Introduction
Henoch-Schönlein purpura (HSP) is a common childhood
systemic vascular inflammatory disease with clinical symptoms
involving the skin, gastrointestinal tract, joints and kidneys. The
severity of renal involvement often directly affects the course and
prognosis of the disease (1,2). Currently, infection is regarded as a
crucial inducing factor for HSP. Abnormal immune function may be
observed at the acute phase of HSP; however, its underlying
mechanism has not yet been fully elucidated.
Toll-like receptors (TLRs) are a class of cell
surface receptors involved in transmembrane signal transduction.
TLRs are able to directly identify and bind pathogen-associated
molecular patterns and then prime the signal transduction pathways
in the host cells, which may promote the synthesis of cytokines and
the activation of T cells and thereby regulate the Th1/Th2 balance
and immune status (3). TLRs serve a
crucial function in the immune and inflammatory responses, and are
reportedly involved in the onset of a variety of diseases,
including bronchial asthma, systemic lupus erythematosus,
rheumatoid arthritis, multiple sclerosis and Kawasaki disease
(4–8). T helper (Th) cells, including Th1, Th2
and Th17, are involved in the pathogenesis of various types of
vascular inflammation (9–11). Th cell subset functional imbalance
and aberrant activation of Th2 and Th17 cells have been observed in
patients with HSP (12–15). However, the association between the
TLR signaling pathways and Th1, Th2 and Th17 in the immune
pathogenesis of HSP remains unknown. Myeloid differentiation factor
88 (MYD88) is the key adaptor molecule in TLR signaling pathways
and the key target molecule for downstream signal transduction
(16,17).
Our previous study (18) indicated that the activation of TLR2
and TLR4 may mediate the pathogenesis of HSP by upregulating the
Th2 immune response. However, the association between TLR6 and the
Th1/Th2 balance and Th17 cells, and whether it has any involvement
in the pathogenesis of HSP remains unclear. The present study was
conducted to elucidate the association between the expression of
TLR6 in the peripheral blood mononuclear cells (PBMCs) and the
subpopulations of Th cells in children with HSP by determining the
expression levels of TLR6 and MYD88 in the PBMCs and the
interleukin (IL)-4, interferon (IFN)-γ and IL-17 levels in serum
samples. This should provide further information concerning the
pathogenesis of HSP in children and may provide novel methods for
the treatment of this disease.
Materials and methods
Patients
A total of 42 children with acute HSP, hospitalized
in the Affiliated Hospital of Qingdao University (Qingdao, China)
were enrolled in the present study between June 2013 and November
2013. The subjects included 25 males and 17 females, with an age
range of 4–13 years (mean age, 6.7 years). All patients met the
2006 diagnostic criteria for allergic purpura defined by EULAR/PReS
(19). Patients were at the time of
first onset and had received no related medications, such as
glucocorticoids, immunodepressant or heparin, in the preceding 4
weeks. A further 30 children receiving physical examination in the
child care health center of the Affiliated Hospital of Qingdao
University during the same period were recruited as the healthy
control group. The healthy control group included 20 males and 10
females, with an age range of 3–12 years (mean age, 6.5 years).
There was no statistical difference in age or gender between the
two groups. This study was conducted in accordance with the
declaration of Helsinki and with the approval of the Ethics
Committee of Qingdao University. Written informed consent was
obtained from the guardians of all participants.
Sampling
A 3-ml peripheral venous blood sample was collected
in a sterile heparin anticoagulant tube for flow cytometry and
fluorescent reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analysis. An additional 1 ml of blood was
collected in a common tube in order to isolate the serum for the
determination of cytokine levels. All serum samples were stored at
−80°C prior to use.
Flow cytometry
Two 100-µl blood samples from each patient treated
with anticoagulant (Fuzhou Maixin Biotechnology Development Co.,
Ltd., Fuzhou, China) were added to two tubes. Subsequently, 10 µl
monoclonal mouse anti-human fluorescein isothiocyanate
(FITC)-labeled CD14 antibody (cat. no. 110419-42; eBioscience,
Inc., San Diego, USA) and 10 µl monoclonal rat anti-human
phycoerythrin (PE)-labeled TLR6 antibody (cat. no. 334708;
BioLegend, Inc., San Diego, USA) were added into one of the two
tubes for determining the expression levels of CD14 and TLR6. Next,
10 µl mouse FITC-labeled IgG2a isotype and 10 µl rat anti-human
PE-labeled isotype (eBioscience, Inc.) were added to the second
tube for each patient to serve as a blank control. Following
incubation at room temperature for 15 min in the dark, 3 ml lysing
solution was added to each tube to lyse the red blood cells, and
the samples were stored at room temperature for a further 15 min.
