Introduction
Endometrial carcinoma, which is also known as corpus
carcinoma, is a common gynecological malignant tumor, with an
incidence that is second only to cervical cancer (1). The five year survival rate of
endometrial carcinoma is 74–91%. Endometrial carcinoma is the
fourth most common cancer in Europe and the United States,
accounting for ~6% of newly diagnosed tumors (2). The etiology of endometrial cancer is
currently not well understood. Preliminary studies have focused on
a single candidate gene or pathway, such as the phosphoinositide
3-kinase (PI3K)/AKT/mammalian target of rapamycin signaling pathway
(2,3); however, to uncover the full range of
genes recurrently mutated in endometrial carcinoma requires whole
genome sequencing of numerous patient samples. The current
immunosuppressive therapy used to treat endometrial carcinoma often
induces anti-tumor immune responses, including antigen-specific T
cell responses (4). However, tumor
evasion of the immune system poses a serious challenge to the
efficacy of immunosuppressive therapies. There are numerous
mechanisms by which tumors may evade the host immune system,
although the mechanisms used by endometrial tumors remain elusive.
A possible immune evasion mechanism includes the suppression of
immune checkpoints. Immune checkpoints maintain the tolerance of
the immune system to self-antigens under normal physiological
conditions, and negatively regulate the immune response, in order
to avoid immune injury (5). In
addition, these molecules have an important role in anti-tumor
immunity, of which the predominant immune checkpoints involved are
cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed
death-ligand (PD-L) 1. At present, numerous inhibitors of these
molecules have been approved for the treatment of cancer by the
Food and Drug Administration, including ant-CTLA4 and anti-PD-1
(6–9).
PD-L1 and PD-L2 are members of the B7-CD28 family
and ligands of programmed death receptor 1 (PD-1), which has a
critical role in central T cell tolerance during the process of T
cell development. PD-L1 is expressed in various tissues, including
the placenta, cells of the heart and spleen, pancreatic island and
white blood cells. Furthermore, PD-L1 has been shown to be highly
expressed in tumor tissue. Conversely, the expression profile of
PD-L2 is very limited; and its expression is predominantly
restricted to dendritic cells (DCs) and macrophages (MФ). However,
the expression of these molecules in numerous immune and non-immune
cells can be induced. The expression levels of PD-L2 have been
shown to vary between diverse types of tumor. The interaction of
PD-1 with PD-L1 has been demonstrated to occur in peripheral T
cells in order to inhibit antigen sensitization; therefore,
protecting normal tissues against immune injury. In addition,
upregulation of PD-L2 has been reported to be stimulated by T
helper cell (Th)-2 cytokines, and is itself involved in the
adjustment of Th2 responses (6).
B7-H4 mRNA has previously been demonstrated to be
abundant in human tissue, although its protein expression levels
were relatively low (10). In
addition, B7-H4 has been detected in various types of human tumor
(10). The soluble B7-H4 protein can
be detected in the sera of patients with tumors, and is able to
inhibit the activation, proliferation, and clonal expansion of
CD4+ and CD8+ T cells (11).
In the present study, the expression levels of
indoleamine 2,3-dioxygenase (IDO), PD-L1, PD-L2, and B7-H4 in
endometrial tumors were detected by immunohistochemical methods.
IDO is a key enzyme for the regulation of adaptive immune
responses; galectin-1 and −3 are involved in cancer and
inflammation (12). In addition, the
expression levels of galectin-1 and galectin-3 were analyzed in a
uterine specimen via ELISA, in order to elucidate the
immunosuppressive mechanisms of endometrial cancer, and to direct
the use of immunosuppressive inhibitors for the treatment of
endometrial carcinoma.
Materials and methods
Specimens
All specimens were collected in the Second
Affiliated Hospital of Zhengzhou University (Zhengzhou, China). A
total of 72 cases of each tumor were collected by surgical
operation, from patients aged 39–74 years old, with an average age
of 54.52±6.47 years. There were 42 cases of primary endometrial
carcinoma, 17 cases of recurrent endometrial carcinoma and 13 cases
of metastatic endometrial carcinoma. In addition, 21 samples of
normal endometrial tissue which was confirmed as benign were
collected. The present study was conducted in accordance with the
declaration of Helsinki, and with approval from the Ethics
Committee of the Second Affiliated Hospital of Zhengzhou
University. Written informed consent was obtained from all
participants.
