Introduction
Gastric cancer is common condition world wide,
although with an incidence rate lower than those of lung, breast
and colorectal cancer (1). Symptoms
of gastric cancer include anemia, weigh loss, appetite loss, easy
fatigability and non-specific symptoms such as abdominal pain
(2). Treatment options for gastric
cancer include endoscopic treatment, surgery, chemotherapy and
radiation (1,3,4). Key
types of endoscopic treatment include endoscopic mucosal resection
(EMR) and endoscopic submucosal dissection (ESD) (5,6). Despite
improvements in treatment efficacy, prognoses for gastric cancer
remain poor (7). A potential reason
for this may be that patients are diagnosed on the basis of
advanced symptoms of late stage gastric cancer, which limits their
treatment options. Therefore, effective screening is essential for
the improvement of outcome of patients with early stage gastric
cancer that exhibits relatively few symptoms (8). Currently, radiographic and endoscopic
screening methods are in use (9).
Endoscopy is the gold standard of diagnosis of
gastric cancer (10). However,
endoscopy is not always performed for screening in countries with a
relatively low number of the patients, and endoscopic resources may
be limited (8). Furthermore,
endoscopy is not always performed for patients with abdominal
symptoms, as these patients are often subjected to transabdominal
ultrasonography (US). US is useful for the diagnosis of diseases in
solid organs, including the liver, biliary system and pancreas
(11). US may be performed for
patients with abdominal pain and diagnose diseases of alimentary
tracts (12). US often reveals
gastrointestinal diseases presenting to the hospital with an acute
abdomen (13,14). In addition, gastrointestinal cancer
is occasionally detected using US (15). Gastric cancer may be incidentally
diagnosed in patients with nonspecific symptoms that undergo US
screening (16).
In the present study, we retrospectively analyzed
the records of patients that were diagnosed with gastric cancer,
and their specimens were available due to surgery or endoscopical
treatment. A variety of factors were investigated that were
associated with the detection of gastric cancer using US, including
the limitations in using US to diagnose gastric cancer.
Materials and methods
Patients
The records of patients admitted to the National
Hospital Organization Shimoshizu Hospital (Yotsukaido, Japan)
between November 2011 and July 2014 were retrospectively analyzed.
Patients included in this study had undergone surgery, EMR or ESD
subsequent to the diagnosis of gastric cancer on the basis of
biopsy specimens, and were subjected to US prior to endoscopy, in
order to diagnose the patient. A total of 13 patients met the
inclusion criteria and were enrolled into this study, including 8
male (mean age ± standard deviation, 69.3±3.8 years) and 5 female
patients (71.4±13.9 years). Patients were divided into the
following two groups: Negative detection group, consisting of
patients in which gastric cancer was not detected using US; and
positive detection group, consisting of patients in which gastric
cancer was detected using US.
This study was subjected to review by the Ethical
Committee of the National Hospital Organization Shimoshizu
Hospital, and was not considered as a clinical trial, since it was
performed as a part of routine clinical practice. The present study
was retrospective and patient anonymity was preserved, thus
informed consent was not required.
Transabdominal US
US was performed using the SSA-700A ultrasound
system (Toshiba Medical Systems Corporation, Ohtawara, Japan) with
a 3.75-MHz curved-array probe (PVT-375BT) or an 8.0-MHz
linear-array probe (PLT-805AT) in the US unit. US was performed by
board-certified fellows of the Japan Society of Ultrasonics in
Medicine (Tokyo, Japan) (http://www.jsum.or.jp/jsum-e/index.html). Gastric
cancer was diagnosed upon detection of irregular wall thickness as
compared with the surrounding lesions, or when loss of
stratification was observed (15).
