Introduction
The inflammatory process plays an important role in
the occurrence and development of ischemic cerebrovascular disease.
Evidence has shown that with the incidence of ischemia, an
inflammatory response also occurs and participates in the injury
and repair of brain tissues. The inflammatory cascade reaction
following cerebral infarction is an important pathophysiological
mechanism of ischemic stroke (1–3). The
acute phase of the post-stroke inflammatory response is a
reflective and non-specific systemic inflammatory response,
accompanied by the accumulation of inflammatory cells, the release
of inflammatory cytokines, the upregulation of leukocyte adhesion
molecules, and the migration of macrophages and neutrophils to
areas of tissue damage. Furthermore, some peripheral inflammatory
indicators also change significantly, for example, white blood cell
counts increase, and serum interleukin-6 and C-reactive protein
(CRP) levels also increase (4–6).
Bacterial or viral infections can induce ischemic stroke;
furthermore, stroke, especially a serious stroke, is often
complicated by pulmonary or urinary tract infections. Bacterial
infection is likely to exacerbate the inflammatory response and
increase brain tissue damage. Studies have confirmed that infection
and fever have a significant correlation with a poor prognosis for
stroke (7,8). Prevention and treatment of bacterial
infections, lowering the body temperature and controlling the
inflammatory response have become treatment strategies for the
acute phase of ischemic stroke. However, so far, little is known
about the causes of the early systemic inflammatory response in
ischemic stroke, and about the dynamic changes of most inflammatory
markers in the acute phase of stroke.
In this study, dynamic changes of inflammatory
indicators, such as body temperature, peripheral blood leukocyte
counts and serum high-sensitivity (hs)-CRP levels in patients with
acute cerebral infarction were observed with the aim of assessing
the following hypotheses: The post-stroke systemic inflammatory
response mainly occurs because of the necrosis of brain tissue,
rather than cryptogenic infection. Successful thrombolytic
treatment may reduce the necrosis of brain tissue and the
inflammatory response, resulting in an earlier return of the
inflammatory indicators to their normal ranges.
Materials and methods
General information
In total, 62 patients with acute cerebral infarction
and intravenous thrombolytic indications were admitted to the
Department of Neurology of the Second Affiliated Hospital of Fujian
Medical University (Quanzhou, China) from January 2009 to February
2013. The patients comprised 36 males and 26 females and ranged
from 35 to 79 years old (mean, 62±10.4 years). The patients were
divided into three groups according to their treatment and
response: i) successful thrombolysis group (n=36); following
thrombolytic therapy, the reduction of National Institutes of
Health Stroke Scale (NIHSS) score within 24 h was ≥4. ii) failed
thrombolysis group (n=12); following thrombolytic therapy, the
reduction of NIHSS score within 24 h was <4; iii) control group
(n=14); no thrombolysis was performed, and only conventional
antiplatelet or anticoagulant therapy was applied. Intravenous
thrombolysis was administered within 3.0 h of stroke incidence in
30 patients, and within 3.0 −4.5 h in 18 cases. This study was
conducted in accordance with the Declaration of Helsinki and with
approval from the Ethics Committee of Fujian Medical University.
Written informed consent was obtained from all participants.
Enrollment standards
The inclusion criteria were as follows: i) aged
18–80 years; ii) signs of brain dysfunction persisted for >1 h,
and the NIHSS score of nerve function was ≥4 points; iii) stroke
incidence was <4.5 h ago; iv) blood pressure <180/100 mmHg;
v) brain computed tomography (CT) excluded intracranial hemorrhage,
and no imaging changes indicative of acute cerebral infarction were
observed; iv) if the patients and their families agreed to
thrombolytic therapy, they were required to sign an informed
consent form, otherwise they were treated with conventional
therapy.
Exclusion criteria
The exclusion criteria were as follows: Intracranial
hemorrhage, including secondary hemorrhage; already existing
post-stroke infectious diseases; trauma and vascular events
occurring 4 weeks before the stroke; chronic inflammation or
malignancy; use of anti-inflammatory drugs, such as adrenal
corticosteroids and non-steroidal anti-inflammatory agents (with
the exception of aspirin); serious heart, liver or renal
insufficiency.