Immunohistochemical staining
For all of the specimens that required staining, the
tissue was sliced to 4.0 µm, formalin-fixed and embedded on a glass
slide. Prior to staining, the sections were dewaxed and re-hydrated
using an ethanol gradient as follows: 90% ethanol, 75% ethanol, 50%
ethanol and 25% ethanol, successively. The staining procedure was
similar. Following heating of the sections at 42°C for antigen
retrieval, 0.2 M HCl or 0.5% H2O2 dissolved
in methanol was added to the specimens to inhibit endogenous
alkaline phosphatase and peroxidase activities. Subsequently, the
appropriate antibodies were added. Tissue sections were visualized
using an AX80 microscope (Olympus Corporation, Tokyo, Japan).
Scoring system
Scoring of each tissue section was preceded by a
routine dying method, in order to authenticate the tumor tissue.
The tissue sections were separated and scored based on a scoring
system outlined in Ino et al (13). Initially, the tissue sections were
allocated into one of three categories (1–25, 25–50 or >50%),
given the scores 1, 2 and 3, respectively. These categories
indicated the total number of tumor cells in the sample expressing
the factor of interest. Subsequently, the tissue sections were
scored according to the strength of the expression: 1, weak; 2,
moderate; and 3, strong. The final value for each tissue section
was dependent on the sum of the two scores: 0–1=0; 2–3=1; 4–5=2 and
6=3. Based on these scores, high and low levels of expression were
distinguished. According to De Jong et al (14), the specimen with a total score of 0–1
is considered a low score, whereas that with a total score of 2–3
is considered a high score. The score of the staining result was
depended on each molecule alone.
Preparation of the lysate
Frozen tissue specimens (150–200 mg) were cut and
decomposed with lysis buffer (250 mm Tris-HCl, 750 mm NaCl, 0.5%
SDS, 2.5% cholic acid, 5% Igepal and 0.01% protease inhibitors;
Sigma-Aldrich, St. Louis, MO, USA). Following incubation at 4°C for
30 min, the lysate underwent centrifugal purification at 15,000 × g
at 4°C for 15 min. The concentration of protein from each specimen
was determined using a Bicinchoninic Acid protein assay.
ELISA
Sheep anti-human galectin-1 [2 µg/ml in
phosphate-buffered saline (PBS); AF1152; R&D Systems, Inc.,
Minneapolis, MN, USA] was used. The tissue specimens were blocked
with PBS solution containing 1% bovine serum albumin (Takara
Biotechnology Co., Ltd., Dalian, China) at room temperature for 1
h, after which the standard protein (recombinant galectin-1; range,
25–0.39 ng/ml; Wuhan Boster Biological Technology, Ltd., Wuhan,
China) was added at room temperature for 2 h. The concentration of
lysate for analysis was 5 mg/ml. Sheep anti-human immunoglobulin G
(200 ng/ml; 110-AG; R&D Systems Inc.), and the horseradish
peroxidase-binding chain enzyme avidin, were used for detection.
Tetramethyl benzidine substrate was added for coloration, and 2 M
H2SO4 was used to terminate the reaction. The
OD value was detected at 450 nm using an ELX80 microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA). Galectin-3 was
detected using a commercial ELISA kit (R&D Systems, Inc.) with
only minor changes, and mouse anti-human galectin-3 (2 µg/ml;
AF1154; R&D Systems, Inc.) was used to detect the expression
levels. The standard concentration of galectin-3 was 62.5–4,000
pg/ml. The detection method was similar to that used for
galectin-1.
Statistical analysis
All data were processed using GraphPad Prism 5
software (GraphPad Software, Inc., La Jolla, LA, USA). All scoring
data were analyzed by the non parametric Kruskal-Wallis test,
followed by Dunn's multiple comparison test. The difference was
calculated by Fisher test. Multiple groups were compared with
two-way analysis of variance or related sample t-test. The survival
curves were compared with log-rank (Mantel-Cox) test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of IDO in endometrial
carcinoma
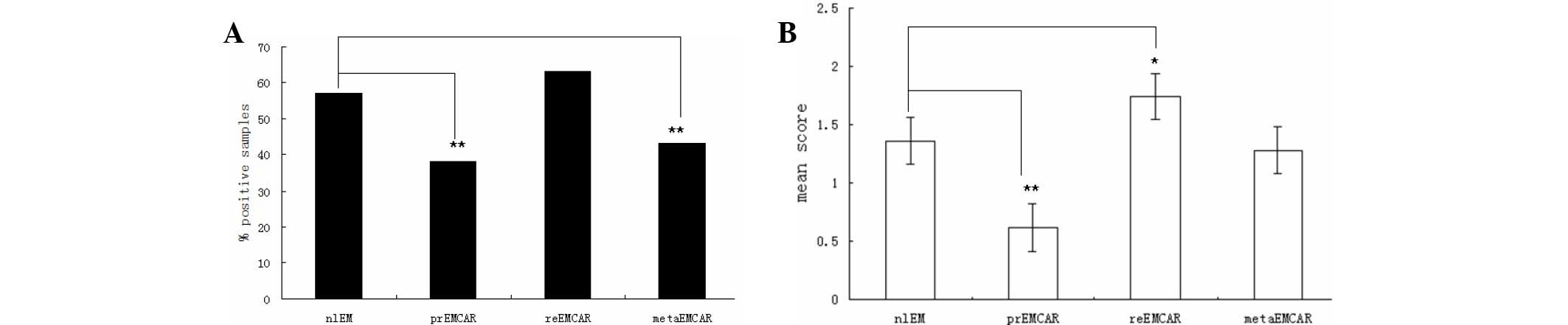
Of the normal endometrial samples, 57% tested
positive for the expression of IDO (Table I and Fig.
