Introduction
Autoimmune hepatitis (AIH) is caused by an immune
attack on the hepatocytes (1).
Patients with AIH are positive for auto-antibodies, such as
anti-nuclear, anti-smooth muscle and liver-kidney microsomal type 1
antibodies (2). Chronic inflammation
in the liver can progress to liver cirrhosis (3). One major problem of AIH is that an
acute presentation or exacerbation of the disease can progress to
liver failure (4,5). Furthermore, patients with AIH can be
susceptible to hepatocellular carcinoma (6); therefore, patients should be
followed-up for exacerbation and hepatocellular carcinoma (7). Primary biliary cirrhosis (PBC) is
characterized by nonsuppurative cholangitis and autoimmune-mediated
destruction of the small or medium-sized bile ducts (8). Inflammation causes cholestasis and the
liver develops cirrhosis (9). AIH is
treated with immunosuppressive drugs, such as prednisolone or
azathioprine (10,11), while PBC is treated with
ursodeoxycholic acid (10). It is
important to differentiate AIH from PBC due to the differences in
the clinical course and management of the two diseases.
Anti-mitochondrial antibody (AMA) can be tested via
indirect immunofluorescence (12).
In this technique, unfixed sections of the kidney and stomach of
Wistar rats are used. AMA is used for the diagnosis of PBC
(13), as 90–95% of patients with
PBC are positive for AMA (14);
however, the use of AMA as a differential test is problematic, as
5% of patients with AIH are positive for AMA (15), and the interpretation of a positive
AMA result in AIH is difficult.
The auto-antigens of AMA have been identified as
pyruvate dehydrogenase complex-E2 (PDC-E2), branched-chain 2-oxo
acid dehydrogenase complex and 2-oxoglutaric acid dehydrogenase
complex (16,17). The antibody to these antigens is
known as AMA-M2 (18). AMA-M2 is
more specific to PBC than AMA, and the determination of its titer
is feasible (19). It is widely
accepted that AMA-M2 is useful for the diagnosis of PBC, and a
scoring system for PBC that uses the antibody has been generated
(20); however, patients with AIH
can still test positive for AMA-M2 (21).
The present study investigates AIH patients who are
AMA-M2(+). Titers of AMA-M2 are compared between AMA-M2(+) AIH
patients and PBC patients, and the changes in AMA-M2 during the
follow-up of the patients with AIH are reported.
Patients and methods
Patients
The records of patients who underwent liver biopsy
between April 2009 and March 2014 were retrospectively reviewed.
Patients were selected, as this study was retrospective. Patents
were included only when they were diagnosed as AIH or PBC with
liver biopsy, and their AMA-M2 was subsequently analyzed. Liver
biopsies were crucial for the histological confirmation of the
diagnosis. The liver biopsy was performed during hospitalization
with an automatic liver biopsy needle, which measured 14 G in
diameter and 150 mm in length (ACE-141501; TSK Laboratory, Tochigi,
Japan) (22). Ten patients were
diagnosed with AIH (2 men and 8 women), and 4 patients were
diagnosed with PBC (all women). No patients were diagnosed with the
PBC-AIH overlap syndrome. This study was reviewed by the ethics
committee of the National Hospital Organization Shimoshizu Hospital
(Yotsukaido, Japan) and was not considered to be a clinical trial,
as it was performed as a part of routine clinical practice. The
committee waived the requirement for informed consent due to the
retrospective nature of the study. Patient anonymity was
preserved.
Diagnosis of AIH
The diagnosis of AIH was based on a scoring system
proposed by the International Autoimmune Hepatitis Group (23). AIH was diagnosed when the
pre-treatment score was >10 or the post-treatment score was
>12 and the pathological findings were consistent with AIH.
Diagnosis of PBC
The diagnosis of PBC was based on guidelines of the
American Association for the Study of Liver Diseases (13). Patients were diagnosed with PBC when
alkaline phosphatase (ALP) levels were elevated, the AMA test was
positive and there was evidence of nonsuppurative cholangitis and
the destruction of small or medium-sized bile ducts. The diagnosis
of PBC-AIH overlap syndrome was based on the Paris criteria
(24).
