Introduction
Parkinson's disease (PD) is predominantly caused by
the death of dopaminergic neurons in the substantia nigra, and
results in deficient striatal dopamine (DA) levels, which are
responsible for the motor symptoms of PD, including bradykinesia,
tremor and rigidity (1). Currently,
the primary treatment for PD is supplemental DA therapy (2). Tyrosine hydroxylase (TH) is a
rate-limiting enzyme of DA synthesis, which catalyzes the
hydroxylation of tyrosine to levodopa (L-dopa) (3). L-dopa is subsequently converted into DA
by aromatic amino acid decarboxylase (AADC) (4). In addition, DA is converted into
3,4-dihydroxyphenylacetic acid (DOPAC) by monoamine oxidase B
(MAO-B) (5), and into homovanillic
acid (HVA) by catechol-O-methyltransferase (COMT) (6). Therefore, L-dopa, DA, DOPAC, HVA, AADC,
MAO-B, COMT and TH may be implicated in the pathogenesis of PD.
It has been demonstrated that L-dopa is the most
effective constituent of typical PD treatment (7). However, the long-term use of L-dopa is
associated with severe side effects, including motor response
fluctuations and the emergence of drug-induced involuntary
movements (8). For instance,
Madopar, which consists of L-dopa and benserazide, is the firstline
treatment option for PD. Long-term administration of Madopar is
complicated by the development of various types of motor response
oscillation, as well as drug-induced dyskinesia, which is a
complication characterized by erratic involuntary movements,
hypotension and psychiatric symptoms (9). Hence, Madopar does not resolve the
adverse effects of L-dopa.
β-asarone (cis-2,4,5-Trimethoxy-1-propenylbenzene)
is a strong fat-soluble substance with a low molecular weight (208
g/mol), which is able to rapidly traverse the blood-brain barrier,
with a peak traversal time of 12 min, and has a half-life of 54 min
(10). Our previous experiments
indicated that β-asarone has a wide range of pharmacological
effects on the central nervous system (CNS) and may be widely
distributed in the rat hippocampus and cortex (11). Notably, we demonstrated that
β-asarone and L-dopa co-administration was able to significantly
increase the striatal levels of DA in healthy rat tissue (11). In addition, the striatum, hippocampus
and cortex were the three important parts of the CNS (12). However, to the best of our knowledge,
there are no prior studies that describe the effects of β-asarone
and L-dopa co-administration on the dynamic changes of
neurotransmitters in the rat striatum, cortex, hippocampus and
plasma.
Therefore, the aim of the present study was to
investigate the dynamic changes in the levels of L-dopa, DA, DOPAC,
HVA and serotonin (5-HT) in the plasma, striatum, hippocampus and
cortex of healthy rats following β-asarone and L-dopa
co-administration, using high-performance liquid chromatography
(HPLC) and fluorescence detection (FD). Furthermore, in order to
observe the dynamic changes in the levels TH, COMT, AADC and MAO-B
after co-administration within 48 h, the levels of these enzymes in
the plasma and striatum were evaluated using an enzyme-linked
immunosorbent assay (ELISA).
Materials and methods
Experimental design
A total of 40 Sprague Dawley rats (20 female and 20
male; weight, 220–250 g) were obtained from the Laboratory Animal
Center of Guangzhou University of Chinese Medicine (ethical code
no. TCMF1-2012028; Guangzhou, China). The animals were housed in a
light- and temperature-controlled room with free access to standard
food and water. The experimental protocols were approved by the
Ethics Committee of Guangzhou University of Chinese Medicine and
were consistent with the international guidelines.
Rats were divided into five groups (n=8 per group):
Control group and four groups that received a single
co-administration of β-asarone and L-dopa, at 15 and 60 mg/kg body
weight, respectively. The rats in the four treatment group rats
were subsequently sacrificed by cervical dislocation at 1, 5, 18
and 48 h, respectively. The control rats received an equal volume
of normal saline vehicle, following the same procedure and were
sacrificed at 48 h.
Preparation of β-asarone
The β-asarone used in this study was extracted from
Acorus tatarinowii Schott according to a previously-reported
procedure (13). The purity of
β-asarone was ~99.55% (14), which
was confirmed by gas chromatography-mass spectrometry, infrared
spectrum and nuclear magnetic resonance detection, which was
conducted at the China National Analytical Center (Guangzhou,
China).
Sample collection
Following the co-administration, rats were
anesthetized with 10% chloral hydrate (3.5 mg/kg, intraperitoneal
injection) at the pre-specified times of 1, 5, 18 and 48 h after
treatment. Next, the limbs of the anesthetized rats were fixed on
an autopsy table and the rat hearts were exposed in the thoracic
cavity by opening the abdominal cavity that is below the xiphoids.
Blood samples were collected from the aortic artery, and the plasma
was separated by centrifugation at 3,000 × g for 10 min and stored
at −80°C for subsequent HPLC analysis. Subsequently, in order to
remove the blood from the rat brain, normal saline was perfused
into the left ventricle and evacuated from the right atrial
appendage. The perfusion was discontinued when the rat eyes and
claws turned pale. The striatum, hippocampus and cortex areas were
then dissected rapidly from the brains on ice and stored at −80°C
for HPLC analysis. Sample collection from the control group rats
was performed following the same procedure.