Pathological analysis
The tumor diameter and depth of invasion were
determined by the pathologists using specimens. Specimens were
obtained through surgery, EMR or ESD, which were performed in our
hospital. Pathological T (pT) staging of the specimens obtained via
surgery, EMR or ESD, was performed by the pathologists, based on
the classification described by the American Joint Committee on
Cancer (7th edition) (17), as
follows: pT1a, lamina propria or muscularis mucosae; pT1b,
submucosa; pT2, muscularis propria; pT3, subserosal connective
tissue without invasion of visceral peritoneum or adjacent
structures; and pT4, seroa (visceral peritoneum) or adjacent
structures. The staging was decided on consensus between two
pathologists.
Statistical analysis
JMP software, version 10.0.2 (SAS Institute, Cary,
NC, USA) was used for statistical analysis. One-way analysis of
variance was applied to variables of patient characteristics and
tumor diameter between the negative and positive detection groups.
The χ2 test was used to determine the association of
depth of invasion and T staging between the negative and positive
detection groups. A P-value of <0.05 was considered to indicate
a statistically significant difference.
Results
US findings
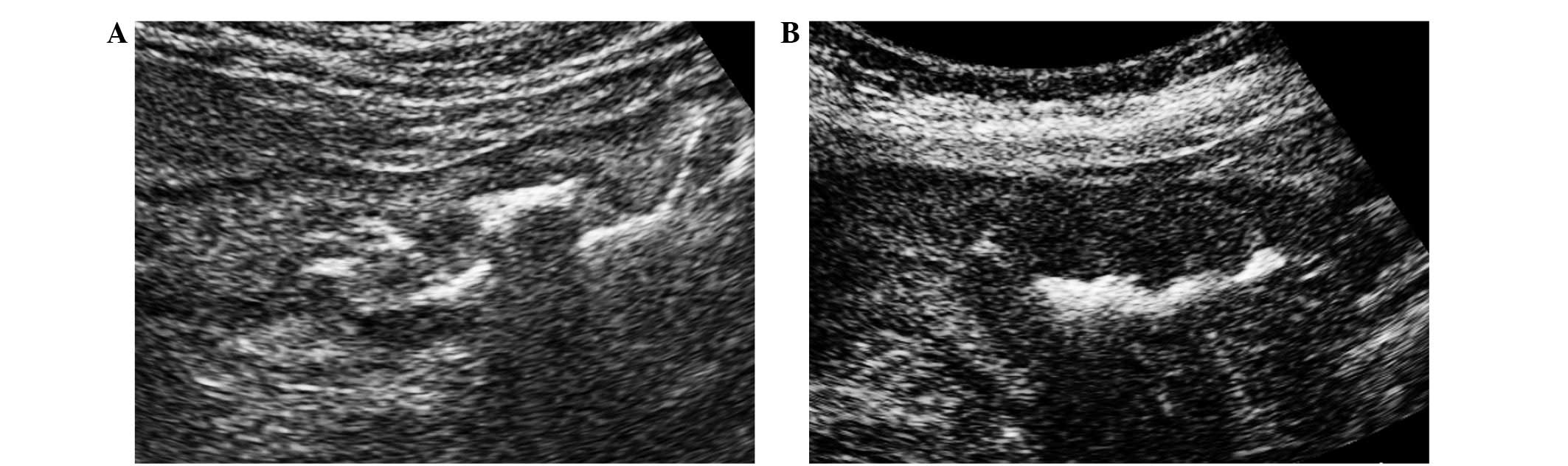
On US images, gastric cancer tumors were detected as
thickening of the gastric wall (Fig. 1A
and B) (8). Gastric wall
thickening may be observed as a symptom of gastric ulcer (18); however, irregularity of the edge of
the thickened wall is a hallmark of gastric cancer (8). All of the present patients with gastric
wall thickening were diagnosed with gastric cancer using upper
gastrointestinal endoscopy. In certain patients, depression of the
center of the gastric wall thickening was clearly demonstrated
(Fig. 1B). A total of 5 patients
were diagnosed with gastric cancer using US, whereas US did not
detect evidence of cancer in 8 others.