Then the tubes were centrifuged at 696 × g for 5 min. After
discarding the supernatant, cells were washed with
phosphate-buffered saline (PBS) twice and centrifuged at 696 × g
for 5 min. Finally, the cells were resuspended in 500 µl PBS and
analyzed using a flow cytometer (FC500; Beckman Coulter, Brea, CA,
USA). The percentage of CD14+TLR6+ cells in
each sample was calculated.
RNA extraction and RT-qPCR
PBMCs from each serum sample were isolated by Ficoll
density-gradient centrifugation (Sigma-Aldrcih, St. Louis, MO, USA)
and dissolved in RNAiso Plus reagent (Takara Biotechnology Co.,
Ltd., Dalian, China) for total RNA extraction, according to the
manufacturer's instruction. The concentration and purity of the
extracted total RNA were determined at a wavelength of 260 nm using
a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific,
Waltham, MA, USA). Next, 2.5 µl total RNA was used as a template to
synthesize the first strand cDNA by reverse transcription (RT),
using a One Step SYBR PrimeScript RT-PCR kit (Takara Biotechnology
Co., Ltd.). The RT-qPCR reaction was performed using an ABI Prism
7000 sequence detection system (Life-Tech, Inc., Williston, VT,
USA) using SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.),
following the manufacturer's instructions. The following
gene-specific primer pairs were used: MYD88, F 5′-GCA CAT GGG CAC
ATA CAG AC-3′ and R 5′-TGG GTC CTT TCC AGA GTT TG-3′; and GAPDH, F
5′-AAC AGC CTC AAG ATC ATC AGC AA-3′ and R 5′-GAC TGT GGT CAT GAG
TCC TTC CA-3′. All samples were normalized against GAPDH reference
gene levels, and the relative expression of MYD88 mRNA was
calculated using the formula: R = 2−ΔΔCT (20). All samples were run in duplicate.
ELISA
Serum levels of interferon (IFN)-γ, IL-4 and IL-17
were determined by ELISA using IFN-γ, IL-4 and IL-17 kits,
following the manufacturer's instructions (Shanghai Yuan-Long
Biotechnology Co., Ltd., Shanghai, China). The plasma was placed at
room temperature for 20 min, until completely thawed. A 96-well
plate consisted of blank control wells, standard wells, and sample
wells. The wells were pre-coated with avidin (Shanghai Yuan-Long
Biotechnology Co., Ltd.). A total of 50 µl standard solution (0, 3,
6, 12, 24 and 48 pg/ml) was added to the standard wells. A total of
10 µl serum sample diluted with 40 µl PBS was added to the sample
wells. Subsequently, 100 µl peroxidase-labeled antibody was added
to the wells containing sample or standard, and 100 µl
non-peroxidase labeled antibody was added to the blank control
wells (the antibodies were provided with the ELISA kits). The
plates were incubated at 37°C for 60 min. The liquid was removed,
and the wells were washed three times with ELISA Wash Buffer (50 mM
Tris-HCl, pH 7.4; 0.2% Tween 20; Shanghai Yuan-Long Biotechnology
Co., Ltd.) and blotted dry with paper towels. A total of 50 µl
substrate A (H2O2) and 50 µl substrate B
(3,3′,5,5′-tetramethylbenzidine; Shanghai Yuan-Long Biotechnology
Co., Ltd.) were added to each well and incubated at 37°C for 15 min
in the dark. Following the addition of 50 µl stop buffer, the
optical density was measured at 450 nm within 15 min using a Sp-MAX
1800 microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
Data are expressed as the mean ± standard deviation
and were processed using SPSS software, version 17.0 (SPSS, Inc.,
Chicago, IL, USA). Independent sample t-test was used to analyze
the difference between the two groups with homogeneity of variance,
while independent sample t'-test was used when the variance was
heterogeneous. Pearson correlation analysis was performed to
analyze the correlation between two parameters. P<0.05 was
considered to indicate a statistically significant difference.