HPLC analysis of DA, L-dopa, DOPAC,
HVA and 5-HT
Briefly, the striatum, hippocampus and cortex were
weighed and homogenized in ice-cold 0.1 M perchloric acid (1:5
g/ml) by sonication at 40 kHz for 5 min. Homogenates were
centrifuged at 12,000 × g for 15 min at 4°C, then the supernatants
were collected and filtered through microporous membrane filters
(0.22 µm), and 20 µl of each sample was injected into the HPLC
column. In addition, 0.1 M HClO4 was added to 500 µl
plasma at a ratio of 1:1 (v/v). The mixture was subjected to vortex
mixing and centrifugation at 13,000 × g for 15 min at 4°C. Next,
the supernatants were collected and filtered through microporous
membrane filters (0.22 µm), and 20 µl of each sample was subjected
to HPLC. The control substances of DA, 5-HT and L-dopa were
obtained from National Institutes for Food and Drug Control
(Beijing, China), while DOPAC and HVA were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
The concentrations of DA and L-dopa were quantified
by HPLC and FD, using a Waters 2695 separations module and a Waters
2475 multiwavelength fluorescence detector (Waters Corporation,
Milford, MA, USA) at an excitation and absorption wavelength of 280
and 330 nm, respectively. Separation was performed using a 5-µm
Hypersil™ ODS2 column (150×4.6 mm; Dalian Elite Analytical
Instruments Co., Ltd., Dalian, China) with column temperature of
30°C and a flow rate of 1 ml/min. The mobile phase was composed of
0.1 M KH2PO4 and methanol. The column was
equilibrated with mobile phase for 30 min prior to analysis. This
method was validated for the determination of neurotransmitter
levels as reported in our previous study (15). Data were analyzed using Empower 2
chromatography data software (Waters Corporation) and the results
were calculated and expressed as µg/g and µg/ml for tissue and
plasma, respectively.
TH, COMT, AADC and MAO-B analyses
The striatum was weighed and homogenized with
ice-cold normal saline (1:3 µl/mg), and then centrifuged at 3,000 ×
g for 10 min to obtain the supernatant. TH (T031FC), COMT (C033FC),
AADC (A036FC) and MAO-B (M035FC) were determined separately using
ELISA kits (Shanghai Saimo Biotechnology Co., Ltd., Shanghai,
China) and an American Hyperion MRIII microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA) according to the
manufacturer's instructions. In addition, ELISA kits from the same
supplier were used to separately determine the plasma levels of
AADC (A036SC), COMT (C033SC) and TH (T031SC).
Statistical analysis
Data are expressed as the mean ± standard deviation
and statistical differences between groups were determined by
one-way analysis of variance followed by Bonferroni post-hoc test
for multiple comparisons at P<0.05. P<0.05 was considered to
indicate a statistically significant difference. Correlations
between the neurotransmitters were performed by Pearson
correlation. All the statistical analyses were performed using SPSS
statistical software, version 17.0 (SPSS, Inc., Chicago, IL,
USA).
Results
Levels of the five neurotransmitters
changed in the rat plasma, hippocampus, cortex and striatum
following treatment
Subsequent to co-administration of β-asarone and
L-dopa, the L-dopa levels increased in the striatum of the rats,
peaking at 1 h, then deceasing markedly at 5 h and remaining stable
between 5 and 48 h. Compared with the control group, L-dopa levels
increased significantly in the 1 h group (P<0.05). DA levels
also increased and peaked at 1 h, showing a slight reduction at 5
h, followed by a further reduction between 5 and 18 h and a
subsequent increase. DA levels demonstrated a significant increase
in the 1, 5, 18 and 48 h groups compared with the control group
(P<0.05). Similarly, DOPAC levels increased significantly in the
1 h group compared with the control group (P<0.01), decreased
linearly between 5 and 18 h (P<0.05), and then exhibited a
gradual increase in the 48 h group. In addition, HVA levels peaked
at 1 h, then declined markedly at 5 h and increased slightly
between 5 and 48 h. Compared to the control group, HVA levels
demonstrated a significant increase in all the treatment groups (1,
5, 18 and 48 h; P<0.05). By contrast, 5-HT levels remained
stable between 1 to 48 h and were not significantly different
compared with the control group (Fig.
1A).