1). In the tumor specimens, IDO was expressed in only 38% of
primary endometrial carcinoma samples, 63% of recurrent endometrial
carcinoma specimens, and 43% of metastatic endometrial carcinoma
samples. Therefore, the percentage of tumor samples that tested
positive for IDO expression was significantly lower (P<0.01), as
compared with the normal endometrium samples. IDO was predominantly
expressed in the cytoplasm, however some tumors exhibited apical
expression. In addition, the infiltrating cells expressing IDO were
in close proximity to the tumor vessels. IDO was highly expressed
in 21% of primary endometrial cancers, indicating that blocking the
expression of IDO may be a useful strategy in the treatment of
primary endometrial carcinoma.
 | Table I.The number and positive rate of
immune checkpoint molecule and indoleamine 2,3-dioxygenase (IDO)
expression in various sample types. |
Table I.
The number and positive rate of
immune checkpoint molecule and indoleamine 2,3-dioxygenase (IDO)
expression in various sample types.
|
| IDO | PD-L1 | PD-L2 | B7-H4 |
|---|
|
|
|
|
|
|
|---|
| Sample | n | % Positive | n | % Positive | n | % Positive | n | % Positive |
|---|
| nlEM | 21 | 57 | 16 | 78 | 23 | 44 | 22 | 100 |
| prEMCAR | 42 | 38 | 29 | 83 | 45 | 40 | 53 | 100 |
| recEMCAR | 17 | 63 | 9 | 68 | 7 | 78 | 17 | 100 |
| metaEMCAR | 13 | 43 | 9 | 100 | 14 | 42 | 15 | 96 |
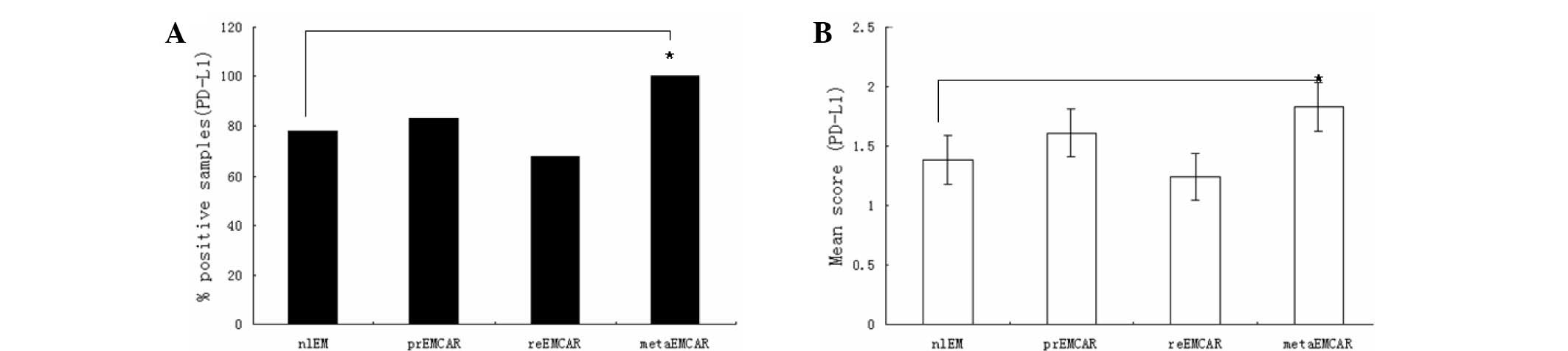
Expression of PD-L1 in endometrial
carcinoma
PD-L1 was expressed in the majority of the specimens
analyzed (Fig. 2), and was
predominantly located in the cytoplasm. PD-L1 was expressed in 78%
of normal endometrium samples, and 70–80% of tumor tissues. The
analyzed specimens and their corresponding percentages are shown in
Table I. As compared with the normal
endometrium, the expression of PD-L1 in tumor samples was
upregulated (P<0.01). The expression levels of PD-L1 across all
samples were medium to high. Of the primary endometrial carcinomas,
72% exhibited expression of PD-L1, suggesting that intervention of
the PD-1/PD-L1 axis may be a promising treatment option for
patients with endometrial cancer.