Results
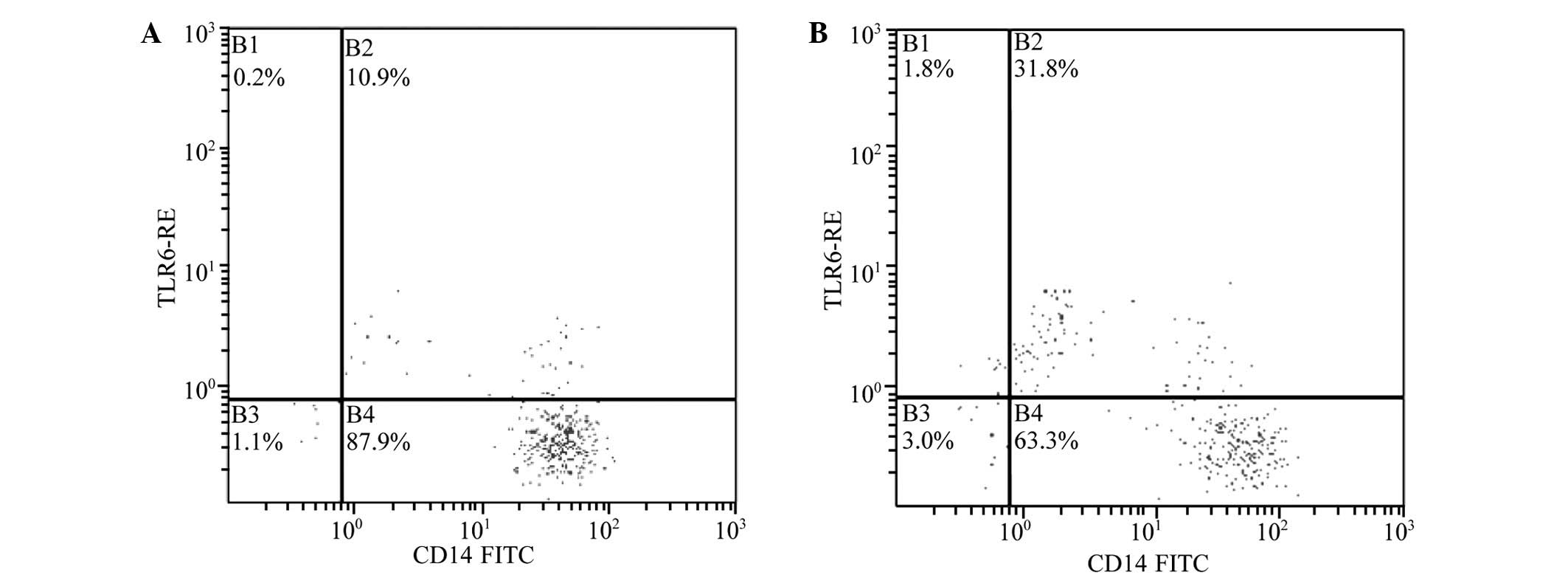
Positive percentage and mean
fluorescence intensity (MFI) of TLR6 and the expression of
MYD88
The positive percentage and MFI of TLR6 in the PBMCs
was determined by fluorescence-activated cell sorting. These values
increased significantly in the patients with HSP compared with
those in the control group (P<0.01; Table I and Fig.
