Introduction
Pulmonary hypertension (PH) is defined as an
increase in pulmonary artery resistance and vascular remodeling,
which can result in right ventricular overload and heart failure.
Although the clinical usage of targeted drugs, such as Sildenafil
and bosentan, may partly ameliorate the symptoms of PH, the
long-term outcomes remain poor. Therefore, a more effective
medicine or therapeutic protocol is required. Previous studies have
indicated that inflammation participates in the pathological
mechanism of PH, and thus may be a promising therapeutic target for
the treatment of PH (1,2). Furthermore, tertiary lymphoid follicles
and inflammatory infiltration has been demonstrated near the
remodeled pulmonary arteries of animal models and patients with PH
(2–5). These inflammatory cells produce
chemokines and cytokines, including tumor necrosis factor (TNF)-α,
and interleukin (IL)-6, and elevated levels of inflammatory
cytokines have been reported to act as predictive factors for the
survival of patients with idiopathic and familial PH (6). Among the elevated inflammatory
cytokines, TNF-α has a crucial role in the hyperproliferation of
pulmonary artery smooth muscle cells (PASMCs) and vascular
remodeling. Furthermore, previous studies have demonstrated that
the calcineurin (CaN)/nuclear factor of activated T-cells (NFAT)
signaling pathway is one of the critical pathways involved in the
proliferation of SMCs in PH (7,8), and
thus may be a therapeutic target for the treatment of PH (8). CaN/NFAT in the cytoplasm of SMCs may be
activated by increased calcium influx, which subsequently initiates
the hyperproliferation of SMCs (9),
resulting in vascular remodeling. As previously demonstrated, TNF-α
may increase calcium influx, which may result in further activation
of CaN/NFAT, and thus promote the hyperproliferation of SMCs
(10). Therefore, the authors of the
present study hypothesized that decreasing the production of TNF-α
may effectively block the activation of CaN/NFAT and SMCs
hyperproliferation, and may be considered a potential treatment for
patients with PH.
Mesenchymal stem cells (MSCs) are a group of
primitive cells, which have self-renewal ability and the potential
to differentiate along various lineages. The clinical application
of MSCs is promising due to the low potential for immunogenicity
and graft rejection reactions. Furthermore, a serum-free culture
system for the expansion of MSCs may further decrease the potential
for zoonotic infections and immunological reactions (11). The authors of the present study have
previously demonstrated that MSCs may produce high levels of
prostaglandin E2 (PGE2) and immunoregulatory cytokines, and
decrease the production of Th1-associated inflammatory cytokines
such as TNF-α and interferon-γ, when co-cultured with activated
CD4+ T cells (12). The
unique immunosuppressive effects produced by MSCs highlight them as
a potential treatment for immune-associated diseases (13,14).
Previous studies have demonstrated that administration of MSCs may
relieve PH in an experimental animal model (15,16);
however, the effects of MSCs on the immune disorder and
hyperproliferation of PASMCs have yet to be elucidated.
In the present study, a rat model of PH was induced
by a single subcutaneous injection of monocrotaline (MCT), prior to
subsequent transplantation of serum-free cultured MSCs derived from
a human umbilical cord. The therapeutic effects, and the expression
levels of TNF-α and CaN/NFAT in the pulmonary arteries were
evaluated, in order to determine the effects of MSCs on the immune
disorder and hyperproliferation of PASMCs associated with PH.
Materials and methods
Human umbilical cord-derived MSCs
The present study was approved by the Institutional
Review Board of Guiyang Medical College (Guiyang, China), and
written informed consent was obtained from all donors prior to the
initiation of this study. Human umbilical cord-derived MSCs were
isolated according to our previous study (17) and were cultured in DMEM/F-12 medium
(Gibco Life Technologies, Carlsbad, CA, USA) supplemented with 100
U/ml penicillin-streptomycin and 10% fetal bovine serum (FBS;
HyClone, GE Healthcare Life Sciences, Logan, UT, USA). On the third
passage, MSC culture medium was exchanged for serum-free Chemically
Defined Mesenchymal Stem Cell Medium (Lonza Inc., Allendale, NJ,
USA). MSCs at passage 8–10 were identified by analyzing the ability
of osteogenic and adipogenic differentiation, as described
previously (11). In addition, the
expression of cell-surface markers, including CD19, CD29, CD31,
CD34, CD44, CD73, CD90, CD105, human leukocyte antigen (HLA)-DR and
HLA-ABC was detected using flow cytometry (BD Biosciences, Franklin
Lakes, NJ, USA) (17).