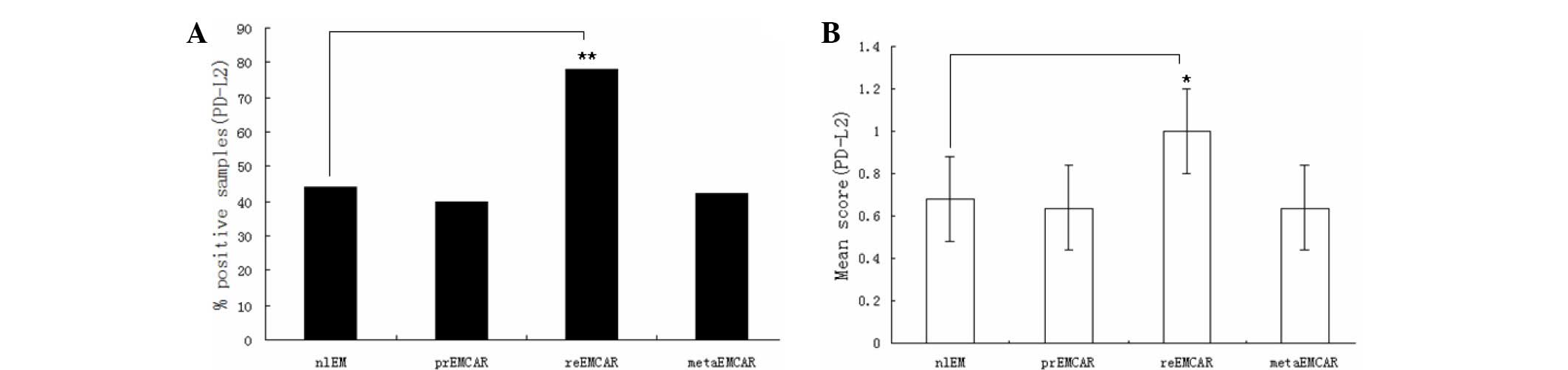
Expression of PD-L2 in endometrial
carcinoma
The expression profile of PD-L2 differed from that
of PD-L1 (Table I). PD-L2 was
expressed in 47% of normal endometrium samples. The positive
percentage of tumor samples ranged from 40–78%. All of the biopsy
specimens were scored from low to medium, and there was no
significant difference among them (Fig.
3). The present study demonstrated that PD-L2 positive cells
were adjacent to tumor cells, which may indicate immune cell
invasion. These immune cells were predominantly distributed in the
tumor, tumor stroma and peripheral tumor. Overall, PD-L2 was highly
expressed in 30% of primary endometrial carcinomas, suggesting that
blocking PD-L2 may be an effective therapeutic strategy in small
numbers of patients with endometrial cancer.