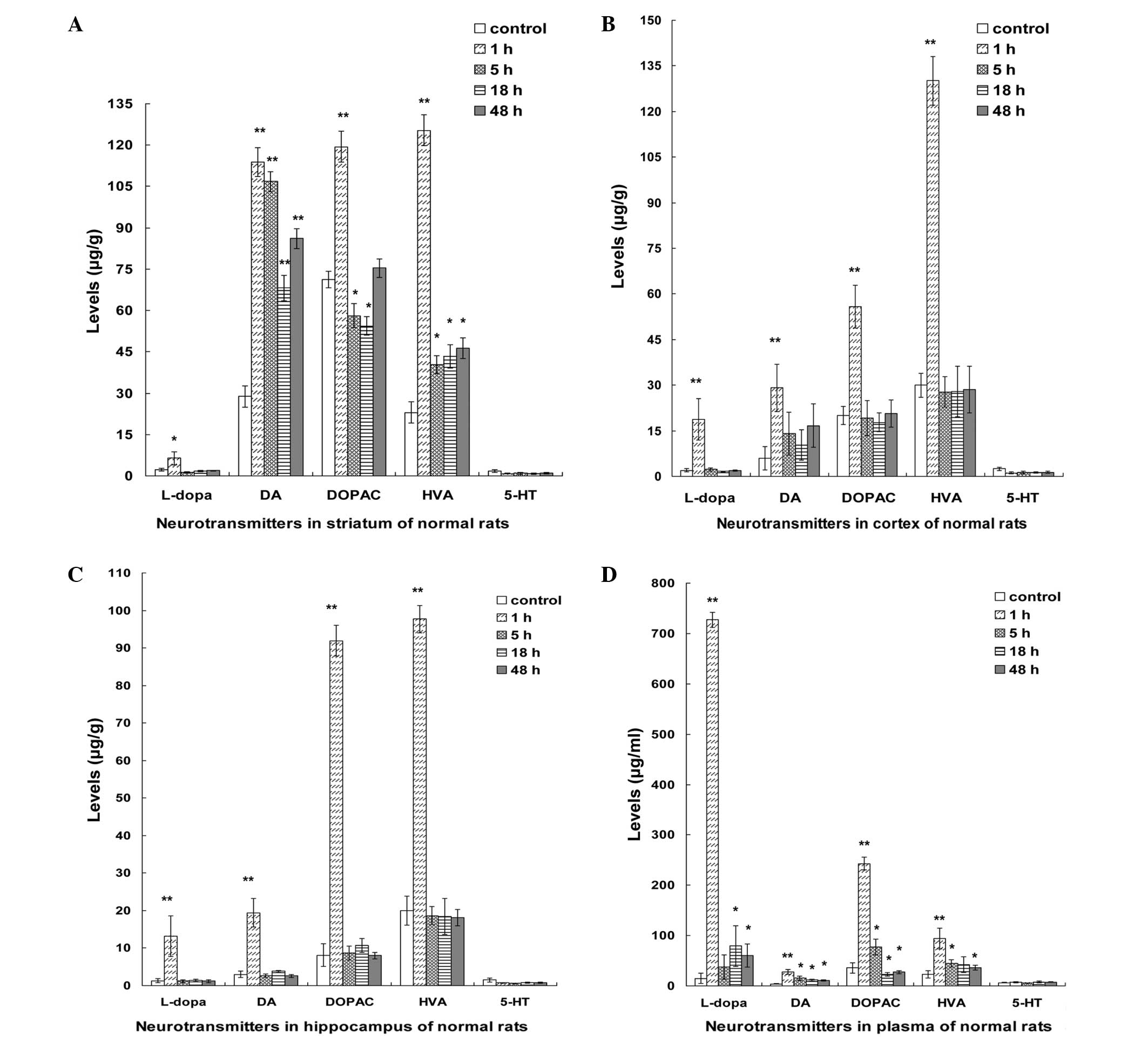 | Figure 1.Dynamic changes in the levels of the
neurotransmitters, L-dopa, DA, DOPAC, HVA and 5-HT, in the plasma
and brain tissues of healthy rats at various time points after the
co-administration of β-asarone and L-dopa. Differences in the
levels of the five neurotransmitters in the (A) striatum, (B)
cortex, (C) hippocampus and (D) plasma of the rats within 48 h
(µg/g tissue weight) are presented. Bars represent the mean ±
standard deviation of 8 rats. *P<0.05 and **P<0.01 vs.
control group (analysis of variance with Bonferroni test). L-dopa,
levodopa; DA, dopamine; DOPAC, 3,4-dihydroxyphenylacetic acid; HVA,
homovanillic acid; 5-HT, serotonin. |
In the cortex and hippocampus, the levels of L-dopa,
DA, DOPAC and HVA peaked at 1 h, followed by a sharp decline at 5 h
and a stable level between 5 and 48 h. Compared with the control
group, the levels of L-dopa, DA, DOPAC and HVA were significantly
increased in the 1 h group (P<0.01); however, the 5-HT levels
remained stable between 1 and 48 h and showed no significant
difference compared with that of the control group (Fig. 1B and C).
In the rat plasma, L-dopa levels increased and
peaked at 1 h, then decreased markedly at 5 h, increased slightly
at 18 h before finally declining slightly at 48 h. In addition, DA
levels increased and peaked at 1 h, and subsequently decreased
slightly between 5 and 48 h. In addition, DOPAC levels increased
and peaked at 1 h, then showed a sharp decline at 5 h, followed by
a further reduction between 18 and 48 h. HVA levels peaked at 1 h,
decreased sharply at 5 h, then declined slowly between 18 and 48 h.