Patient characteristics
Patient characteristics are presented in Table I. The hemoglobin level was
significantly lower in the positive detection patients compared
with that in the negative detection patients (P=0.0455). This was
probably due to tumor bleeding (19). No statistically significant
differences in the other parameters were detected between the two
groups. Gastric wall thickness was 3.7+1.0 mm in negative detection
and 12.2±5.9 mm in positive detection (P<0.01). Larsen et
al reported that gastric wall thickness in normal healthy
subjects is 3.27±0.42 mm (20). It
was clear that the gastric wall was thicker in the positive
detection patients compared with the normal subjects.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Normal range | Negative
detectiona | Positive
detectiona | P-value |
|---|
| Patients (n) | – | 8 | 5 | – |
| Gender
(male:female) | – | 6:2 | 2:3 | – |
| Mean age (years) | – | 72.8±5.3 |
65.8±11.6 | 0.1654 |
| Age range
(years) | – |
67–81 |
47–76 | – |
| WBC (per µl) | 3500–8500 | 7,366±2647 |
7380±2464 | 0.9904 |
| CRP (mg/dl) | <0.3 |
1.08±1.70 |
2.18±2.75 | 0.3053 |
| Hb (g/dl) | 13.5–17.0 | 13.8±2.5 | 10.9±3.6 | 0.0455 |
| T-Bil (mg/dl) | 0.30–1.20 |
0.73±0.52 |
0.60±0.32 | 0.5360 |
| ALP (IU/l) | 115–359 | 242±75 | 246±64 | 0.9072 |
| AST (IU/l) | 13–33 | 23.3±8.3 |
25.9±15.6 | 0.6271 |
| ALT (IU/l) | 8–42 |
20.8±12.2 |
22.2±19.8 | 0.8447 |
| g-GTP (IU/l) | 10–47 |
36.8±22.0 |
59.7±63.1 | 0.2608 |
| CEA (ng/ml) | <5.0 |
148±435 |
180±437 | 0.8824 |
| CA19-9 (U/ml) | <37 |
16.6±15.1 |
1541±4266 | 0.2985 |
| Wall thickness, mean
(mm) | – |
3.7±1.0 | 12.2±5.9 | 0.0008 |
| Wall thickness, range
(mm) | – | 2–5 | 7–20 | – |
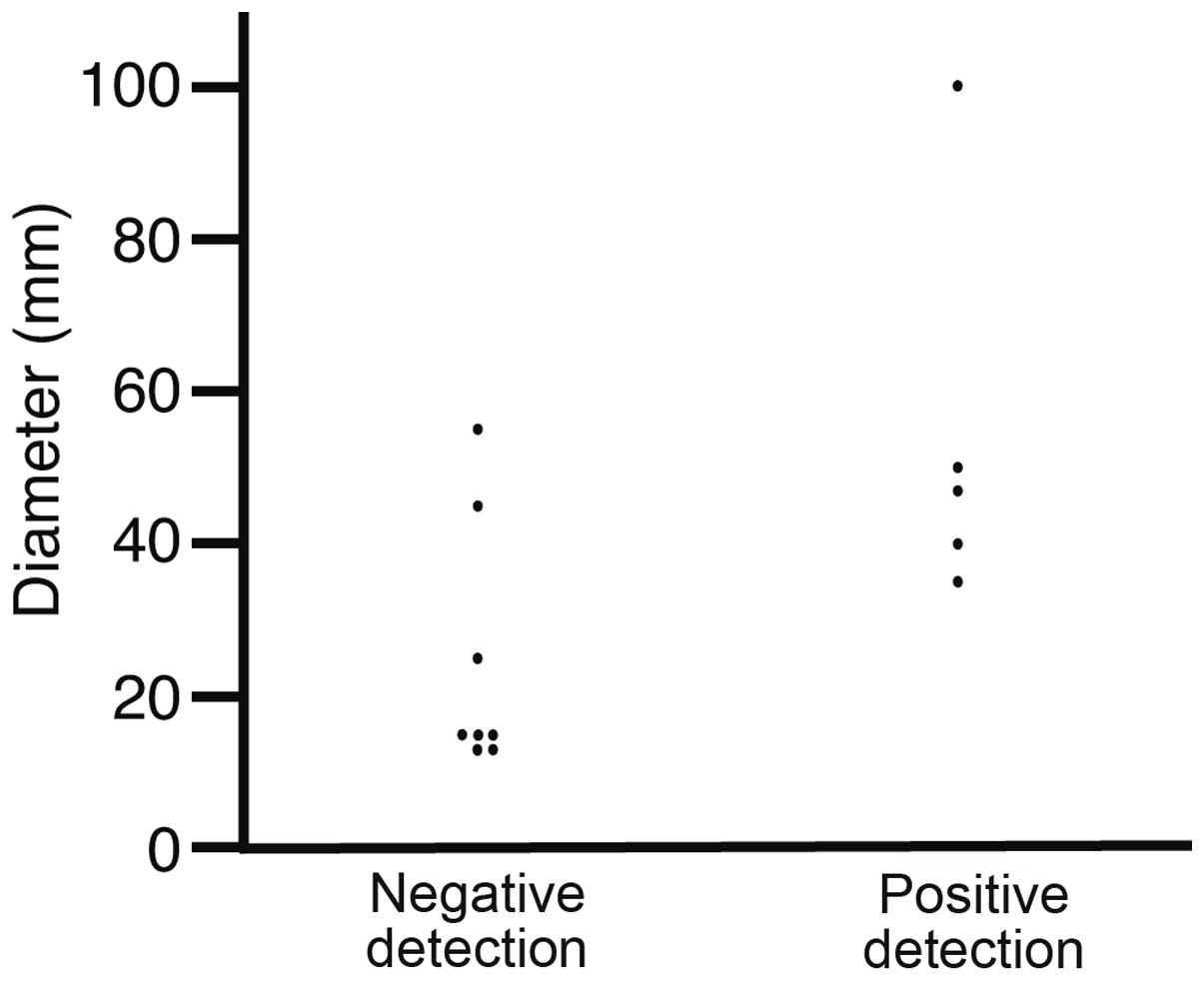
Tumor diameter
The tumor diameters were analyzed in the specimens
obtained via surgery, EMR or ESD (Fig.
2). The diameters of the negative and positive detection
patients were 24.5±16.4 and 54.4±26.2 mm, respectively (P=0.0266).