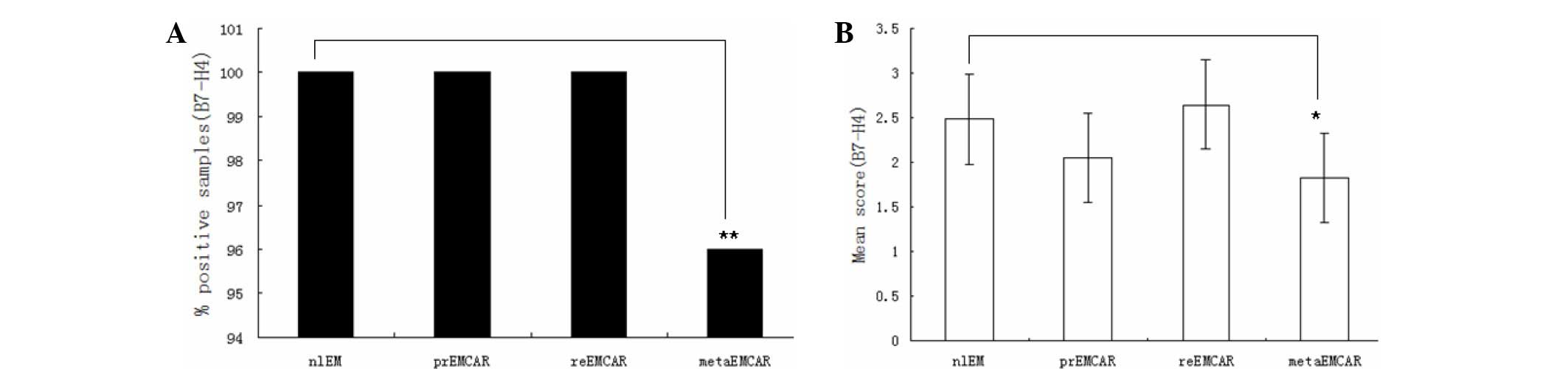
Expression of B7-H4 in endometrial
carcinoma
B7-H4 was expressed in the majority of biopsy
specimens (Table I). Statistical
analysis demonstrated that there was no significant difference in
the expression levels of B7-H4 between the normal and tumor samples
(Fig. 4). In addition, B7-H4 was
predominantly expressed in the cytoplasm. Identifying the
infiltrating immune cells was complicated by dyeing effects. The
present study demonstrated that B7-H4 was highly expressed in 90%
of primary endometrial carcinomas, suggesting that B7-H4 may be an
attractive candidate target for therapeutic drugs.
Detection of galectin-1 and galectin-3
levels
The expression levels of galectin-1 and galectin-3
were detected in tumor lysates from 29 endometrial cancer samples,
21 normal endometrium samples and 18 leiomyosarcoma samples via
ELISA. Galectin-1 was detected in all of the samples, and there was
no significant difference in the expression levels of galectin-1 or
galectin-3 between all of the groups (Table II).
 | Table II.Expression levels of galectin-1 and
galectin-3 in tumor lysates were detected by ELISA. |
Table II.
Expression levels of galectin-1 and
galectin-3 in tumor lysates were detected by ELISA.
| Items | Galectin-1
(ng/ml) | Galectin-3
(pg/ml) |
|---|
| nlEM |
8.3±1.4 |
860±53.2 |
| EMCAR |
7.2±1.3 |
1780±125 |
| nlMYOM |
12.8±2.1 |
920±86 |
Discussion
In the present study, the expression levels of
PD-L1, PD-L2, B7-H4, IDO, galectin-1 and galectin-3 were evaluated
in endometrial carcinoma samples. Previous studies have
demonstrated that the ligands of PD-1, PD-L1 and PD-L2, are
expressed in numerous types of tumor, including melanoma, brain
tumor, lung cancer, cancer of the urinary tract and pancreatic
cancer (6–9). PD-L1 is generally expressed in 65% of
soft tissue tumor cases (15).
However, to the best of our current knowledge, there are few
reports associating PD-L1 and PD-L2 with gynecological tumors. In
the present study, there were no significant differences between
the expression levels of PD-L1 and PD-L2 in normal tissues, as
compared with tumor tissues. Contrary to expectations, the results
of the present study suggested that high expression levels of PD-L1
are associated with an overall longer survival rate. However, this
phenomenon was similarly identified in previous studies of
melanoma, Merkel cellular tumor and mismatch repair-proliferated
rectal cancer (16–18). Interferon-γ and CD8+ T
cells are able to promote the upregulation of PD-L1; therefore, the
expression of PD-L1 may reflect an ongoing endogenous anti-tumor
immune response, which may represent a negative feedback loop
dependent on the invasion of the immune response (9).
Previous studies suggested that PD-L2 is exclusively
expressed in DCs and MФ (19,20);
however, it has since been demonstrated that PD-L2 is expressed in
somatic tissues and tumors, including tumor-associated fibroblasts
(21), pharyngeal tissue (22) and hepatic cell carcinoma (23). The majority of biopsy specimens in
the present study exhibited only low levels of PD-L2 expression,
although there was a high rate of expression of PD-L2 in the
recurrent endometrial carcinoma group. Previous studies have
detected only low levels of PD-L2 in the majority of tumors
(4,24), which is concordant with the results
of the present study. Furthermore, a previous study demonstrated
that PD-L2 expression was negative in the majority of ovarian
carcinomas (25). However, PD-L1 and
PD-L2 have been detected in cervical cancer, although the results
suggested that PD-L1 and PD-L2 were only expressed in a small
number of tumors (26).