Experimental animals
A total of 24 female Sprague-Dawley rats (body
weight, 200 g) were purchased and housed in specific pathogen-free
units at the Laboratory Animals Center at Tianjin Blood Diseases
Hospital (Tianjin, China). The rats were maintained at 25°C in 70%
relative humidity, with a 12-h light/dark cycle. All animal studies
were approved by the Institutional Animal Care and Use Committee of
Guiyang Medical College. The 24 rats were randomly divided into
three equal groups: The model group [(MCT and phosphate-buffered
saline (PBS)], the treatment group (MCT and MSCs), and the control
group (PBS). PH was induced in the rats by a single subcutaneous
injection of 60 mg/kg MCT (Sigma-Aldrich, St. Louis, MO, USA)
(18), with PBS administered as a
control. A total of 5 days after MCT injection, PBS or MSCs were
administered via the caudal vein. Briefly, at passage 8–10, the
MSCs were detached for transplantation using 0.25% trypsin (Gibco
Life Technologies) and 0.53 mM EDTA (Sigma-Aldrich). Following
subsequent washing with PBS twice, 106 cells were
resuspended in 1 ml PBS, and transplanted into the rats via the
caudal vein, 5 days after injection of MCT. Furthermore, in order
to observe the distribution of MSCs in the lungs, two additional
rats received transplantation of CM-Dil-labeled (Invitrogen Life
Technologies, Carlsbad, CA, USA) MSCs under the same protocol.
Examination of hemodynamics
At day 21, the rats were anesthetized by
intraperitoneal injection of 50 mg/kg pentobarbital
(Sigma-Aldrich). Polygraph examination (Nihon Kohden Corporation,
Tokyo, Japan) of the mean aortic pressure (MAoP) was performed by
inserting a polyethylene catheter into the ascending aorta via the
right carotid artery. The right ventricular systolic pressure
(RVSP) was subsequently tested by inserting a polyethylene catheter
into the right ventricle via the right external jugular vein.
Subsequently, blood samples were collected from the external
jugular vein. Plasma was segregated by centrifugation at 2,000 × g
for 10 min at 4°C, and stored at −80°C. The rats were subsequently
sacrificed by decapitation, prior to harvesting of the lung tissue
and pulmonary arteries. The pulmonary arteries were immersed in
RNAsafer Stabilizer Reagent (Tianjin Baorui Biological Technology
Co., Ltd., Tianjin, China), and the remaining pulmonary lobes were
fixed in 10% paraformaldehyde (Sigma-Aldrich) at room
temperature.
Histological examination
Following fixation in 10% paraformaldehyde for 24 h,
the lung tissue was embedded in paraffin, and serial 5 µm sections
were stained with hematoxylin and eosin (Yuanmu Biotechnology Co.,
Ltd., Shanghai, China), prior to observation under a fluorescence
microscope (Olympus Corporation, Tokyo, Japan). The medial wall
thickness of the pulmonary arteriole is expressed as: Wall
thickness (WT; %)=[(medial thickness × 2)/external diameter] × 100
(19).
TNF-α expression in the lung
tissue
Paraffin sections fixed on gelatin-coated slides
were deparaffinized and rehydrated prior to sequential incubation
with 0.3% Triton X-100 (Sigma-Aldrich), 3% hydrogen peroxide (Santa
Cruz Biotechnology Inc., Dallas, TX, USA) and 2% bovine serum
albumin (MP Biomedicals, Santa Ana, CA, USA). The sections were
subsequently incubated overnight at 4°C with a goat polyclonal
primary antibody against TNF-α (1:400; sc-1350; Santa Cruz
Biotechnology Inc.). The following day, the lung tissue was
incubated with a biotinylated rabbit anti-goat secondary antibody
(Wuhan Boster Biotechnology Co., Ltd., Wuhan, China) for 30 min,
prior to immunoreactivity detection using a
3-amino-9-ethylcarbazole peroxidase substrate kit (Wuhan Boster
Biotechnology Co., Ltd.).