No gastric cancer tumors <30 mm were detected, indicating that
US detected gastric cancer tumors >30 mm.
Correlation of gastric cancer
detection with pT staging and depth of invasion
The effect of pT staging and depth of invasion on
the detection of gastric cancer using US was also analyzed
(Table II). Diagnosis was
successful using US for gastric cancer tumors above stage pT2
(P=0.0242). By contrast, stage pT1 gastric cancer tumors remained
undetected. Tumors invading deeper than the submucosa were also
diagnosed using US (P=0.0242), whereas cases of gastric cancer
limited to the mucosa remained undetected.
 | Table II.Correlation of gastric cancer
detection using ultrasonography with depth of invasion or
pathological T staging. |
Table II.
Correlation of gastric cancer
detection using ultrasonography with depth of invasion or
pathological T staging.
|
| pT staging
(P=0.0242) | Invasion depth
(P=0.0242) |
|
|---|
|
|
|
|
|
|---|
| Group | >pT2 | pT1a | >SM | M | Total |
|---|
| Positive
detectiona | 5 | 0 | 5 | 0 | 5 |
| Negative
detectiona | 3 | 5 | 3 | 5 | 8 |
| Total | 8 | 5 | 8 | 5 | 13 |
Discussion
Gastric cancer can be detected during US screening
(15) and such tumors are diagnosed
upon observation of a thickened gastric wall, destruction of the
wall structure (loss of stratification) and, occasionally, a
hypoechoic mass (16). If patients
drink water prior to undergoing a US scan, the gastric wall is
visualized as a five-layered structure (21). Loss of stratification indicates
destruction of the normal structure of the gastric wall. The
presence of gastric cancer should be considered when a wall
thickness of >10 mm is observed (22). In the present study, wall thickness
ranged between 7 and 20 mm (mean, 12.2±5.9 mm). Certain patients
were diagnosed with gastric cancer when a wall thickness of <10
mm was detected, which was due to the presence of irregular-shaped
wall thickness or loss of stratification compared with the
surrounding tissues.