B7-H4 has been demonstrated to be expressed in
various tumors, including breast and ovarian cancer (10); and previous research detected B7-H4
in ovarian tumor cells and tumor-associated MФ (27,28). In
the present study, the levels of B7-H4 were correlated with patient
prognosis. Previous studies have detected B7-H4 expression in
endometrial carcinoma (29,30). Although these studies also detected
B7-H4 expression in normal endometrium, B7-H4 staining demonstrated
that its expression increased from normal to malignant endometrial
carcinoma, which is inconsistent with the results of the present
study, in which B7-H4 was found to be highly expressed in both
benign and malignant tissue. This divergence in results may be due
to the use of different antibodies, methods and scoring
systems.
Galectin-1 and galectin-3 upregulation has
previously been demonstrated in numerous tumors, including bladder
cancer (31,32), head and neck squamous cell carcinoma
(33) and ovarian cancer (34,35).
Numerous studies have detected galectin-1 and galectin-3 expression
in endometrial carcinoma, although the results have been
conflicting (4,36). van den Brûle et al (37) reported that the expression levels of
galectin-1, but not galectin-3, were upregulated in endometrial
carcinoma, as compared with normal endometrium. Similarly,
galectin-1 expression levels have been demonstrated to be
upregulated in undifferentiated cancer, as compared with
differentiated cancer (38).
Conversely, Brustmann et al (38) reported that the expression levels of
galectin-3 were upregulated in endometrial carcinoma, as compared
with normal endometrium, whereas Ege et al (39) reported that the expression levels of
galectin-3 were decreased in endometrial carcinoma, as compared
with normal endometrium. The results of the present study suggested
that the expression levels of galectin-1 and galectin-3 were not
significantly different between normal and tumor tissues.
The levels of galectin-1 in leiomyosarcoma tumor
lysate have been shown to be significantly upregulated, as compared
with that of the myometrium, tumor and other tissue types.
Furthermore, the present study demonstrated galectin-3 expression
in the membrane and cytoplasm of primary tumors, and the single
cell suspension liquid of cervical cancer cell lines (26). The localization of galectin-3 has
been reported to alter between various cancers (39). Brustmann et al (38) demonstrated increased expression of
galectin-3 in the nucleus of endometrial carcinoma specimens,
whereas Mylonas et al (40)
reported galectin-3 expression in the cytoplasm and nucleus.
However, the expression of galectin-3 in the cytoplasm has
previously been associated with the invasion of the muscle layer
near endometrial carcinoma (37),
although this correlation was not demonstrated in the present
study. Furthermore, the results of the present study did not
demonstrate a difference in the expression levels of galectin-1 and
galectin-3 between normal endometrium and endometrial carcinoma.
This difference may be partially due to the use of divergent
methods.
In conclusion, the present study reports the
expression levels of immune checkpoint molecules PD-L1, PD-L2 and
B7-H4, and the immunosuppressive molecules galectin-1 and
galectin-3, in endometrial tumor specimens. The results of the
present study, combined with positive reports from ongoing clinical
trials (4,41), suggest that inhibitors of these
molecules may be considered as treatments for patients with
endometrial tumors. Furthermore, the present study demonstrated
that PD-L2 may be a useful target for immune suppression in a small
number of patients with endometrial carcinoma, whereas both PD-L1
and B7-H4 were highly expressed in two of the analyzed tumor types,
and therefore may be ideal candidate drug targets.
References
|
1
|
Nguyen TT, Hachisuga T, Urabe R, Kurita T,
Kagami S, Kawagoe T, Shimajiri S and Nabeshima K: Significance of
p53 expression in background endometrium in endometrial carcinoma.
Virchows Arch. 466:695–702. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Murali R, Soslow RA and Weigelt B:
Classification of endometrial carcinoma: More than two types.
Lancet Oncol. 15:e268–e278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharp ZD and Bartke A: Evidence for
down-regulation of phosphoinositide 3-kinase/Akt/mammalian target
of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory
signaling pathways in Ames dwarf mice. J Gerontol A Biol Sci Med
Sci. 60:293–300. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vanderstraeten A, Luyten C, Verbist G,
Tuyaerts S and Amant F: Mapping the immunosuppressive environment
in uterine tumors: Implications for immunotherapy. Cancer Immunol
Immunother. 63:545–557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kübler K, Ayub TH, Weber SK, Zivanovic O,
Abramian A, Keyver-Paik MD, Mallmann MR, Kaiser C, Serçe NB, Kuhn W
and Rudlowshi C: Prognostic significance of tumor-associated
macrophages in endometrial adenocarcinoma. Gynecol Oncol.