Treatment
The patients in the successful thrombolysis group
and failed thrombolysis group were treated intravenously with
recombinant tissue-type plasminogen activator (alteplase/Actilyse;
Boehringer Ingelheim, Ingelheim am Rhein, Germany) at a total
dosage of 0.9 mg/kg body weight (maximum dosage, 90 mg); 10% of the
dosage was intravenously injected in the first 1 min, the rest was
then intravenously injected uniformly over 60 min. Aspirin 100
mg/day or clopidogrel 75 mg/day was given orally 24 h after the
thrombolysis. Patients with atrial fibrillation were also given
4,000 IU low-molecular-weight heparin by abdominal subcutaneous
injection twice/day (9,10). Patients in the control group were
treated orally with aspirin 100 mg/day or clopidogrel 75 mg/day.
Patients with atrial fibrillation were also given 4,000–5,000 IU
low-molecular-weight heparin, by abdominal subcutaneous injection
twice/day.
Laboratory tests
Routine cranial CT scanning was performed on
enrollment and 24 h after the initiation of treatment. If the
disease developed, CT scanning or cranial magnetic resonance
imaging was performed in a timely manner. Daily measurements of
body temperature were taken (axillary) at least 4 times, and the
body temperature and daily maximum temperature were adopted as
statistical data. The peripheral blood leukocyte counts were
determined on the day of admission, and on days 1, 3, 5 and 7 after
this, using conventional laboratory methods (normal range,
4×109−10×109/l). An immunoturbidimetry method
(Wide Range C-Reactive Protein reagent; Siemens Healthcare
Diagnostics Inc., Malvern, PA USA) was used for the determination
of hs-CRP concentration (normal range, 1.4–11.0 mg/l).
Efficacy evaluation
The NIHSS was used to score the neurological
deficits, and the scores were recorded on the day of admission and
on days 1, 3, 5 and 7, respectively.
Statistical analysis
SPSS statistical software package version 11.0
(SPSS, Inc., Chicago, IL, USA) was used, and data are presented as
the mean ± standard deviation. Comparisons between groups are
performed using a Student's t-test, while more than two groups were
compared by one-way analysis of variance. Spearman's correlation
analysis was applied to determine the correlation of two
non-normally distributed variables. P<0.05 was considered to
indicate a statistically significant result.
Results
Baseline clinical characteristics
The baseline clinical characteristics, including
gender, age, hypertension, diabetes, auricular fibrillation, stroke
type and thrombolysis/treatment time in the three groups are shown
in Table I. There were no
significant differences between gender ratio and age among the
three groups.
 | Table I.Basic clinical characteristics of the
62 cases. |
Table I.
Basic clinical characteristics of the
62 cases.
| Items | Successful
thrombolysis (%) | Failed thrombolysis
(%) | Control (%) |
|---|
| Total number | 36 | 12 | 14 |
| Male | 20 (55.6) | 7 (58.3) | 9 (64.3) |
| Female | 16 (44.4) | 5 (41.7) | 5 (35.7) |
| Age, mean ± SD | 61.5±10.0 | 62.8±11.2 | 63.5±10.4 |
| Hypertensive
disease | 12 (33.3) | 7 (58.3) | 8 (57.14) |
| Diabetics | 8
(22.2) | 4 (33.3) | 6 (42.9) |
| Auricular
fibrillation | 10 (27.8) | 6 (50.0) | 6 (42.9) |
| Stroke type
(TOAST)a |
|
|
|
|
Atherosclerosis | 6
(16.7) | 2 (16.7) | 2 (14.3) |
|
Cardiogenic embolism | 10 (27.8) | 6 (50.0) | 6 (42.9) |
| Arterial
embolism | 8
(22.2) | 2 (16.7) | 3 (21.4) |
| Other
reason | 2 (5.6) | 0 | 0 |
| Unknown
reason | 10 (27.8) | 2 (16.7) | 3 (21.4) |
|
Thrombolysis/treatment within 0–3 h | 24 (66.7) | 6 (50.0) | 5 (35.7) |
|
Thrombolysis/treatment within 3–4.5 h | 12 (33.3) | 6 (50.0) | 9 (4.3) |
Inflammatory indicators in 62 cases of
acute cerebral infarction
The inflammatory indicators, including body
temperature, peripheral blood leukocyte count and hs-CRP in the 62
cases at various time-points are shown in Table II. Spearman's correlation analysis
demonstrated that the NIHSS score of body temperature on
post-stroke day 3 was significantly higher, as comparde with on the
day of admission (prior to treatment) (P<0.05). The NIHSS scores
of peripheral blood leukocyte count on days 1 and 3 were
significantly higher, as compared with on the day of admission
(P<0.05). The NIHSS scores of hs-CRP on days 3, 5 and 7 were
significantly higher, as compared with on the day of admission
(P<0.05).