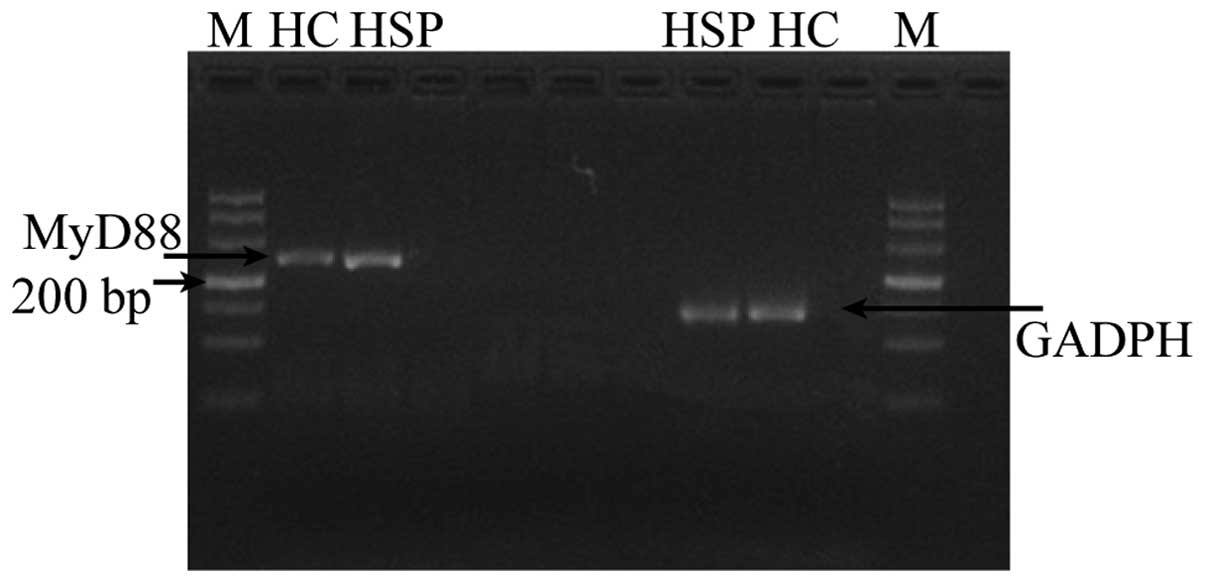
1). Furthermore, the mRNA expression levels of MYD88 in the
PBMCs of the HSP group were markedly increased compared with those
in the control group PBMCs (P<0.01; Table I and Fig.
2).
 | Table I.Expression of TLR6 protein and MyD88
mRNA in the PBMCs of the two groups. |
Table I.
Expression of TLR6 protein and MyD88
mRNA in the PBMCs of the two groups.
| Parameter | Cases | TLR6 MFI | TLR6 protein (%) | MYD88 mRNA |
|---|
| HSP | 42 | 12.56±4.09 | 29.13±10.17 | 1.28±0.42 |
| Control | 30 | 2.97±1.83 | 6.86±4.05 | 0.99±0.25 |
| t'-value | – | 9.40 | 10.49 | 2.46 |
| P-value | – | <0.01 | <0.01 | <0.01 |
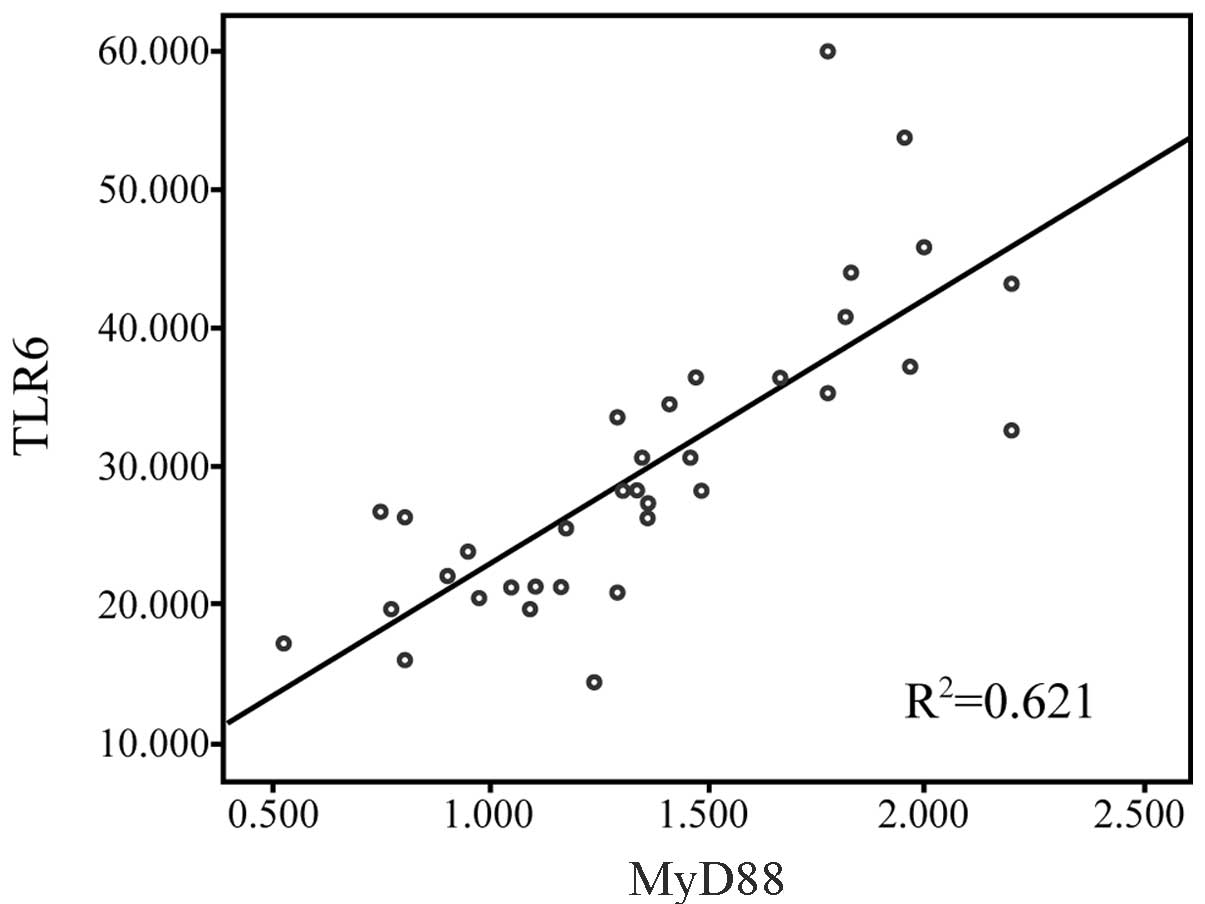
Correlation between TLR6 and MyD88
expression
Pearson correlation analysis indicated that the MFI
of TLR6 protein in the PBMCs was positively correlated with the
mRNA expression of MyD88 in the patients with HSP (r=0.79;
P<0.01; Fig. 3).
Serum levels of IFN-γ, IL-4 and IL-17,
and the IFN-γ/IL-4 ratio
As determined by ELISA, the serum levels of IFN-γ,
IL-4 and IL-17 in the HSP group were significantly higher compared
with those in the normal control group. However, the ratio of IFN-γ