Laboratory data
The laboratory data analyzed in the present study
were ALP, aspartate aminotransferase (AST), alanine
aminotransferase (ALT), γ-glutamyl transpeptidase (γ-GTP) and
AMA-M2. The data were collected upon admission of the patients to
the hospital. Detection of AMA-M2 was outsourced to the LSI
Medience Corp. (Tokyo, Japan). AMA-M2 reacted with the PDC-E2, the
branched-chain 2-oxo acid dehydrogenase complex and the
2-oxoglutaric acid dehydrogenase complex (16). The cut-off value for AMA-M2 was 5.
Patients with values <5 were considered negative for AMA-M2;
patients with values >5 were considered positive.
Statistical analysis
Results are presented as the mean ± standard
deviation. One-way analysis of variance was performed for the
analysis of the laboratory data using the statistical software JMP
10.0.2 (SAS Institute, Inc., Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of AMA-M2(+) AIH patients
and PBC patients
The laboratory data of the AMA-M2(+) AIH patients
and PBC patients were compared to confirm that the patients had
different diseases (Table I). ALP
levels tended to be higher in the patients with PBC than those in
the AMA-M2(+) AIH patients. AST and ALT levels were higher in the
AMA-M2(+) AIH patients than those in the PBC patients. No
significant differences were found between the groups (P>0.05).
Higher levels of ALP indicate bile duct obstruction, a
characteristic of PBC (13). The
higher AST and ALT levels in the AMA-M2(+) AIH patients were due to
the acute presentation.
 | Table I.Blood analysis comparison between
AMA-M2(+) AIH patients and PBC patients. |
Table I.
Blood analysis comparison between
AMA-M2(+) AIH patients and PBC patients.
| Parameter | AMA-M2(+) AIH
patients | PBC patients | P-value |
|---|
| Age (years) |
59.3±14.8 |
61.8±19.9 | 0.8467 |
| ALP (IU/l) |
457±126 |
780±349 | 0.1312 |
| AST (IU/l) |
631±212 |
59±22 | 0.1044 |
| ALT (IU/l) |
663±777 |
66±22 | 0.1752 |
| γ-GTP (IU/l) |
258±135 |
298±339 | 0.8340 |
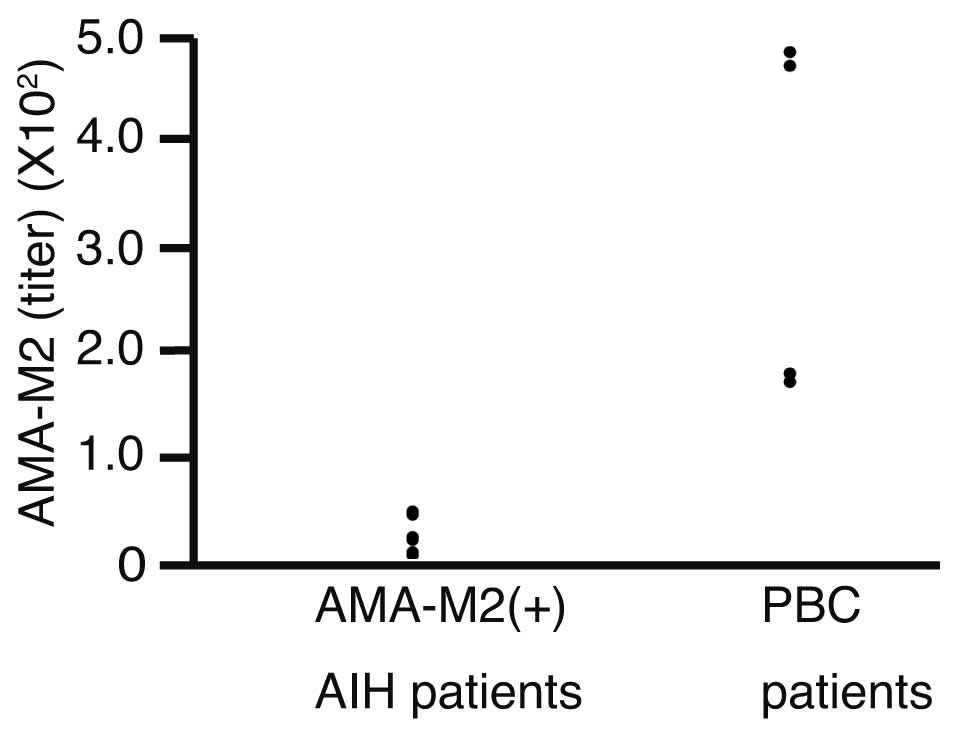
Titers of AMA-M2 were compared between the AMA-M2(+)
AIH patients and the patients with PBC (Fig. 1). Titers of AMA-M2 were 24.8±14.8 in
the AMA-M2(+) AIH patients and 324±174 in the PBC patients. The
difference was statistically significant (P=0.0138).