 | Table II.Inflammatory indicators in 62 cases of
acute cerebral infarction. |
Table II.
Inflammatory indicators in 62 cases of
acute cerebral infarction.
| Time-point | Body temperature
(°C) | Leukocytes
(x109/l) | hs-CRP (mg/l) |
|---|
| On admission | 36.5±0.5 |
8.8±1.8 | 8.9±6.8 |
| Post-stroke day
1 | 36.6±0.6 | 11.1±2.1a | 10.2±7.6 |
| Post-stroke day
3 | 37.1±0.8a | 12.4±2.5a |
65.8±12.8a |
| Post-stroke day
5 | 36.8±0.5 |
8.9±1.6 |
38.2±9.9a |
| Post-stroke day
7 | 36.5±0.6 |
8.6±1.5 |
25.3±8.4a |
Correlation analysis
The correlations of NIHSS score at admission and
inflammatory indicators are shown in Table III. Spearman's correlation analysis
demonstarted that the NIHSS score on the day of admission (prior to
treatment) was significantly positively correlated with peripheral
blood leukocyte count on post-stroke days 1 and 3 (P<0.05). It
was also significantly positively correlated with the hs-CRP level
on days 3, 5 and 7 (P<0.05). The NIHSS score was only
significantly positively correlated with body temperature on day 3
(P<0.05).
 | Table III.Correlation of NIHSS score at
admission and inflammatory indicators. |
Table III.
Correlation of NIHSS score at
admission and inflammatory indicators.
| Inflammatory
indicator | N | Spearman's rho | P-value |
|---|
| Body
temperature |
|
|
|
| On
admission | 62 | 0.115 |
0.250 |
|
Post-stroke day 1 | 62 | 0.122 |
0.195 |
|
Post-stroke day 3 | 62 | 0.558 | <0.001 |
|
Post-stroke day 5 | 62 | 0.152 |
0.145 |
|
Post-stroke day 7 | 62 | 0.136 |
0.182 |
| Leukocyte |
|
|
|
| On
admission | 62 | 0.145 |
0.150 |
|
Post-stroke day 1 | 62 | 0.232 |
0.035 |
|
Post-stroke day 3 | 62 | 0.355 |
0.002 |
|
Post-stroke day 5 | 62 | 0.150 |
0.166 |
|
Post-stroke day 7 | 62 | 0.130 |
0.194 |
| hs-CRP |
|
|
|
| On
admission | 62 | 0.155 |
0.139 |
|
Post-stroke day 1 | 62 | 0.162 |
0.110 |
|
Post-stroke day 3 | 62 | 0.625 | <0.001 |
|
Post-stroke day 5 | 62 | 0.457 | <0.001 |
|
Post-stroke day 7 | 62 | 0.340 |
0.003 |
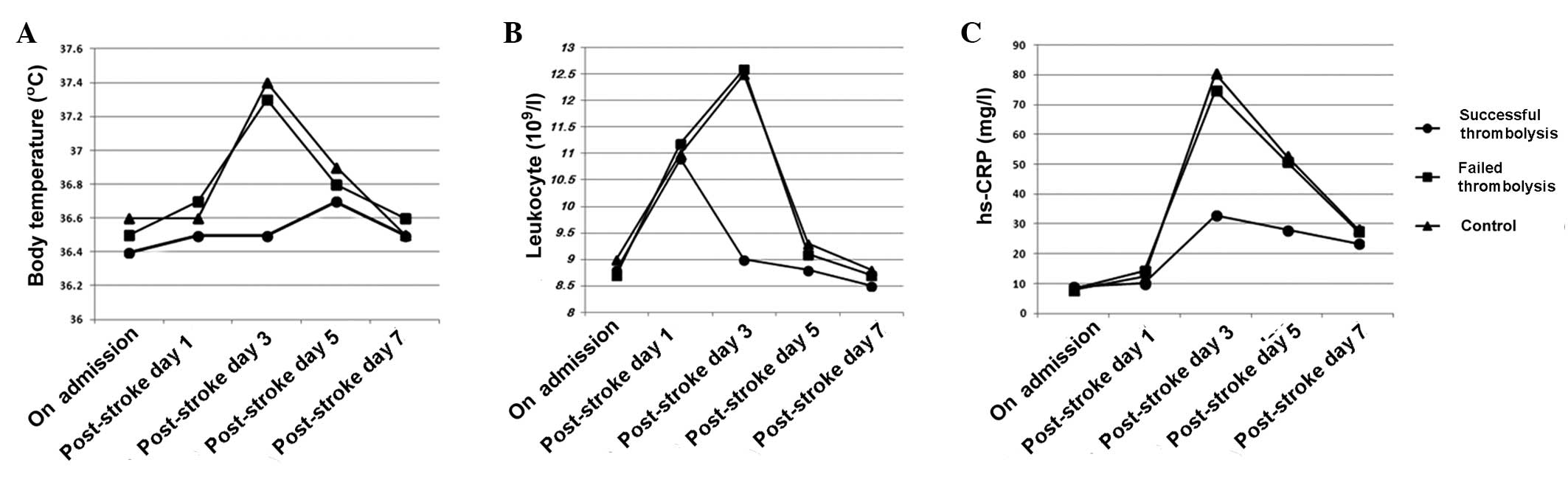
Comparison of the three groups
No significant difference was observed among the
three groups in the NIHSS scores of neurological deficit prior to
treatment. However, the NIHSS score of the successful thrombolysis
group was decreased significantly compared with that in the failed
thrombolysis and control groups on days 1 and 7 (P<0.001). The
body temperature of the successful thrombolysis group was