to IL-4 in the HSP group was lower than that in the control group
(Table II).
 | Table II.Serum levels of IFN-γ, IL-4 and IL-17
and the IFN-γ/IL-4 ratio in the two groups. |
Table II.
Serum levels of IFN-γ, IL-4 and IL-17
and the IFN-γ/IL-4 ratio in the two groups.
| Parameter | Cases | IFN-γ (pg/ml) | IL-4 (pg/ml) | IFN-γ/IL-4 | IL-17 (pg/ml) |
|---|
| HSP | 42 | 195.86±84.16 | 8.18±3.48 | 25.67±11.47 | 13.17±2.84 |
| Control | 30 | 140.83±77.78 | 5.05±1.88 | 47.90±10.06 | 11.09±1.77 |
| t'-value | – | 2.44 | 3.44 | 2.27 | 2.64 |
| P-value | – | <0.05 | <0.01 | <0.05 | <0.01 |
Correlation of TLR6 with IFN-γ, IL-4
and IL-17 levels and the IFN-γ/IL-4 ratio
The MFI of TLR6 in the PBMCs of the HSP group showed
a marked positive correlation with serum levels of IL-4 (r=0.69;
P<0.01) and IL-17 (r=0.36; P<0.05). However, the TLR6 MFI was
negatively correlated with the ratio of IFN-γ to IL-4 (r=-0.38,
P<0.05). Furthermore, no significant correlation was detected
between the MFI of TLR6 in the PBMCs and serum IFN-γ levels
(P>0.05; Table III).
 | Table III.Correlation between TLR6 MFI in
patients with HSP and serum levels of IFN-γ, IL-4 and IL-17, and
the IFN-γ/IL-4 ratio. |
Table III.