Comparison of AMA-M2(-) and AMA-M2(+)
AIH patients
Next, the laboratory data of the AMA-M2(-) and
AMA-M2(+) AIH patients were compared to assess any differences
(Table II). AST and ALT levels were
higher in the AMA-M2(+) AIH patients than those in the AMA-M2(-)
AIH patients, but the difference was not significant.
 | Table II.Comparison between AMA-M2(-) and
AMA-M2(+) AIH patients. |
Table II.
Comparison between AMA-M2(-) and
AMA-M2(+) AIH patients.
| Parameter | AMA-M2(-) AIH
patients | AMA-M2(+) AIH
patients | P-value |
|---|
| Males:females | 2:4 | 0:4 | NA |
| Age (years) |
52.3±15.2 |
59.3±14.8 | 0.4974 |
| ALP (IU/l) |
367±140 |
457±126 | 0.3304 |
| AST (IU/l) |
198±103 |
631±212 | 0.1115 |
| ALT (IU/l) |
271±144 |
663±777 | 0.2500 |
| γ-GTP (IU/l) |
235±116 |
258±135 | 0.7800 |
Follow-up
Three AMA-M2(+) AIH patients were followed-up after
liver biopsy (Table III). The
levels of AMA-M2, AST, ALT and γ-GTP had decreased in all the
patients. Two patients became negative for AMA-M2.
 | Table III.Follow-up of AMA-M2(+) patients with
autoimmune hepatitis. |
Table III.
Follow-up of AMA-M2(+) patients with
autoimmune hepatitis.
| Parameter | Patient 1 | Patient 2 | Patient 3 |
|---|
| Gender | Female | Female | Female |
| Age (years) |
38 |
65 |
72 |
| ALP (IU/l) |
|
|
|
| 0
months | 589 | 542 | 531 |
|
Follow-upa | 202 | 395 | 158 |
| AST (IU/l) |
|
|
|
| 0
months | 1,460 | 107 | 662 |
|
Follow-upa |
14 |
62 |
18 |
| ALT (IU/l) |
|
|
|
| 0
months | 1,780 |
55 |
585 |
|
Follow-upa |
12 |
37 |
15 |
| γ-GTP (IU/l) |
|
|
|
| 0
months |
264 |
70 |
387 |
|
Follow-upa |
32 |
53 |
65 |
| AMA-M2 |
|
|
|
| 0
months |
21.2 |
9.4 |
44.9 |
|
Follow-upa | (-) | (-) |
13.1 |
Discussion
It is established that AMA-M2 is more specific to
PBC than to AIH (13); therefore,
the AMA-M2 titer is a common test for PBC (25,26). A
problem with this is that patients with AIH can still test positive
for AMA-M2 (21). In a study by
Yanagawa et al (27), 9 out
of 55 patients with AIH were found to be positive for AMA-M2. In
the present study, 4 AIH patients were found to be positive for
AMA-M2 on admission. The difference in the percentage of patients
positive for AMA-M2 may have been dependent on the antigens with
which AMA-M2 reacted. The method used by Yanagawa et al
(27) detected PDC-E2, whereas the
method used in the present study detected branched-chain 2-oxo acid
dehydrogenase complex and 2-oxoglutaric acid dehydrogenase complex,
in addition to PDC-E2. Another reason for the difference may have
been the method utilized to detect positivity. Yanagawa et
al used western blot analysis, while the outsourced laboratory
in the present study used a chemiluminescent enzyme immunoassay
(CLEIA). The CLEIA is more sensitive than western blot analysis
(28).
The titers of AMA-M2 in AIH patients have not been
previously reported, to the best of our knowledge. In the present
study, the titers of AMA-M2 were significantly lower in the AIH
patients than those in the PBC patients, suggesting that a higher
AMA-M2 titer is specific to PBC (26). AMA-M2 has been found to persist or
disappear after long-term follow-up in AIH patients (29). In the present study, titers of AMA-M2
decreased in the AMA-M2(+) AIH patients.