significantly lower than that in the other two groups on day 3
(P<0.005), with a significant reduction in the number of
peripheral blood leukocytes (P<0.001). The level of hs-CRP was
significantly reduced in the successful thrombolysis group on days
3 and 5, when compared with the failed thrombolysis and control
groups (P<0.001; Table IV;
Fig. 1). No significant differences
were observed between the failed thrombolysis group and the control
group (P>0.05).
 | Table IV.NIHSS scores and inflammatory
indicators before and after treatment. |
Table IV.
NIHSS scores and inflammatory
indicators before and after treatment.
| Indicators | Successful
thrombolysis (n=36) | Failed thrombolysis
(n=12) | ta |
P-valuea | Control (n=14) | tb |
P-valueb |
|---|
| NIHSS score |
|
| On
admission | 10.0±4.8 | 10.8±5.0 | 0.495 | 0.623 | 9.8±4.5 | 0.135 | 0.893 |
|
Post-stroke day 1 | 4.0±2.2 | 8.7±3.4 | 5.274 | <0.001 | 9.0±3.2 | 6.323 | <0.001 |
|
Post-stroke day 7 | 2.2±1.6 | 6.0±2.4 | 6.252 | <0.001 | 7.0±2.1 | 8.710 | <0.001 |
| Body temperature
(°C) |
|
| On
admission | 36.4±0.5 | 36.5±0.4 | 0.628 | 0.533 | 36.6±0.5 | 1.270 | 0.210 |
|
Post-stroke day 1 | 36.5±0.5 | 36.7±0.6 | 1.141 | 0.260 | 36.6±0.5 | 0.635 | 0.528 |
|
Post-stroke day 3 | 36.5±0.7 | 37.3±0.9 | 3.189 | 0.003 | 37.4±0.9 | 3.763 | <0.001 |
|
Post-stroke day 5 | 36.7±0.6 | 36.8±0.5 | 0.519 | 0.606 | 36.9±0.6 | 1.058 | 0.295 |
|
Post-stroke day 7 | 36.5±0.6 | 36.6±0.6 | 0.500 | 0.619 | 36.5±0.5 | 0.553 | 0.583 |
| Leukocyte
(109/l) |
|
| On
admission | 8.8±1.4 | 8.7±2.0 | 0.192 | 0.849 | 9.0±1.9 | 0.406 | 0.687 |
|
Post-stroke day 1 | 10.9±1.8 | 11.2±2.3 | 0.466 | 0.643 | 11.0±2.0 | 0.171 | 0.865 |
|
Post-stroke day 3 | 9.0±2.0 | 12.6±2.5 | 5.070 | <0.001 | 12.5±2.6 | 5.100 | <0.001 |
|
Post-stroke day 5 | 8.8±1.2 | 9.1±1.8 | 0.658 | 0.514 | 9.3±2.0 | 1.087 | 0.282 |
|
Post-stroke day 7 | 8.5±1.4 | 8.7±1.5 | 0.421 | 0.676 | 8.8±1.8 | 0.627 | 0.534 |
| hs-CRP (mg/l) |
|
| On
admission | 9.0±6.4 | 8.1±6.6 | 0.419 | 0.677 | 7.9±7.0 | 0.532 | 0.597 |
|
Post-stroke day 1 | 10.1±7.4 | 14.3±8.7 | 1.630 | 0.110 | 12.3±7.0 | 0.958 | 0.343 |
|
Post-stroke day 3 | 32.8±9.9 | 74.6±13.1 | 11.663 | <0.001 | 80.4±11.6 | 14.548 | <0.001 |
|
Post-stroke day 5 | 28.0±8.4 | 50.8±10.6 | 7.621 | <0.001 | 52.7±10.0 | 8.849 | <0.001 |
|
Post-stroke day 7 | 23.5±7.6 | 27.6±9.8 | 1.504 | 0.139 | 28.2±8.8 | 1.879 | 0.066 |
Discussion