5-HT levels remained a stable between 1 and 48 h and showed no
significant difference compared with the control group.
Furthermore, the levels of L-dopa, DA, DOPAC and HVA demonstrated a
marked increase in the 1 h group compared with the control group
(P<0.01; Fig. 1D).
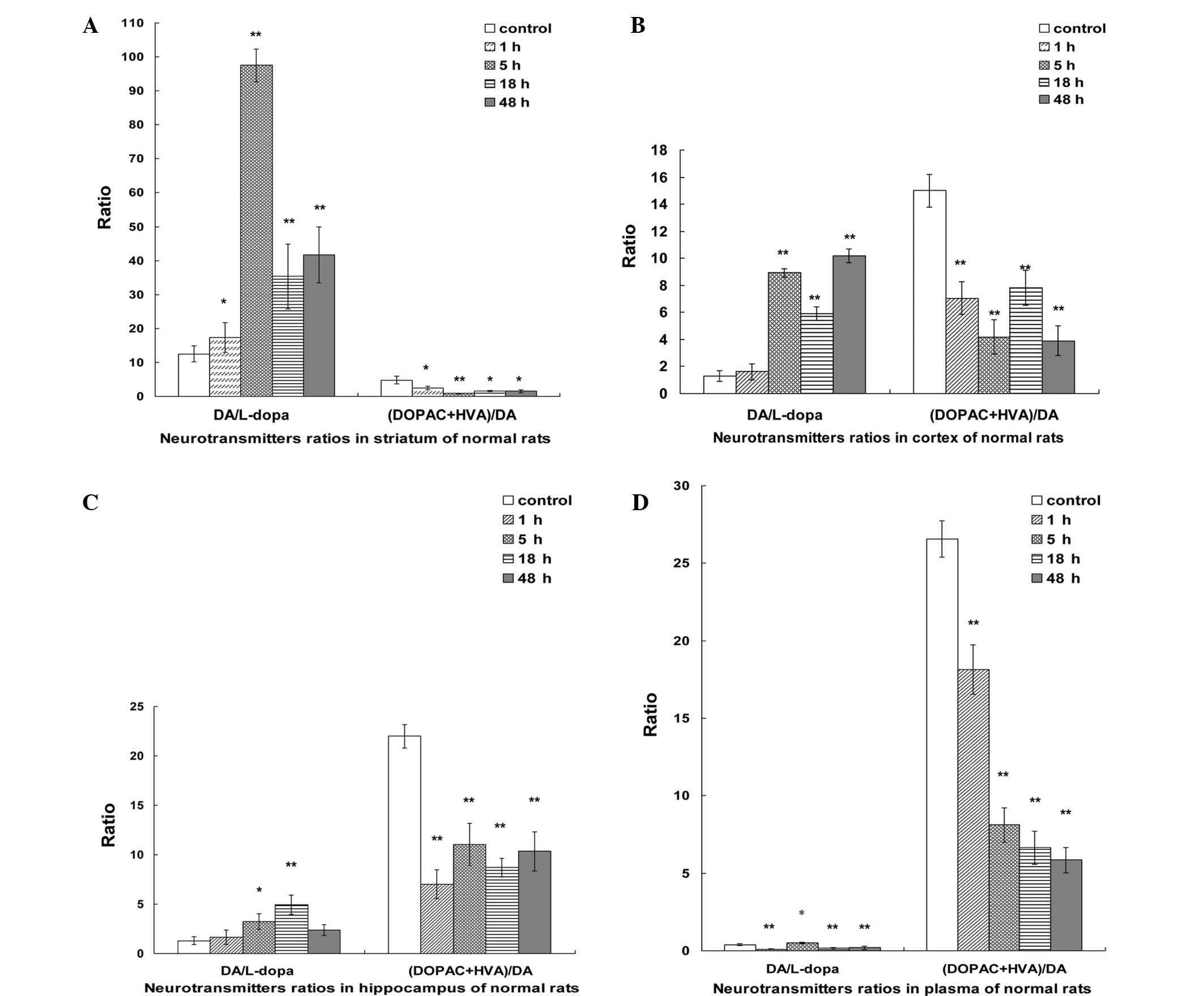
Comparisons of DA/L-dopa and (DOPAC +
HVA)/DA ratios in the plasma, striatum, hippocampus and cortex
In the striatum, the DA/L-dopa ratio showed a
significant increase in the initial 5 h, followed by a sharp
reduction at 18 h, and then increased slightly at 48 h. However,
the (DOPAC + HVA)/DA ratio exhibited a marked reduction at 1 h,
reaching a minimum at 5 h and then increasing slightly between 18
and 48 h (Fig. 2A).
In the cortex, the DA/L-dopa ratio demonstrated a
sharp increase in the first 5 h, followed by a rapid decline at 18
h, and then increased significantly at 48 h. By contrast, the
(DOPAC + HVA)/DA ratio showed a sharp decline in the first 5 h,
followed by a marked increase at 18 h, and subsequently decreased
at 48 h (Fig. 2B).