One major limitation of the present study was that
it included a small number of patients. Lower ALP and higher
AST/ALT values are characteristics of AIH as compared with PBC
(21). The same tendency was found
in this study, but the differences were not statistically
significant. Despite the small number of patients, the titers of
AMA-M2 were found to be significantly higher in the patients with
PBC than those in the AMA-M2(+) AIH patients; however, a larger
sample of patients is required to confirm these results. Fig. 1 shows a gap in the AMA-M2 titers
between the AMA-M2(+) AIH patients and the PBC patients, which
indicates that threshold values may exist to differentiate between
AMA-M2(+) AIH patients and patients with PBC. A study with a larger
sample size is required to investigate this further.
In conclusion, certain patients with AIH were found
to be positive for AMA-M2 in the present study, but the titers were
significantly lower than those in the PBC patients. AMA-M2 titers
were decreased in the AIH patients at follow-up. Future studies
should include a larger sample size and investigate liver biopsies,
focusing on bile duct lesions.
References
|
1
|
Liberal R, Grant CR, Mieli-Vergani G and
Vergani D: Autoimmune hepatitis: A comprehensive review. J
Autoimmun. 41:126–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liberal R, Mieli-Vergani G and Vergani D:
Clinical significance of autoantibodies in autoimmune hepatitis. J
Autoimmun. 46:17–24. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Krawitt EL: Autoimmune hepatitis. N Engl J
Med. 354:54–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oketani M, Ido A, Nakayama N, Takikawa Y,
Naiki T, Yamagishi Y, Ichida T, Mochida S, Onishi S and Tsubouchi
H: Intractable Hepato-Biliary Diseases Study Group Of Japan:
Etiology and prognosis of fulminant hepatitis and late-onset
hepatic failure in Japan: Summary of the annual nationwide survey
between 2004 and 2009. Hepatol Res. 43:97–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Czaja AJ: Acute and acute severe
(fulminant) autoimmune hepatitis. Dig Dis Sci. 58:897–914. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Montano Loza, Carpenter HA and Czaja AJ:
Predictive factors for hepatocellular carcinoma in type 1
autoimmune hepatitis. Am J Gastroenterol. 103:1944–1951. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshizawa K, Matsumoto A, Ichijo T,
Umemura T, Joshita S, Komatsu M, Tanaka N, Tanaka E, Ota M,
Katsuyama Y, et al: Long-term outcome of Japanese patients with
type 1 autoimmune hepatitis. Hepatology. 56:668–676. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bowlus CL and Gershwin ME: The diagnosis
of primary biliary cirrhosis. Autoimmun Rev. 13:441–444. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kakuda Y, Harada K, Sawada-Kitamura S,
Ikeda H, Sato Y, Sasaki M, Okafuji H, Mizukoshi E, Terasaki S, Ohta
H, et al: Evaluation of a new histologic staging and grading system
for primary biliary cirrhosis in comparison with classical systems.
Hum Pathol. 44:1107–1117. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manns MP, Czaja AJ, Gorham JD, Krawitt EL,
Mieli-Vergani G, Vergani D and Vierling JM: American Association
For The Study Of Liver Diseases: Diagnosis and management of
autoimmune hepatitis. Hepatology. 51:2193–2213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Verslype C, George C, Buchel E, Nevens F,
van Steenbergen W and Fevery J: Diagnosis and treatment of
autoimmune hepatitis at age 65 and older. Aliment Pharmacol Ther.
21:695–699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chantran Y, Ballot É and Johanet C:
Autoantibodies in primary biliary cirrhosis: Antimitochondrial
autoantibodies. Clin Res Hepatol Gastroenterol. 37:431–433. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindor KD, Gershwin ME, Poupon R, Kaplan
M, Bergasa NV and Heathcote EJ: American Association For Study Of
Liver Diseases: Primary biliary cirrhosis. Hepatology. 50:291–308.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gershwin ME, Mackay IR, Sturgess A and
Coppel RL: Identification and specificity of a cDNA encoding the 70
kd mitochondrial antigen recognized in primary biliary cirrhosis. J
Immunol. 138:3525–3531. 1987.PubMed/NCBI
|
|
15
|
Czaja AJ, Carpenter HA, Santrach PJ, Moore
SB and Homburger HA: The nature and prognosis of severe cryptogenic
chronic active hepatitis. Gastroenterology. 104:1755–1761.