TNF-α plasma levels
The plasma levels of TNF-α were determined using an
ELISA kit (PeproTech, Inc., Rocky Hill, NJ, USA), according to the
manufacturer's instructions.
CaN and NFATc2 expression in the
pulmonary arteries
Total RNA was extracted from the pulmonary arteries
using an E.Z.N.A. Total RNA I kit (Omega Biotek, Inc., Norcross,
GA, USA), which included tissue lysing and homogenization,
according to the manufacturers instructions. Subsequently, the
total RNA was reverse transcribed into cDNA using a Moloney Murine
Leukemia Virus Reverse Transcriptase kit (Invitrogen Life
Technologies). Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analyses for CaN and NFATc2 were performed on an
7300 Real-Time PCR system (Applied Biosystems Life Technologies,
Foster City, CA, USA) using Platinum® SYBR® Green qPCR SuperMix-UDG
with ROX (Invitrogen Life Technologies). The primer sequences were
as follows: GAPDH, forward 5′-CCA TTC TTC CAC CTT TGA TGCT-3′,
reverse 5′-TGT TGC TGT AGC CAT ATT CAT TGT-3′; CaN, forward 5′-CAG
AGG GTG CTT CGA TTCTC-3′, reverse 5′-CCC CTA AGA AGA GGT AGC GA-3′;
and NFATc2, forward 5′-CAG CAG ATT TGG GAG ATG GAA G-3′, and
reverse 5′-GAC TGG GTG GTA AGT AAA GTG C-3′. PCR cycling conditions
were as follow: 95°C for 2 min, and 95°C for 15 sec, and 60°C for
30 sec, for 40 cycles. The relative expression levels were
quantified by the 2−ΔΔCt method.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA) was used
to perform statistical analyses. Differences were compared using
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference.
Results
Identification of MSCs
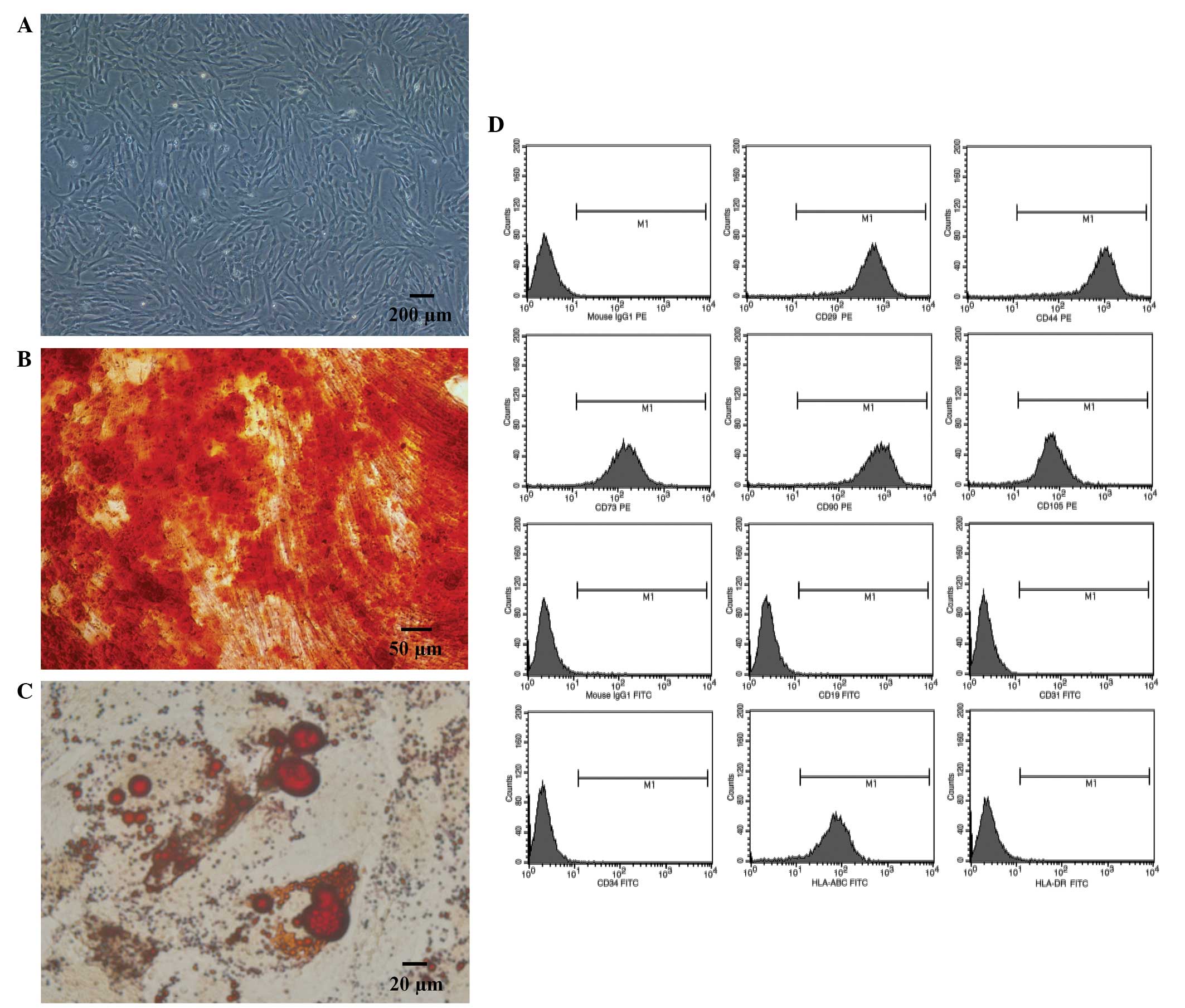
Human umbilical cord-derived MSCs proliferated well
when cultured in serum-free medium. Furthermore, the MSCs exhibited
a typical fibroblast shape (Fig.
1A), and demonstrated osteogenic (Fig. 1B) and adipogenic (Fig. 1C) differentiation abilities, when
cultured in specific conditioned medium. Flow cytometric analysis