Stroke induces the body to produce an inflammatory
response against brain tissue damage. Acute ischemic stroke, due to
the interruption of regional cerebral blood flow, reperfusion and
the destruction of the blood-brain barrier, causes peripheral white
blood cells to migrate and infiltrate into the brain, where they
activate microglia, initiate inflammatory cascade reactions,
release a series of inflammatory indicators and increase brain
damage. This inflammatory response is not limited to the local
brain tissue at the early stages, but is a general, non-specific
and systemic inflammatory reaction. Biological markers, such as
temperature and blood pressure, and physiologic parameters, such as
peripheral blood leukocytes, CRP and interleukin-6, are important
indicators of the systemic inflammatory response (3,4).
Post-stroke fever is a common clinical phenomenon, which might be
not only the early systemic inflammatory response against necrotic
brain tissue, but also caused by infection, deep vein thrombosis
and other complications. Previous studies have shown that body
temperature elevation is associated with the clinical severity and
prognosis of stroke (12–14). Elevated body temperature is also a
risk factor for the hemorrhagic transformation of acute ischemic
stroke when recombinant tissue-type plasminogen activator treatment
is not applied to patients (15).
Some studies have found that the higher and sooner the body
temperature increases in the acute phase of ischemic stroke, the
more severe the brain damage is likely to be; however, this
correlation exists only within the first 24 h of stroke (16,17).
Other studies have indicated that it is not admissional body
temperature that is associated with adverse outcomes of stroke, but
the temperature peak occurring a few days after the stroke
(18–20). The present study revealed that the
more serious the stroke and the higher the NIHSS score, the more
the body temperature increased. The NIHSS score and body
temperature showed a significant correlation on day 3 after
admission, but no significant correlation on days 1 and 2. The
difference between these findings might be due to the fact that the
studies were mostly retrospective analyses, which could not
completely rule out concomitant infection. In addition, many
factors are able to affect the body temperature measurement; oral
or anal temperature readings would be more accurate.