Correlation between TLR6 MFI in
patients with HSP and serum levels of IFN-γ, IL-4 and IL-17, and
the IFN-γ/IL-4 ratio.
| Parameter | IFN-γ | IL-4 | IFN-γ/IL-4 | IL-17 |
|---|
| r | 0.097 | 0.69 | −0.38 | 0.36 |
| P-value | >0.05 | <0.01 | <0.05 | <0.05 |
Discussion
To date, the pathogenesis of allergic purpura
remains unclear, although it has been proposed that the
pathogenesis of the disease may be associated with humoral and
cellular immune disorders, blood coagulation and fibrinolysis
disorders and genetic susceptibility. No clear regulatory mechanism
has been identified to explain the abnormal immune function
observed in patients with allergic purpura. Previous studies
indicate that cellular immune dysfunction, manifesting as a Th1/Th2
imbalance in the acute phase and dominant activation of Th2 cells,
is present in a significant proportion of pediatric patients with
HSP (12–15). Furthermore, Th17 cells are activated
abnormally in these patients, and regulatory T (Treg) cells are
reduced in number or exhibit reduced activity. In the present
study, it was observed that Th1 cytokine IFN-γ and Th2 cytokine
IL-4 increased significantly in the PBMCs of the patients with HSP
at the acute phase. However, the IFN-γ/IL-4 ratio decreased
markedly in the peripheral blood, suggesting that a Th1/Th2
imbalance is present in the patients with HSP, in particular a
dominance of Th2. The upregulation of IFN-γ may be a compensatory
response to the observed immunological imbalances in vivo.
Th17 is a novel subset of CD4+ helper T cells identified
in recent years, and is characterized by the secretion of various
cytokines, including IL-17, IL-21 and IL-22 (21,22).
IL-17 induces monocytes/macrophages, smooth muscle cells,
epithelial cells and endothelial cells to produce a variety of
inflammatory cytokines and chemokines that are involved in the
inflammatory response or autoimmune reaction. The present results
suggest that the serum IL-17 levels in the patients with HSP were
elevated markedly, indicating that Th17 cells serve a crucial
function in the pathogenesis of HSP.
TLRs are a key variety of pattern recognition
receptors, a bridge that link the innate immune response with the
adaptive immune response. Following the activation of a TLR by its
specific ligand, it may activate numerous transcription factors via
MYD88-dependent or-independent pathways, including interferon
regulatory factor 3 (IRF3), IRF7, activator protein 1 and nuclear
factor-κB. In addition, a TLR may promote the gene expression of
IL-6, IL-1β, IL-12, tumor necrosis factor-β and other inflammatory
factors. TLRs may also upregulate the expression of co-stimulating
molecules on the surface of antigen-presenting cells, leading to
the production of a variety of cytokines and promoting the
activation of Th cells, thereby priming the adaptive immune
response (23–25). The excessive activation of TLRs may
lead to the occurrence and development of a variety of autoimmune
diseases (26,27). However, the function served by TLRs
in the pathogenesis of HSP is not yet clear.
The upregulation of co-stimulating molecules and the
formation of a microenvironment induced by the activated TLR
signaling pathways may cause naïve T cells to transform into
CD4+, such as Th1, Th2, Th17 and
CD4+CD25+Foxp3+ Treg cells. Under
certain conditions, the binding of specific ligands to TLRs may
lead to the occurrence of Th2 or Th17 immune responses, although
the majority of TLR ligands induce the Th1 immune response. Kim
et al (28) observed that the
overexpression of TLR4 and TLR9 may be involved in the
immunopathogenesis of multiple dermatomyositis by activating Th1,
Th2 and Th17 immune responses. Morgan et al (29) confirmed that TLR6 activation is able
to promote Th1 and Th17 immune responses in gastrointestinal
lymphoid tissue in inflammatory bowel disease. Zhao et al
(30) observed that TLR2 and TLR4
levels are increased in the peripheral blood and bronchoalveolar
lavage fluid of mice that have inhaled various doses of fine
particulate matter (PM2.5). Furthermore, Zhao et al
demonstrated that the levels of IL-5 and IL-10 were elevated in the
alveolar lavage fluid and peripheral blood of these mice, while
IL-4 levels were increased in the peripheral blood only, suggesting
that TLR2 and TLR4 may induce the Th2 immune response in the
inflammatory reaction caused by the inhalation of PM2.5
particulates.