135:176–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Topalian SL, Drake CG and Pardoll DM:
Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor
immunity. Curr Opin Immunol. 24:207–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He C, Qiao H, Jiang H and Sun X: The
inhibitory role of b7-h4 in antitumor immunity: Association with
cancer progression and survival. Clin Dev Immunol. 2011:6958342011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yi KH and Chen L: Fine tuning the immune
response through B7-H3 and B7-H4. Immunol Rev. 229:145–151. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Terness P, Kallikourdis M, Betz AG,
Rabinovich GA, Saito S and Clark DA: Tolerance signaling molecules
and pregnancy: IDO, galectins, and the renaissance of regulatory T
cells. Am J Reprod Immunol. 58:238–254. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ino K, Yoshida N, Kajiyama H, Shibata K,
Yamamoto E, Kidokoro K, Takahashi N, Terauchi M, Nawa A, Nomura S,
et al: Indoleamine 2,3-dioxygenase is a novel prognostic indicator
for endometrial cancer. Br J Cancer. 95:1555–1561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Jong RA, Kema IP, Boerma A, Boezen HM,
van der Want JJ, Gooden MJ, Hollema H and Nijman HW: Prognostic
role of indoleamine 2,3-dioxygenase in endometrial carcinoma.
Gynecol Oncol. 126:474–480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S,
Kim KM, Park HS, Lee H, Moon WS, Chung MJ, et al: Tumor
infiltrating PD1-positive lymphocytes and the expression of PD-L1
predict poor prognosis of soft tissue sarcomas. PLoS One.
8:e828702013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Taube JM, Anders RA, Young GD, Xu H,
Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL
and Chen L: Colocalization of inflammatory response with B7-h1
expression in human melanocytic lesions supports an adaptive
resistance mechanism of immune escape. Sci Transl Med.
4:127ra372012.PubMed/NCBI
|
|
17
|
Droeser RA, Hirt C, Viehl CT, Frey DM,
Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A,
Rosso R, et al: Clinical impact of programmed cell death ligand 1
expression in colorectal cancer. Eur J Cancer. 49:2233–2242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lipson EJ, Vincent JG, Loyo M, Kagohara
LT, Luber BS, Wang H, Xu H, Nayar SK, Wang TS, Sidransky D, et al:
PD-L1 expression in the Merkel cell carcinoma microenvironment:
Association with inflammation, Merkel cell polyomavirus and overall
survival. Cancer Immunol Res. 1:54–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Y, Zhao Y, Ran X and Wang C:
Increased expression of herpesvirus entry mediator in
1,25-dihydroxyvitamin D3-treated mouse bone marrow-derived
dendritic cells promotes the generation of
CD4+CD25+Foxp3+regulatory T cells. Mol Med Rep. 9:813–818.
2014.PubMed/NCBI
|
|
20
|
Togno-Peirce C, Nava-Castro K, Terrazas LI
and Morales-Montor J: Sex-associated expression of co-stimulatory
molecules CD80, CD86, and accessory molecules, PDL-1, PDL-2 and
MHC-II, in F480+ macrophages during murine cysticercosis. Biomed
Res Int. 2013:5701582013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rozali EN, Hato SV, Robinson BW, Lake RA
and Lesterhuis WJ: Programmed death ligand 2 in cancer-induced
immune suppression. Clin Dev Immunol. 2012:6563402012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohigashi Y, Sho M, Yamada Y, Tsurui Y,
Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, et al:
Clinical significance of programmed death-1 ligand-1 and programmed
death-1 ligand-2 expression in human esophageal cancer. Clin Cancer
Res. 11:2947–2953. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M,
Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, et al: Overexpression
of PD-L1 significantly associates with tumor aggressiveness and
postoperative recurrence in human hepatocellular carcinoma. Clin
Cancer Res. 15:971–979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kojima M, Murata S, Mekata E, Takebayashi
K, Jaffee EM and Tani T: Fusion protein of mutant B7-DC and Fc
enhances the antitumor immune effect of GM-CSF-secreting whole-cell
vaccine. J Immunother. 37:147–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hamanishi J, Mandai M, Iwasaki M, Okazaki
T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N,
et al: Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+
T lymphocytes are prognostic factors of human ovarian cancer. Proc
Natl Acad Sci USA. 104:3360–3365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Karim R, Jordanova ES, Piersma SJ, Kenter
GG, Chen L, Boer JM, Melief CJ and van der Burg SH: Tumor-expressed
B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and
survival of patients with cervical carcinoma. Clin Cancer Res.