1993.PubMed/NCBI
|
|
16
|
Moteki S, Leung PS, Dickson ER, Van Thiel
DH, Galperin C, Buch T, Alarcon-Segovia D, Kershenobich D, Kawano
K, Coppel RL, et al: Epitope mapping and reactivity of
autoantibodies to the E2 component of 2-oxoglutarate dehydrogenase
complex in primary biliary cirrhosis using recombinant
2-oxoglutarate dehydrogenase complex. Hepatology. 23:436–444. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gershwin ME, Rowley M, Davis PA, Leung P,
Coppel R and Mackay IR: Molecular biology of the 2-oxo-acid
dehydrogenase complexes and anti-mitochondrial antibodies. Prog
Liver Dis. 10:47–61. 1992.PubMed/NCBI
|
|
18
|
Leung PS, Chuang DT, Wynn RM, Cha S,
Danner DJ, Ansari A, Coppel RL and Gershwin ME: Autoantibodies to
BCOADC-E2 in patients with primary biliary cirrhosis recognize a
conformational epitope. Hepatology. 22:505–513. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamagiwa S, Kamimura H, Takamura M and
Aoyagi Y: Autoantibodies in primary biliary cirrhosis: Recent
progress in research on the pathogenetic and clinical significance.
World J Gastroenterol. 20:2606–2612. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamamoto K, Terada R, Okamoto R, Hiasa Y,
Abe M, Onji M and Tsuji T: A scoring system for primary biliary
cirrhosis and its application for variant forms of autoimmune liver
disease. J Gastroenterol. 38:52–59. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Farias AQ, Gonçalves LL, Bittencourt PL,
De Melo ES, Abrantes-Lemos CP, Porta G, Nakhle MC, Carrilho FJ and
Cancado EL: Applicability of the IAIHG scoring system to the
diagnosis of antimitochondrial/anti-M2 seropositive variant form of
autoimmune hepatitis. J Gastroenterol Hepatol. 21:887–893. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki A, Brunt EM, Kleiner DE, Miquel R,
Smyrk TC, Andrade RJ, Lucena MI, Castiella A, Lindor K and
Björnsson E: The use of liver biopsy evaluation in discrimination
of idiopathic autoimmune hepatitis versus drug-induced liver
injury. Hepatology. 54:931–939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alvarez F, Berg PA, Bianchi FB, Bianchi L,
Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet
VJ, et al: International autoimmune hepatitis group report: Review
of criteria for diagnosis of autoimmune hepatitis. J Hepatol.
31:929–938. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chazouilleres O, Wendum D, Serfaty L,
Montembault S, Rosmorduc O and Poupon R: Primary biliary
cirrhosis-autoimmune hepatitis overlap syndrome: Clinical features
and response to therapy. Hepatology. 28:296–301. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dellavance A, Cancado EL, Abrantes-Lemos
CP, Harriz M, Marvulle V and Andrade LE: Humoral autoimmune
response heterogeneity in the spectrum of primary biliary
cirrhosis. Hepatol Int. 7:775–784. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miyakawa H, Kitazawa E, Fujikawa H,
Kikuchi K, Abe K, Kawaguchi N and Kako M: Analysis of two major
anti-M2 antibodies (anti-PDC-E2/anti-BCOADC-E2) in primary biliary
cirrhosis: Relationship to titers of immunofluorescent
anti-mitochondrial antibody. Hepatol Res. 18:1–9. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yanagawa T, Miyakawa H, Shibata M,
Kawaguchi N, Ishibashi M, Goto N and Mitamura K: Immunoreactivity
to pyruvate dehydrogenase complex-E2 in well-defined patients with
autoimmune hepatitis: Western blot analysis. Hepatol Res. 26:81–86.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roda A, Pasini P, Guardigli M, Baraldini
M, Musiani M and Mirasoli M: Bio- and chemiluminescence in
bioanalysis. Fresenius J Anal Chem. 366:752–759. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Brien C, Joshi S, Feld JJ, Guindi M,
Dienes HP and Heathcote EJ: Long-term follow-up of
antimitochondrial antibody-positive autoimmune hepatitis.
Hepatology. 48:550–556. 2008. View Article : Google Scholar : PubMed/NCBI
|