In the acute stage of cerebral infarction, when the
brain tissue is in a state of ischemia and hypoxia, stimulated
leukocyte adhere to and aggregate on the vessel wall, releasing
oxygen free radicals and other harmful substances, causing or
aggravating tissue damage (1–3). Animal
models have shown that a lack of white blood cells can reduce
cerebral infarction volume, and thus reduce the inflammatory
response (21). Therefore, many
scholars consider that an increase in white blood cell counts is a
risk factor for stroke, and is associated with poor prognosis
(22,23). The present study demonstrated that
the higher the pre-treatment NIHSS score, the greater the number of
peripheral blood leukocytes, particularly on days 1 and 3 after
stroke. This result is also consistent with the aggregation
phenomenon of lymphocytes in damaged brain tissues, which had been
observed by Akopov et al (24).
CRP is an acute-phase reactant synthesized by liver
cells and epithelial cells when stimulated by inflammatory factors.
As an important inflammatory indicator, CRP is not only associated
with systemic atherosclerosis and coronary heart disease, but also
closely associated with the incidence, development and prognosis of
cerebral infarction (25,26). High levels of CRP have been found to
be positively correlated with the severity and long-term mortality
of ischemic stroke (27,28). The present study found that the more
serious the stroke on admission, as indicated by a higher NIHSS
score, the higher the serum level of hs-CRP, particularly on days
3, 5 and 7 after the onset of the disease.
A series of pathophysiological changes occur
following acute cerebral infarction. An ischemic penumbra forms; if
the blood flow recovers rapidly, cell function can be restored to
normal; if the ischemia increases, the range of infarction extends.
Necrotic brain tissue would activate the inflammatory response
through cells, body fluids and metabolic mechanisms, exhibited as
fever, leukocytosis and increasing CRP levels. Previous studies
have found a positive correlation between lesion size and changes
in inflammatory indicators (6,22).
Thrombolytic therapy can facilitate the rapid recanalization of
occlusive vasculature, restore blood flow and reduce ischemic area
or infarct volume; therefore, it is regarded as the most important
measure to restore blood flow. Recombinant tissue-type plasminogen
activator (Alteplase) is the main thrombolytic drug currently used,
with an effective therapeutic time window of 4.5 h (29). The present study showed that the
clinical neurological functions of the patients undergoing
successful intravenous thrombolytic therapy improved significantly,
with a significant reduction of the inflammatory response. With
regard to the detailed clinical presentation, compared with the
failed thrombolytic treatment and control groups, the patients in
the successful thrombolytic group exhibited NIHSS neurological
deficit scores that were significantly reduced on days 1 and 7
after treatment. In addition, they exhibited a significant
reduction in body temperature on day 3, accompanied by a
significant reduction in the number of peripheral blood leukocytes,
and a significant reduction in hs-CRP levels on days 3 and 5. These
results are consistent with previous studies (30–33), and
suggest that the systemic inflammatory response following acute
cerebral infarction arises mainly because of ischemic injury of
local brain tissue, rather than cryptogenic infection. and that
successful thrombolytic therapy could reduce the necrosis of brain
tissue, reduce inflammation and induce tissue repair.
In summary, the present study observed that acute
ischemic stroke induces a systemic inflammatory response due to the
necrosis of brain tissue, causing increases in peripheral
inflammatory indicators (body temperature, peripheral blood
leukocyte counts and hs-CRP levels). Changes in the inflammatory
indicators are associated with the severity of stroke. Ultra-early
and effective thrombolytic therapy can significantly reduce the
systemic inflammatory response and improve nerve function.
