. 302:356–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao X, Li LP, Qin XH, Li SH, Zhang M, Wang
Q, Hu HH, Fang YY, Gao YB, Li XW, et al: Astrocytic adenosine
5′-triphosphate release regulates the proliferation of neural stem
cells in the adult hippocampus. Stem Cells. 31:1633–1643. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cognato GP, Vuaden FC, Savio LE, Bellaver
B, Casali E, Boqo MR, Souza DO, Sevigny J and Bonan CD: Nucleoside
triphosphate diphosphohydrolases role in the pathophysiology of
cognitive impairment induced by seizure in early age. Neuroscience.
180:191–200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu L, Tang L, Wei W, Hong Y, Chen H, Ying
W and Chen S: Nicotinamide mononucleotide improves energy activity
and survival rate in an in vitro model of Parkinson's disease. Exp
Ther Med. 8:943–950. 2014.PubMed/NCBI
|