The results of this study suggest that TLR6 protein
expression and MYD88 mRNA expression levels in the PBMCs of
pediatric patients with HSP were significantly elevated compared
with those in control subjects, and that the expression of TLR6 was
significantly positively correlated with MYD88 mRNA expression
(P<0.01). The observations suggest that TLR6 mediates the
production of inflammatory cytokines and is involved in the
pathogenesis of HSP, potentially via MYD88-dependent signal
transduction pathways. Furthermore, the present results indicate
that TLR6 protein expression is significantly positively correlated
with serum IL-4 and IL-17 levels, but negatively correlated with
the IFN-γ/IL-4 ratio in patients with HSP. These results suggest
that TLR signal transduction pathways are activated by the binding
of their specific ligands, which results in the production of a
variety of inflammatory cytokines. However, this binding results
predominantly in the activation of Th2 and Th17, through a variety
of immunological mechanisms, thereby leading to the excessive
production IL-4. This overproduction may result in a Th1/Th2
imbalance and the overexpression of IL-17, which induces the onset
of HSP.
In conclusion, TLR6 protein and MYD88 mRNA
expression levels are increased in the PBMCs of pediatric patients
with HSP, and are significantly positively correlated. Th1/Th2
imbalance, excessive activation of Th17 and positive correlation of
TLR6 protein expression with Th17 cells and the Th2 immune response
were observed in the children with HSP. These findings suggest that
the activation of TLR6 may mediate the immunopathogenesis of HSP by
promoting Th2 and Th17 immune responses.
References
|
1
|
Jauhola O, Ronkainen J, Koskimies O,
Ala-Houhala M, Arikoski P, Hölttä T, Jahnukainen T, Rajantie J,
Ormälä T and Nuutinen M: Clinical course of extrarenal symptoms in
Henoch-Schonlein purpura: A 6-month prospective study. Arch Dis
Child. 95:871–876. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Punnoose AR, Lynm C and Golub RM: JAMA
patient page. Henoch-Schönlein purpura. JAMA. 307:7422012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawasaki T and Kawai T: Toll-like receptor
signaling pathways. Front Immunol. 5:4612014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miranda-Hernandez S and Baxter AG: Role of
toll-like receptors in multiple sclerosis. Am J Clin Exp Immunol.
2:75–93. 2013.PubMed/NCBI
|
|
5
|
Klaassen EM, van de Kant KD, Soeteman M,
Damoiseaux J, van Eys G, Stobberingh EE, Stelma FF, Quaak M, van
Schayck OC, Jöbsis Q, et al: CD14/Toll-like receptors interact with
bacteria and regulatory T-cells in the development of childhood
asthma. Eur Respir J. 44:799–802. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kirchner M, Sonnenschein A, Schoofs S,
Schmidtke P, Umlauf VN and Mannhardt-Laakmann W: Surface expression
and genotypes of toll-like receptors 2 and 4 in patients with
juvenile idiopathic arthritis and systemic lupus erythematosus.
Pediatr Rheumatol Online J. 11:92013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin IC, Kuo HC, Lin YJ, Wang FS, Wang L,
Huang SC, Chien SJ, Huang CF, Wang CL, Yu HR, et al: Augmented TLR2
expression on monocytes in both human Kawasaki disease and a mouse
model of coronary arteritis. PLoS One. 7:e386352012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang H, Gou SJ, Zhao MH and Chen M: The
expression of Toll-like receptors 2, 4 and 9 in kidneys of patients
with anti-neutrophil cytoplasmic antibody (ANCA)-associated
vasculitis. Clin Exp Immunol. 177:603–610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Na SY, Park MJ, Park S and Lee ES:
Up-regulation of Th17 and related cytokines in Behçet's disease
corresponding to disease activity. Clin Exp Rheumatol. 31:32–40.
2013.PubMed/NCBI
|
|
10
|
Kato H and Perl A: Mechanistic target of
rapamycin complex 1 expands Th17 and
IL-4+CD4−CD8− double-negative T
cells and contracts regulatory T cells in systemic lupus
erythematosus. J Immunol. 192:4134–4144. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park SJ and Shin JI: Another beneficial
effect of rituximab on refractory ANCA-associated vasculitis: The
role of interleukin-17 suppression? Scand J Immunol. 77:2212013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li YY, Li CR, Wang GB, Yang J and Zu Y:
Investigation of the change in CD4+ T cell subset in
children with Henoch-Schonlein purpura. Rheumatol Int.