determined the cells were positive for HLA-ABC, CD29, CD44, CD73,
CD90 and CD105, whereas they were negative for: CD19, CD31, CD34
and HLA-DR (Fig. 1D).
Transplantation of MSCs improves
PH
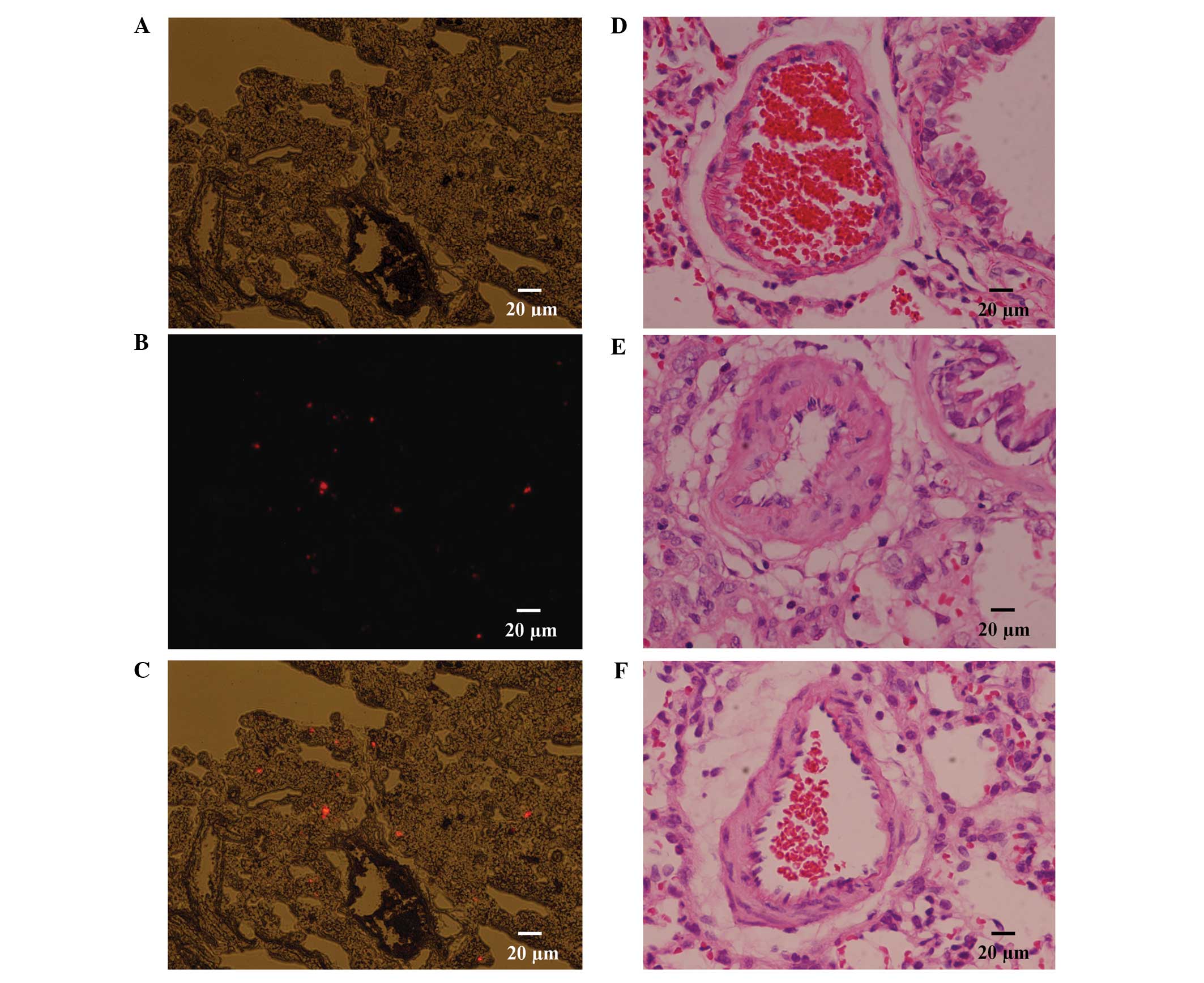
As described, two rats were transplanted with CM-Dil
labeled MSCs; and following a period of 2 days, the rats were
sacrificed by decapitation, and the lung tissues were harvested.
Serial 5 µm cryosections were prepared and observed under a
fluorescence microscope; the results of which revealed an abundance
of CM-Dil positive cells distributed in the lungs, which may
confirm that transplanted MSCs localize to injured lung tissue
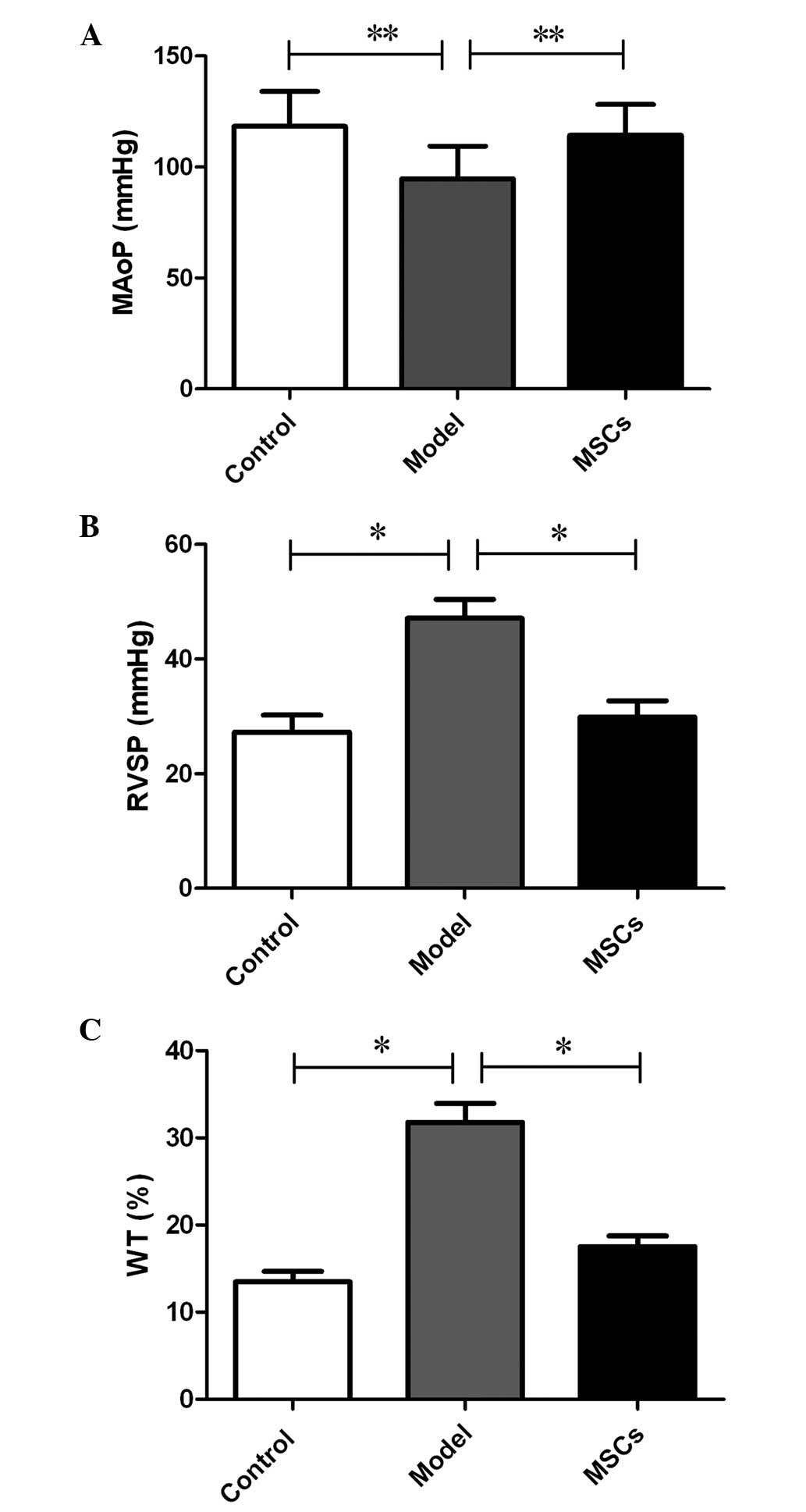
following transplantation, via a peripheral vessel (Fig. 2A-C). A hemodynamic examination was
performed 21 days after subcutaneous injection of MCT, the results
of which indicated that the MAoP in the model group was
significantly decreased, as compared with that in the control group
(94.64±14.83 vs. 118.34±15.67 mmHg; P<0.05) (Fig. 3A). The RVSP increased from 27.28±2.96
mmHg in the control group to 47.13±3.31 mmHg in the model group
(P<0.01) (Fig. 3B). Furthermore,
compared with that of the model group, the MAoP recovered to
114.25±13.93 mmHg (P<0.05; Fig.
3A), whereas the RVSP recovered to 29.86±2.87 mmHg (P<0.01;
Fig. 3B) following MSC treatment.
These results suggest that the MSC transplantation treatment
improved hemodynamic abnormalities.
In addition to the hemodynamic assessment, a
histological examination detected medial hypertrophy of the
pulmonary muscular arterioles, in the model group. In the model
group, the medial hypertrophy of the pulmonary muscular arterioles
(Fig. 2E) was evident compared with
the control group (Fig. 2D). the WT
increased from 13.48±1.23 to 31.78±2.16% (P<0.01); whereas the
medial hypertrophy improved following transplantation with the MSCs
(Fig. 2F), (WT, 17.53±1.24 vs.