References
|
1
|
Zierath D, Thullbery M, Hadwin J, Gee JM,
Savos A, Kalil A and Becker KJ: CNS immune responses following
experimental stroke. Neurocrit Care. 12:274–284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakase T, Yamazaki T, Ogura N, Suzuki A
and Nagata K: The impact of inflammation on the pathogenesis and
prognosis of ischemic stroke. J Neurol Sci. 271:104–109. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Q, Tang XN and Yenari MA: The
inflammatory response in stroke. J Neuroimmunol. 184:53–68. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
ShenharTsarfaty S, Assayag EB, Bova I,
Shopin L, Berliner S, Shapira I and Bornstein NM: Early signaling
of inflammation in acute ischemic stroke: Clinical and rheological
implications. Thromb Res. 122:167–173. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McColl BW, Allan SM and Rothwell NJ:
Systemic inflammation and stroke: Aetiology, pathology and targets
for therapy. Biochem Soc Trans. 35:1163–1165. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sotgiu S, Zanda B, Marchetti B, Fois ML,
Arru G, Pes GM, Salaris FS, Arru A, Pirisi A and Rosati G:
Inflammatory biomarkers in blood of patients with acute brain
ischemia. Eur J Neurol. 13:505–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harms H, Prass K, Meisel C, Klehmet J,
Rogge W, Drenckhahn C, Göhler J, Bereswill S, Göbel U, Wernecke KD,
et al: Preventive antibacterial therapy in acute ischemic stroke: A
randomized controlled trial. PLoS One. 3:e21582008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schwarz S, Al-Shajlawi F, Sick C, Meairs S
and Hennerici MG: Effects of prophylactic antibiotic therapy with
mezlocillin plus sulbactam on the incidence and height of fever
after severe acute ischemic stroke: The Mannheim Infection in
Stroke Study (MISS). Stroke. 39:1220–1227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ad Hoc Committee representing the National
Stroke Foundation and the Stroke Society of Australasia: The
implementation of intravenous tissue plasminogen activator in acute
ischaemic stroke - a scientific position statement from the
National Stroke Foundation and the Stroke Society of Australasia.
Intern Med J. 39:317–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
AdamsHP Jr, del Zoppo G, Alberts MJ, Bhatt
DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C,
et al: Guidelines for the early management of adults with ischaemic
stroke: A guideline from the American Heart Association/American
Stroke Association Stroke Council, Clinical Cardiology Council,
Cardiovascular Radiology and Intervention Council and the
Atherosclerotic Peripheral Vascular Disease and Quality of Care
Outcomes in Research Interdisciplinary Working Groups: The American
Academy of Neurology affirms the value of this guideline as an
educational tool for neurologists. Stroke. 38:1655–1711. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
AdamsHP Jr, Bendixen BH, Kappelle LJ,
Biller J, Love BB, Gordon DL and Marsh EE III: Classification of
subtype of acute ischemic stroke. Definitions for use in a
multicenter clinical trial. TOAST. Trial of Org 10172 in Acute
Stroke Treatment. Stroke. 24:35–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naess H, Idicula T, Lagallo N, Brogger J,
WajeAndreassen U and Thomassen L: Inverse relationship of baseline
body temperature and outcome between ischemic stroke patients
treated and not treated with thrombolysis: The Bergen stroke study.
Acta Neurol Scand. 122:414–417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saini M, Saqqur M, Kamruzzaman A, Lees KR
and Shuaib A: VISTA Investigators: Effect of hyperthermia on
prognosis after acute ischemic stroke. Stroke. 40:3051–3059. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greer DM, Funk SE, Reaven NL, Ouzounelli M
and Uman GC: Impact of fever on outcome in patients with stroke and
neurologic injury: A comprehensive meta-analysis. Stroke.
39:3029–3035. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leira R, Sobrino T, Blanco M, Campos F,
Rodríguez-Yáñez M, Castellanos M, Moldes O, Millán M, Dávalos A and
Castillo J: A higher body temperature is associated with
haemorrhagic transformation in patients with acute stroke untreated
with recombinant tissue-type plasminogen activator (rtPA). Clin Sci
(Lond). 122:113–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Lim LL, Levi C, Heller RF and
Fisher J: Influence of admission body temperature on stroke
mortality. Stroke. 31:404–409. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kammersgaard LP, Jørgensen HS, Rungby JA,
Reith J, Nakayama H, Weber UJ, Houth J and Olsen TS: Admission body
temperature predicts long-term mortality after acute stroke: The
Copenhagen Stroke Study. Stroke. 33:1759–1762. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Karaszewski B, Thomas RG, Dennis MS and
Wardlaw JM: Temporal profile of body temperature in acute ischemic
stroke: Relation to stroke severity and outcome. BMC Neurol.