In the present study, tumor diameters were larger in
cases of gastric cancer detected using US compared with cases in
which cancer was not detectable using US. In addition, the
hemoglobin level was lower in gastric cancer cases detected using
US compared with the negative detection patients, possibly due to
tumor bleeding (19). These results
indicated that gastric cancers that were detected using US were at
a more advanced stage compared with those that were not detectable
using US. The advancement of gastric cancer is represented with T
staging (23), which can be
evaluated using transabdominal US with patients drinking water
prior to the scan, or using endoscopic US (24,25). In
the current study, it was difficult to evaluate pT staging using US
as the patients did not consume water prior to screening, and thus
pT staging was evaluated subsequent to surgical resection. The
results clearly indicated that cases in which gastric cancer was
detected using US were at a more advanced stage of the disease
compared with those in which gastric cancer was not detectable
using US, and no pT1a stage gastric cancer cases were detected
using US. In addition, T staging is determined on the basis of the
depth of invasion; thus, a pathological analysis of the correlation
between the detection of gastric cancer and the depth of invasion
was conducted. Gastric cancer that was detected using US invaded
deeper than the submucosa. However, none of the gastric cancer
cases limited to the mucosa were detectable using US.
In conclusion, the present study demonstrated that
the detection of gastric cancer using US correlated with the tumor
diameter, pT staging and depth of invasion, and that US was able to
detect advanced gastric cancer. In future studies, more patients
should be enrolled, and loss of stratification should be
investigated with color Doppler imaging and contrast enhancement
(26,27).
References
|
1
|
Marrelli D, Polom K, de Manzoni G,
Morgagni P, Baiocchi GL and Roviello F: Multimodal treatment of
gastric cancer in the west: Where are we going? World J
Gastroenterol. 21:7954–7969. 2015.PubMed/NCBI
|
|
2
|
Takahashi T, Saikawa Y and Kitagawa Y:
Gastric cancer: Current status of diagnosis and treatment. Cancers
(Basel). 5:48–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamamoto M, Rashid OM and Wong J: Surgical
management of gastric cancer: The East vs. West perspective. J
Gastrointest Oncol. 6:79–88. 2015.PubMed/NCBI
|
|
4
|
Papadimitriou K, Antoniou G, Rolfo C,
Russo A, Bronte G, Vassiliou V, Papamichael D, Peeters M and
Kountourakis P: Adjuvant chemoradiation therapy in gastric cancer:
Critically reviewing the past and visualizing the next step
forward. Gastroenterol Res Pract. 2015:6508462015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peng LJ, Tian SN, Lu L, Chen H, Ouyang YY
and Wu YJ: Outcome of endoscopic submucosal dissection for early
gastric cancer of conventional and expanded indications: Systematic
review and meta-analysis. J Dig Dis. 16:67–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Facciorusso A, Antonino M, Di Maso M and
Muscatiello N: Endoscopic submucosal dissection vs endoscopic
mucosal resection for early gastric cancer: A meta-analysis. World
J Gastrointest Endosc. 6:555–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosa F, Alfieri S, Tortorelli AP, Fiorillo
C, Costamagna G and Doglietto GB: Trends in clinical features,
postoperative outcomes, and long-term survival for gastric cancer:
A Western experience with 1,278 patients over 30 years. World J
Surg Oncol. 12:2172014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi IJ: Endoscopic gastric cancer
screening and surveillance in high-risk groups. Clin Endosc.
47:497–503. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi KS and Suh M: Screening for gastric
cancer: the usefulness of endoscopy. Clin Endosc. 47:490–496. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moon HS: Improving the endoscopic
detection rate in patients with early gastric cancer. Clin Endosc.