31.78±2.16%; P<0.01) (Fig. 3C),
which was similar to the effects observed of MSCs on
hemodynamics.
MSCs decrease the expression levels of
TNF-α in lung tissue and plasma
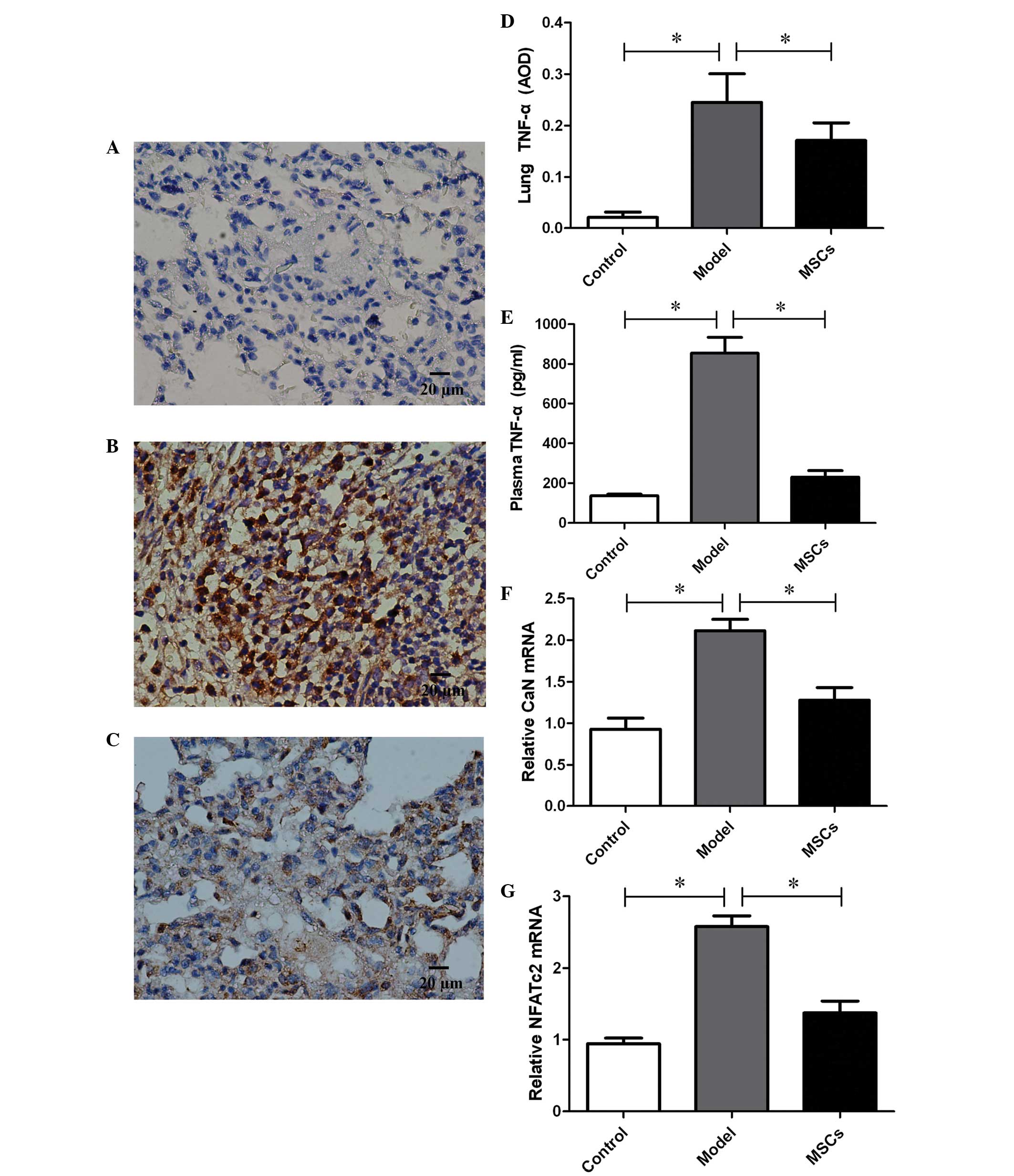
TNF-α expression was detected in the lung tissue of
the PH rat model 21 days after injection with MCT.