12:1232012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
den Hertog HM, van der Worp HB, van Gemert
HM, Algra A, Kappelle LJ, van Gijn J, Koudstaal PJ and Dippel DW:
An early rise in body temperature is related to unfavorable outcome
after stroke: Data from the PAIS study. J Neurol. 258:302–307.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leira R, Rodríguez-Yáñez M, Castellanos M,
Blanco M, Nombela F, Sobrino T, Lizasoain I, Dávalos A and Castillo
J: Hyperthermia is a surrogate marker of inflammation-mediated
cause of brain damage in acute ischaemic stroke. J Intern Med.
260:343–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hurn PD, Subramanian S, Parker SM,
Afentoulis ME, Kaler LJ, Vandenbark AA and Offner H: T- and
B-cell-deficient mice with experimental stroke have reduced lesion
size and inflammation. J Cereb Blood Flow Metab. 27:1798–1805.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smith CJ, Emsley HC, Gavin CM, Georgiou
RF, Vail A, Barberan EM, del Zoppo GJ, Hallenbeck JM, Rothwell NJ,
Hopkins SJ and Tyrrell PJ: Peak plasma interleukin-6 and other
peripheral markers of inflammation in the first week of ischaemic
stroke correlate with brain infarct volume, stroke severity and
long-term outcome. BMC Neurol. 4:22004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Christensen H and Boysen G: C-reactive
protein and white blood cell count increases in the first 24 h
after acute stroke. Cerebrovasc Dis. 18:214–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Akopov SE, Simonian NA and Grigorian GS:
Dynamics of polymorphonuclear leukocyte accumulation in acute
cerebral infarction and their correlation with brain tissue damage.
Stroke. 27:1739–1743. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
EliasSmale SE, Kardys I, Oudkerk M, Hofman
A and Witteman JC: C-reactive protein is related to extent and
progression of coronary and extra-coronary atherosclerosis; results
from the Rotterdam study. Atherosclerosis. 195:e195–e202. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ridker PM, Danielson E, Fonseca FA, Genest
J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ,
MacFadyen JG, et al: Rosuvastatin to prevent vascular events in men
and women with elevated C-reactive protein. N Engl J Med.
359:2195–2207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Idicula TT, Brogger J, Naess H,
WajeAndreassen U and Thomassen L: Admission C-reactive protein
after acute ischemic stroke is associated with stroke severity and
mortality: The ‘Bergen stroke study’. BMC Neurol. 9:182009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
den Hertog HM, van Rossum JA, van der Worp
HB, van Gemert HM, de Jonge R, Koudstaal PJ and Dippel DW: PAIS
investigators: C-reactive protein in the very early phase of acute
ischemic stroke: Association with poor outcome and death. J Neurol.
256:2003–2008. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hacke W, Kaste M, Bluhmki E, Brozman M,
Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et
al: Thrombolysis with alteplase 3 to 4.5 h after acute ischemic
stroke. N Engl J Med. 359:1317–1329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Audebert HJ, Rott MM, Eck T and Haberl RL:
Systemic inflammatory response depends on initial stroke severity
but is attenuated by successful thrombolysis. Stroke. 35:2128–2133.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hemmen TM, Raman R, Guluma KZ, Meyer BC,
Gomes JA, CruzFlores S, Wijman CA, Rapp KS, Grotta JC and Lyden PD:
ICTuS-L Investigators: Intravenous thrombolysis plus hypothermia
for acute treatment of ischemic stroke (ICTuS-L): Final results.
Stroke. 41:2265–2270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Idicula TT, WajeAndreassen U, Brogger J,
Naess H, Lundstadsveen MT and Thomassen L: The effect of
physiologic derangement in patients with stroke treated with
thrombolysis. J Stroke Cerebrovasc Dis. 17:141–146. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Millan M, Grau L, Castellanos M,
Rodríguez-Yáñez M, Arenillas JF, Nombela F, Pérez de la Ossa N,
López-Manzanares L, Serena J, Castillo J and Dávalos A: Body
temperature and response to thrombolytic therapy in acute ischaemic
stroke. Eur J Neurol. 15:1384–1389. 2008. View Article : Google Scholar : PubMed/NCBI
|