In the hippocampus, the DA/L-dopa ratio exhibited an
increasing tendency in the first 18 h, and then returned to the
normal levels. In addition, the (DOPAC + HVA)/DA ratio showed a
sharp decline in the first 1 h, followed by a slight increase
between 5 and 48 h (Fig. 2C).
In the plasma, when compared with the control group,
the DA/L-dopa ratio showed a significant decline in the first 1 h,
and then peaked at 5 h followed by a further reduction between 18
and 48 h. Furthermore, the (DOPAC + HVA)/DA ratio presented a sharp
reduction after 48 h, when compared with the control group
(Fig. 2D).
Comparison of DA, L-dopa, DOPAC, HVA
and 5-HT alterations in the plasma, striatum, hippocampus and
cortex following the co-administration
Following co-administration of β-asarone and L-dopa,
the L-dopa levels increased and peaked at 1 h, followed by a sharp
decline at 5 h, and remained stable between 5 and 48 h in the
plasma, cortex, hippocampus and striatum. Among these, the L-dopa
levels in the plasma were the highest. Furthermore, DA levels
increased and peaked at 1 h in the plasma, hippocampus, cortex and
striatum. Among these, the striatal levels of DA were the highest
within 48 h, showing a slight reduction at 5 h, followed by a
further reduction at 18 h and subsequent increase. However, DA
levels in the cortex, hippocampus and plasma demonstrated a sharp
decline at 5 h, followed by stable levels between 5 and 48 h. In
addition, the striatal levels of DOPAC peaked at 1 h, followed by a
sharp decline at 5 h, and remained stable until 18 h, prior to
increasing at 48 h. DOPAC levels in the cortex, hippocampus and
plasma exhibited a rapid decrease at 5 h, followed by a steady
level between 5 and 48 h. Furthermore, striatal levels of HVA
showed a marked reduction between 1 and 5 h, followed by a gradual
increase between 5 and 48 h. Similarly, HVA levels in the cortex,
hippocampus and plasma displayed a sharp reduction between 1 and 5
h, but remained stable between 5 and 48 h. By contrast, the 5-HT
levels remained stable and showed no statistically significant
differences from the control group levels within 48 h among the
plasma, striatum, cortex and hippocampus.
Neurotransmitter ratio alterations in
the plasma and brain following the co-administration
The DA/L-dopa ratio in the striatum and cortex
showed a marked increase in the first 5 h after treatment, followed
by a sharp reduction at 18 h, and subsequently returned to normal
levels. In addition, the DA/L-dopa ratio in the hippocampus
exhibited a rapid increase in the first 18 h, followed by a sharp
reduction at 48 h. Furthermore, the DA/L-dopa ratio in the plasma
increased and peaked at 5 h, followed by a rapid reduction at 18 h,
and remained stable between 18 and 48 h.
The (DOPAC + HVA)/DA ratio in the plasma and
striatum showed a rapid reduction within 48 h. In addition, the
(DOPAC + HVA)/DA ratio in the cortex demonstrated a reduction in
the first 5 h, followed by a rapid increase at 18 h. Similarly, the
(DOPAC + HVA)/DA ratio in the hippocampus showed a sharp decline at
1 h, followed by a reduction at 18 h, and subsequently returned to
normal levels.
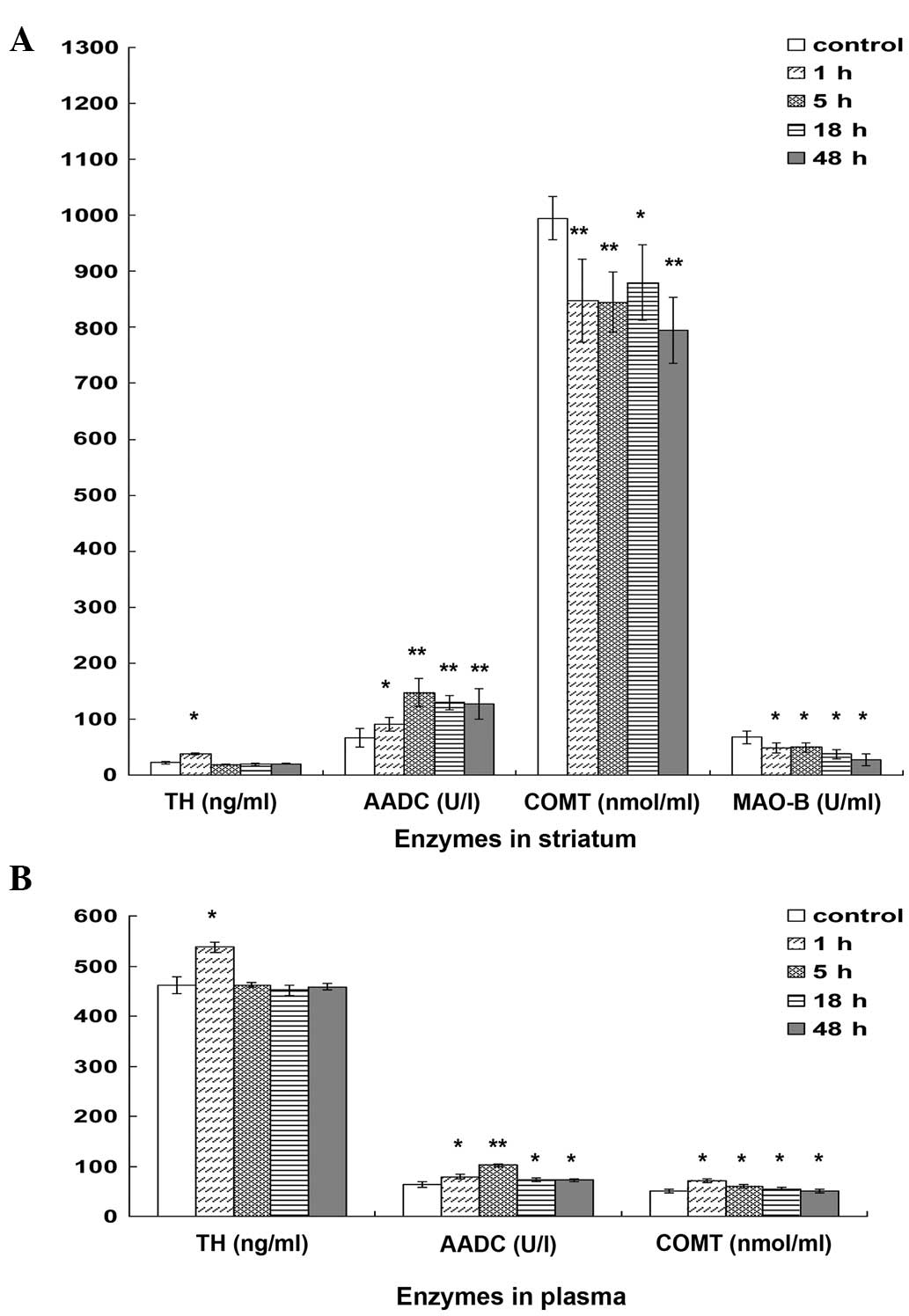
Alterations in TH, COMT, AADC and
MAO-B levels at various time points
In the striatum, TH levels increased and peaked at 1
h, followed by a marked reduction at 5 h, remaining stable between