48:291–296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beggs AD and Thomas PR: Point of use
ultrasound by general surgeons: review of the literature and
suggestions for future practice. Int J Surg. 11:12–17. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goto R, Arai K, Kitada H, Ogoshi K and
Hamashima C: Labor resource use for endoscopic gastric cancer
screening in Japanese primary care settings: A work sampling study.
PLoS One. 9:e881132014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Puylaert JB: Ultrasound of acute GI tract
conditions. Eur Radiol. 11:1867–1877. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lameris W, van Randen A, Dijkgraaf MG,
Bossuyt PM, Stoker J and Boermeester MA: Optimization of diagnostic
imaging use in patients with acute abdominal pain (OPTIMA): Design
and rationale. BMC Emerg Med. 7:92007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomizawa M, Shinozaki F, Hasegawa R, Fugo
K, Shirai Y, Ichiki N, Sugiyama T, Yamamoto S, Sueishi M and
Yoshida T: Screening ultrasonography is useful for the diagnosis of
gastric and colorectal cancer. Hepatogastroenterology. 60:517–521.
2013.PubMed/NCBI
|
|
16
|
O'Malley ME and Wilson SR: US of
gastrointestinal tract abnormalities with CT correlation.
Radiographics. 23:59–72. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
American Joint Committee on Cancer (AJCC):
AJCC Cancer Staging Handbook. 7th. Springer-Verlag; New York, USA:
2010
|
|
18
|
Allen BC, Tirman P, Tobben JP, Evans JA
and Leyendecker JR: Gastroduodenal ulcers on CT: Forgotten, but not
gone. Abdom Imaging. 40:19–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomizawa M, Shinozaki F, Hasegawa R,
Togawa A, Shirai Y, Ichiki N, Motoyoshi Y, Sugiyama T, Yamamoto S
and Sueishi M: Reduced hemoglobin and increased C-reactive protein
are associated with upper gastrointestinal bleeding. World J
Gastroenterol. 20:1311–1317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Larsen MC, Yan BM, Morton J and Van Dam J:
Determination of the relationship between gastric wall thickness
and body mass index with endoscopic ultrasound. Obes Surg.
21:300–304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kimmey MB, Martin RW, Haggitt RC, Wang KY,
Franklin DW and Silverstein FE: Histologic correlates of
gastrointestinal ultrasound images. Gastroenterology. 96:433–441.
1989.PubMed/NCBI
|
|
22
|
Martínez-Ares D, Alonso-Aguirre PA,
Souto-Ruzo J, Martín-Granizo-Barrenechea I, Pereira-Bueno S,
Cid-Gómez L and Rodríguez-Prada JI: Ultrasonography is an accurate
technique for the diagnosis of gastrointestinal tumors in patients
without localizing symptoms. Rev Esp Enferm Dig. 101:773–786. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liao SR, Dai Y, Huo L, Yan K, Zhang L,
Zhang H, Gao W and Chen MH: Transabdominal ultrasonography in
preoperative staging of gastric cancer. World J Gastroenterol.
10:3399–3404. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jürgensen C, Brand J, Nothnagel M, Arit A,
Neser F, Habeck JO, Schreiber S, Stölzel U, Zeitz M and Hampe J:
Prognostic relevance of gastric cancer staging by endoscopic
ultrasound. Surg Endosc. 27:1124–1129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei F, Huang P, Li S, Chen J, Zhang Y,
Hong Y, Wei S and Cosgrove D: Enhancement patterns of gastric
carcinoma on contrast-enhanced ultrasonography: Relationship with
clinicopathological features. PLoS One. 8:e730502013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen CN, Lin JJ, Lee H, Cheng YM, Chang
KJ, Hsieh FJ, Lai HS, Chang CC and Lee PH: Association between
color doppler vascularity index, angiogenesis-related molecules,
and clinical outcomes in gastric cancer. J Surg Oncol. 99:402–408.
2009. View Article : Google Scholar : PubMed/NCBI
|