15:6341–6347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng L, Jiang J, Gao R, Wei S, Nan F, Li
S and Kong B: B7-H4 expression promotes tumorigenesis in ovarian
cancer. Int J Gynecol Cancer. 19:1481–1486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen C, Qu QX, Shen Y, Mu CY, Zhu YB,
Zhang XG and Huang JA: Induced expression of B7-H4 on the surface
of lung cancer cell by the tumor-associated macrophages: A
potential mechanism of immune escape. Cancer Lett. 317:99–105.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyatake T, Tringler B, Liu W, Liu SH,
Papkoff J, Enomoto T, Torkko KC, Dehn DL, Swisher A and Shroyer KR:
B7-H4 (DD-O110) is overexpressed in high risk uterine endometrioid
adenocarcinomas and inversely correlated with tumor T-cell
infiltration. Gynecol Oncol. 106:119–127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qian Y, Shen L, Cheng L, Wu Z and Yao H:
B7-H4 expression in various tumors determined using a novel
developed monoclonal antibody. Clin Exp Med. 11:163–170. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cindolo L, Benvenuto G, Salvatore P, Pero
R, Salvatore G, Mirone V, Prezioso D, Altieri V, Bruni CB and
Chiariotti L: Galectin-1 and galectin-3 expression in human bladder
transitional-cell carcinomas. Int J Cancer. 84:39–43. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Demydenko D and Berest I: Expression of
galectin-1 in malignant tumors. Exp Oncol. 31:74–79.
2009.PubMed/NCBI
|
|
33
|
Saussez S, Lorfevre F, Lequeux T, Laurent
G, Chantrain G, Vertongen F, Toubeau G, Decaestecker C and Kiss R:
The determination of the levels of circulating galectin-1 and −3 in
HNSCC patients could be used to monitor tumor progression and/or
responses to therapy. Oral Oncol. 44:86–93. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim MK, Sung CO, Do IG, Jeon HK, Song TJ,
Park HS, Lee YY, Kim BG, Lee JW and Bae DS: Overexpression of
Galectin-3 and its clinical significance in ovarian carcinoma. Int
J Clin Oncol. 16:352–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van den Brûle F, Califice S, Garnier F,
Fernandez PL, Berchuck A and Castronovo V: Galectin-1 accumulation
in the ovary carcinoma peritumoral stroma is induced by ovary
carcinoma cells and affects both cancer cell proliferation and
adhesion to laminin-1 and fibronectin. Lab Invest. 83:377–386.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stewart CJ and Crook ML: Galectin-3
expression in uterine endometrioid adenocarcinoma: comparison of
staining in conventional tumor glands and in areas of MELF pattern
myometrial invasion. Int J Gynecol Pathol. 29:555–561. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van den Brule FA, Buicu C, Berchuck A,
Bast RC, Deprez M, Liu FT, Cooper DN, Pieters C, Sobel ME and
Castronovo V: Expression of the 67-kD laminin receptor, galectin-1,
and galectin-3 in advanced human uterine adenocarcinoma. Hum
Pathol. 27:1185–1191. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brustmann H, Riss D and Naudé S:
Galectin-3 expression in normal, hyperplastic, and neoplastic
endometrial tissues. Pathol Res Pract. 199:151–158. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ege CB, Akbulut M, Zekioğlu O and Ozdemir
N: Investigation of galectin-3 and heparanase in endometrioid and
serous carcinomas of the endometrium and correlation with known
predictors of survival. Arch Gynecol Obstet. 284:1231–1239. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mylonas I, Mayr D, Walzel H, Shabani N,
Dian D, Kuhn C, Kunze S, Jeschke U and Friese K: Mucin 1,
Thomsen-Friedenreich expression and galectin-1 binding in
endometrioid adenocarcinoma: An immunohistochemical analysis.
Anticancer Res. 27:1975–1980. 2007.PubMed/NCBI
|
|
41
|
Howitt BE, Shukla SA, Sholl LM,
Ritterhouse LL, Watkins JC, Rodig S, Stover E, Strickland KC,
D'Andrea AD, Wu CJ, et al: Association of polymerase e-mutated and
microsatellite-instable endometrial cancers with neoantigen load,
number of tumor-Infiltrating lymphocytes, and expression of PD-1
and PD-L1. JAMA Oncol. Jul 9–2015.((Epub ahead of print)).
PubMed/NCBI
|