5 and 48 h. The AADC levels demonstrated a rapid increase in the
first 5 h followed by a further reduction at 18 h. By contrast, the
COMT levels showed a sharp reduction at 1 h, followed by a further
reduction at 48 h. In addition, MAO-B levels showed a slight
reduction between 1 and 48 h (Fig.
3A).
In the plasma, TH levels increased at 1 h, followed
by a rapid reduction at 5 h, and remained at a stable level between
5 and 48 h. AADC levels exhibited a gradual increase in the first 5
h and were subsequently reduced at 18 h, followed by a stable level
between 18 and 48 h. Furthermore, AADC levels within 48 h were
elevated compared with the control group, while COMT levels showed
a significant reduction between 1 and 48 h (Fig. 3B).
Correlation between DA and 5-HT levels
in the plasma, hippocampus, cortex and striatum following the
co-administration
In order to investigate the association between the
neurotransmitters following the co-administration of β-asarone and
L-dopa, the correlations between DA and 5-HT in the cortex,
striatum, hippocampus and plasma were analyzed using Pearson
correlation analysis (Table I). The
correlation between DA and 5-HT was positive and statistically
significant in the striatum at 5 h (r=0.961, P<0.05). In
addition, the correlations between DA and 5-HT in the hippocampus
at 18 and 48 h were 0.976 and 0.965, respectively (P<0.05).
Furthermore, the correlation between DA and 5-HT in the plasma at
18 h was 0.989 (P<0.05). The results indicated that there were
significant positive correlations between DA and 5-HT in the
striatum at 5 h, in the hippocampus at 18 and 48 h and in the
plasma at 18 h.
 | Table I.Correlation between DA and 5-HT in rat
plasma and brain tissues within 48 h. |
Table I.
Correlation between DA and 5-HT in rat
plasma and brain tissues within 48 h.
|
| r-value |
|---|
|
|
|
|---|
| Group | Striatum | Cortex | Hippocampus | Plasma |
|---|
| 1 h | 0.289 | 0.871 | 0.501 | 0.214 |
| 5 h |
0.961a | 0.178 | 0.831 | 0.182 |
| 18 h | 0.296 | 0.700 |
0.976a |
0.989a |
| 48 h | 0.579 | 0.692 |
0.965a | 0.944 |
Discussion
β-asarone is a major component of Acorus
tatarinowii Schott, and has a significant pharmacological
effect in attenuating neuronal apoptosis, thus protecting against
neurotoxicity (16). Furthermore,
L-dopa remains the most effective medicine for the treatment of PD
(7). To date, researchers have
combined L-dopa with other medicines, such as carbidopa, for the
treatment of PD, in order to mitigate the side-effects associated
with L-dopa (17,18); however, such combinations have not
resolved the adverse reactions that result from chronic use of
L-dopa. Notably, we demonstrated in a previous study that β-asarone
and L-dopa co-administration is able to significantly increase the
striatal dopamine (DA) levels in healthy rats (11). However, the dynamic changes in the
levels of DA, L-dopa, DOPAC, HVA and 5-HT in the plasma and brain
of healthy rats within 48 h of the co-administration treatment
remain unknown. Therefore, a quantitative HPLC method was employed
to analyze the levels of these five neurotransmitters in the
striatum, hippocampus, cortex and plasma of rats at 1, 5, 18 and 48
h following treatment. In addition, we determined the dynamic
change in a number of enzymes associated with these
neurotransmitters in the plasma and striatum within 48 h.