32:3785–3792. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jen HY, Chuang YH, Lin SC, Chiang BL and
Yang YH: Increased serum interleukin-17 and peripheral Th17 cells
in children with acute Henoch-Schönlein purpura. Pediatr Allergy
Immunol. 22:862–868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen O, Zhu XB, Ren H, Wang YB and Sun R:
The imbalance of Th17/Treg in Chinese children with
Henoch-Schonlein purpura. Int Immunopharmacol. 16:67–71. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Donadio ME, Loiacono E, Peruzzi L, Amore
A, Camilla R, Chiale F, Vergano L, Boido A, Conrieri M, Bianciotto
M, et al: Toll-like receptors, immunoproteasome and regulatory T
cells in children with Henoch-Schönlein purpura and primary IgA
nephropathy. Pediatr Nephrol. 29:1545–1551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Akira S: Mammalian Toll-like receptors.
Curr Opin Immunol. 15:5–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kogut MH, Iqbal M, He H, Philbin V, Kaiser
P and Smith A: Expression and function of Toll-like receptors in
chicken heterophils. Dev Comp Immunol. 29:791–807. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang H, Zhang QY, Cheng N, Zhang SQ and
Lin Y: Study on expression of TLR2 and TLR4 in peripheral blood
mononuclear cells and their relationship with Th1/Th2 immune
resposnse in patients with Henoch-Schonlein pupra. Zhong Hua Wei
Sheng Wu Xue He Mian Yi Xue Za Zhi. 33:839–844. 2013.(In
Chinese).
|
|
19
|
Ozen S, Ruperto N, Dillon MJ, Bagga A,
Barron K, Davin JC, Kawasaki T, Lindsley C, Petty RE, et al:
EULAR/PReS endorsed consensus criteria for the classification of
childhood vasculitides. Ann Rheum Dis. 65:936–941. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Raphael I, Nalawade S, Eager TN and
Forsthuber TG: T cell subsets and their signature cytokines in
autoimmune and inflammatory diseases. Cytokine. 74:5–17. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song X, Gao H and Qian Y: Th17
differentiation and their pro-inflammation function. Adv Exp Med
Biol. 841:99–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brown J, Wang H, Hajishengallis GN and
Martin M: TLR-signaling networks: An integration of adaptor
molecules, kinases and cross-talk. J Dent Res. 90:417–427. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin B, Sun T, Yu XH, Yang YX and Yeo AE:
The effects of TLR activation on T-cell development and
differentiation. Clin Dev Immunol. 2012:8364852012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qian C and Cao X: Regulation of toll-like
receptor signaling pathways in innate immune responses. Ann N Y
Acad Sci. 1283:67–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mills KH: TLR-dependent T cell activation
in autoimmunity. Nat Rev Immunol. 11:807–822. 2011.PubMed/NCBI
|
|
27
|
Coppo R, Camilla R, Amore A, Peruzzi L,
Daprà V, Loiacono E, Vatrano S, Rollino C, Sepe V, Rampino T and
Dal Canton A: Toll-like receptor 4 expression is increased in
circulating mononuclear cells of patients with immunoglobulin A
nephropathy. Clin Exp Immunol. 159:73–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim GT, Cho ML, Park YE, Yoo WH, Kim JH,
Oh HJ, Kim DS, Baek SH, Lee SH, Lee JH, et al: Expression of TLR2,
TLR4 and TLR9 in dermatomyositis and polymyositis. Clin Rheumatol.
29:273–279. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morgan ME, Koelink PJ, Zheng B, den Brok
MH, van de Kant HJ, Verspaget HW, Folkerts G, Adema GJ and
Kraneveld AD: Toll-like receptor 6 stimulation promotes T-helper 1
and 17 responses in gastrointestinal-associated lymphoid tissue and
modulates murine experimental colitis. Mucosal Immunol.
7:1266–1277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao C, Liao J, Chu W, Wang S, Yang T, Tao
Y and Wang G: Involvement of TLR2 and TLR4 and Th1/Th2 shift in
inflammatory responses induced by fine ambient particulate matter
in mice. Inhal Toxicol. 24:918–927. 2012. View Article : Google Scholar : PubMed/NCBI
|