In the present study, it was observed that the
striatal levels of L-dopa, DA, DOPAC and HVA increased after the
co-administration and peaked at 1 h, followed by reduction or
return to the normal levels between 5 and 48 h. Notably, DA
metabolism occurs primarily in the striatum (19). The present results suggested that
striatal DA levels were increased compared with those in the
hippocampus, cortex and plasma within 48 h, indicating that the
co-administration may aid in maintaining DA levels in the
striatum.
Compared with the control group, the DA/L-dopa ratio
in the striatum exhibited a rapid increase and peaked within the
initial 5 h after treatment, followed by a sharp decline at 18 h,
and subsequent increase. In addition, the DA/L-dopa ratio in the
striatum was higher compared with that in the hippocampus and
cortex between 1 and 48 h. These results indicated that the
transformation of L-dopa to DA occurred primarily in the striatum
after co-administration. In addition, the (DOPAC + HVA)/DA ratios
in the striatum, hippocampus and cortex exhibited a significant
reduction compared with the control group. These results indicated
that the co-administration of β-asarone and L-dopa may be able to
reduce the metabolism of DA.
In order to identify the mechanism through which DA
levels are elevated predominantly in the striatum, the functions of
TH, AADC, COMT and MAO-B were investigated. TH is the primary
regulator and rate-limiting enzyme of L-dopa synthesis (3). The results of the present study showed
that striatal TH levels increased and peaked at 1 h, and
subsequently returned to the normal levels at 5 h. This may explain
why L-dopa level at in the striatum were higher at 1 h compared
with at 5–48 h. In addition, L-dopa is known to be converted into
DA by AADC (4). The current results
indicated that striatal AADC levels in the treatment groups
increased within 48 h compared with the control group, which was
consistent with the observation that striatal DA levels within 48 h
were elevated compared with levels in the hippocampus and cortex.
Furthermore, DA may be degraded into DOPAC by MAO-B and into HVA by
COMT (5,6). In the present study, striatal levels of
MAO-B and COMT in the treatment groups were reduced compared with
the control group, which may explain why the (DOPAC + HVA)/DA ratio
in the striatum was reduced compared with the control group during
48 h.
In the plasma, the levels of L-dopa, DA, DOPAC and
HVA increased and peaked at 1 h after the co-administration, and
then showed a sharp decrease between 5 and 48 h. Based on the
ratios of DA/L-dopa and (DOPAC + HVA)/DA, the turnover rate of
L-dopa was found to peak at 5 h after co-administration, followed
by a rapid decline between 5 and 48 h. However, the turnover rate
of DA exhibited a sharp decrease between 1 and 48 h compared with
control group. In addition, TH levels increased and peaked at 1 h
prior to returning to the normal levels. AADC levels peaked at 5 h,
and were higher compared with the control group. In addition, COMT
levels exhibited a decreasing trend within 48 h compared with the
control group. Thus, collectively the present results suggest that
the co-administration may promote the generation of DA by enhancing
the activity of AADC, in addition to reducing the metabolism of DA
by inhibiting the activity of COMT.
In the present study, the 5-HT levels in the plasma,
striatum, hippocampus and cortex of the rats exhibited no
statistically significant differences when compared with the
control group rats. However, the correlation between DA and 5-HT
was positive and significant in the striatum, cortex, hippocampus
and plasma at 5, 1, 18–48 and 18 h, respectively. The results
indicated that the statistically significant correlation identified
between DA and 5-HT was associated with the brain and plasma areas
in healthy rats following the co-administration.
In conclusion, the present study reported the
dynamic changes in the levels of L-dopa, DA, DOPAC, HVA and 5-HT in
the striatum, cortex, hippocampus and plasma of rats within 48 h of
the co-administration of β-asarone and L-dopa. Notably, the
co-administration exerted the effect of maintaining a steady DA
level in the striatum during the 48-h period. Furthermore, the
co-administration appeared to promote the generation of DA by
enhancing the activity of AADC, while reducing the metabolism of DA
by inhibiting the activity of COMT. Therefore, the
co-administration of β-asarone and L-dopa may provide a beneficial
intervention for the treatment of PD.
Acknowledgements
This study was supported by grants from the
Guangdong Natural Science Foundation of China (No. S2012010010625)
and the First Clinical Medical College of Guangzhou University of
Chinese Medicine Excellence Doctoral Dissertation Cultivation
Project (No. YB201403).
References
|
1
|
Nutt JG and Wooten GF: Clinical practice.
Diagnosis and initial management of Parkinson's disease. N Engl J
Med. 353:1021–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Calabresi P, Giacomini P, Centonze D and
Bernardi G: Levodopa-induced dyskinesia: A pathological form of
striatal synaptic plasticity? Ann Neurol. 47:S60–S68.
2000.PubMed/NCBI
|
|
3
|
Baier CJ, Pallarés ME, Adrover E, Katunar
MR, Raisman-Vozari R and Antonelli MC: Intrastriatal 6-OHDA lesion
differentially affects dopaminergic neurons in the ventral
tegmental area of prenatally stressed rats. Neurotox Res.
26:274–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ren J, Zhang Y, Jin H, Yu J, Zhou Y, Wu F
and Zhang W: Novel inhibitors of human DOPA decarboxylase extracted
from Euonymus glabra Roxb. ACS Chem Biol. 9:897–903. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bolea I, Colivicchi MA, Ballini C,
Marco-Contelles J, Tipton KF, Unzeta M and Della Corte L:
Neuroprotective effects of the MAO-B inhibitor, PF9601N, in an
in vivo model of excitotoxicity. CNS Neurosci Ther.
20:641–650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Onzawa Y, Kimura Y, Uzuhashi K, Shirasuna
M, Hirosawa T, Taogoshi T and Kihira K: Effects of
3-O-methyldopa, L-3,4-dihydroxyphenylalanine metabolite, on
locomotor activity and dopamine turnover in rats. Biol Pharm Bull.
35:1244–1248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Noack C, Schroeder C, Heusser K and Lipp
A: Cardiovascular effects of levodopa in Parkinson's disease.
Parkinsonism Relat Disord. 20:815–818. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hong JY, Oh JS, Lee I, Sunwoo MK, Ham JH,
Lee JE, Sohn YH, Kim JS and Lee PH: Presynaptic dopamine depletion
predicts levodopa-induced dyskinesia in de novo Parkinson
disease. Neurology. 82:1597–1604. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alachkar A, Brotchie JM and Jones OT:
Locomotor response to L-DOPA in reserpine-treated rats following
central inhibition of aromatic L-amino acid decarboxylase: Further
evidence for non-dopaminergic actions of L-DOPA and its
metabolites. Neurosci Res. 68:44–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu HB and Fang YQ: Pharmacokinetics of
beta-asarone in rats. Yao Xue Xue Bao. 39:836–838. 2004.(In
Chinese). PubMed/NCBI
|
|
11
|
Huang L, Deng M, Zhang S, Fang Y and Li L:
Coadministration of β-asarone and levodopa increases dopamine in
rat brain by accelerating transformation of levodopa: A different
mechanism from Madopar. Clin Exp Pharmacol Physiol. 41:685–690.
2014.PubMed/NCBI
|
|
12
|
Veloso A, Fernández R, Astigarraga E,
Barreda-Gómez G, Manuel I, Giralt MT, Ferrer I, Ochoa B,
Rodríguez-Puertas R and Fernández JA: Distribution of lipids in
human brain. Anal Bioanal Chem. 401:89–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu L and Fang YQ: Analysis of the
distribution of β-asarone in rat hippocampus, brainstem, cortex and
cerebellum with GC-MS. J Med Plants Res. 9:1728–1734. 2011.
|
|
14
|
Fang YQ, Shi C, Liu L and Fang RM:
Analysis of transformation and excretion of β-asarone in rabbits
with GC-MS. Eur J Drug Metab Pharmacokinet. 37:187–190. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang S, Gui XH, Xue ZF, Huang LP, Fang
RM, Ke XH, Li L and Fang YQ: Dynamic of neurochemical alterations
in striatum, hippocampus and cortex after the 6-OHDA mesostriatal
lesion. Int J Dev Neurosci. 36:32–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei G, Chen YB, Chen DF, Lai XP, Liu DH,
Deng RD, Zhou JH, Zhang SX, Li YW, Lii H, et al: β-Asarone inhibits
neuronal apoptosis via the CaMKII/CREB/Bcl-2 signaling pathway in
an in vitro model and AβPP/PS1 mice. J Alzheimers Dis. 33:863–880.
2013.PubMed/NCBI
|
|
17
|
Takáts A, Nagy H, Radics P, Tóth A and
Tamás G: Treatment possibilities in advanced Parkinson's disease.
Ideggyogy Sz. 66:365–371. 2013.(In Hungarian). PubMed/NCBI
|
|
18
|
Lazzara CA, Riley RR, Rane A, Andersen JK
and Kim YH: The combination of lithium and I-Dopa/Carbidopa reduces
MPTP-induced abnormal involuntary movements (AIMs) via calpain-1
inhibition in a mouse model: Relevance for Parkinsons disease
therapy. Brain Res. June 26–2015.(epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu T, Liu J, Zhang H, Hallett M, Zheng Z
and Chan P: Attention to automatic movements in Parkinson's
disease: Modified automatic mode in the striatum. Cereb Cortex.
June 12–2014.(epub ahead of print). View Article : Google Scholar
|