Immunohistochemistry demonstrated a downregulation of TNF-α
expression in the lung tissue of rats transplanted with MSCs, and
the average optical density (AOD) decreased from 0.25±0.06 in the
model group to 0.17±0.03 (P<0.01) (Fig. 4A–D). In addition, plasma TNF-α levels
were also measured, and were markedly increased in the model group,
as compared with the controls (854.49±80.15 vs. 135.96±8.98,
P<0.01) (Fig. 4E). Consistent
with the changes in TNF-α expression observed in the lung tissue,
TNF-α expression in the plasma of the MSCs treatment group
decreased significantly, as compared with the model group
(230.04±33.22 vs. 854.49±80.15, P<0.01), and no differences were
found in comparison with the controls (P>0.05) (Fig. 4E).
MSCs decrease the expression levels of
CaN and NFATc2
In order to assess the proliferation of PASMCs, the
expression levels of CaN and NFATc2, which are considered to be
critical factors in the proliferation of SMCs, were assessed by
RT-qPCR. A total of 21 days after injection with MTC, CaN and
NFATc2 expression levels in the pulmonary arteries were
significantly upregulated in the model group, as compared with that
of the control group (Fig. 4F and
G). However, the high expression levels of CaN and NFATc2 were
inhibited following transplantation with the MSCs.
Discussion
Using a rat model of MCT-induced PH, the present
study has demonstrated that TNF-α expression was increased in the
plasma and lung tissue. Furthermore, in addition to the medial
hypertrophy of the pulmonary muscular arterioles and the
hemodynamic alterations observed, the expression levels of CaN and
NFATc2 in the pulmonary arteries were significantly upregulated.
The transplantation of MSCs cultured in serum-free medium decreased
TNF-α expression levels in both the lung tissue and plasma of rats
with MCT-induced PH; and the expression levels of CaN and NFATc2 in
the pulmonary arteries were also downregulated. The results of the
present study indicated that transplantation with MSCs may improve
the medial hypertrophy of pulmonary muscular arterioles and
hemodynamic abnormalities associated with PH. Furthermore, the
administration of MSCs may provide a potential treatment for
patients with PH by suppressing the production of TNF-α and
downregulating CaN and NFATc2 expression in the pulmonary arteries,
in order to prevent PASMCs hyperproliferation.
The persistent contraction and remodeling of
pulmonary arterioles is the main pathological and
physiopathological characteristic of PH, and high blood pressure in
the pulmonary circulation may result in of right ventricular
overload. With the progression of the disease, right ventricular
hypertrophy and dilatation ensue. Despite the development of
targeted drugs that may improve the symptoms of PH and decrease the
occurrence of heart attack to a certain extent, heart failure and
mortality remain the inevitable fate of numerous patients with PH,
as drug efficacy remains limited in the long-term (20,21).
Therefore, it is crucial that more effective therapeutic protocols
are explored, particularly those that directly target the
pathomechanism of PH. With the advancement of studies exploring the
mechanisms of PH, various studies have demonstrated that
inflammation and hyperproliferation of PASMCs may be a promising
target for the treatment of PH, as they are considered to be the
major pathological features of PH (1,8).
Concordantly, the present study demonstrated that the expression
levels of TNF-α in the lung tissue and plasma were increased in the
rat model of MCT-induced PH. Furthermore, as pivotal regulatory
factors of the proliferation of SMCs, the expression levels of CaN
and NFATc2 in pulmonary arteries were significantly upregulated.
Medial hypertrophy of the pulmonary muscular arterioles accompanies
these changes, as pulmonary circulation pressure is increased. As a
previous study has demonstrated, it is possible that TNF-α, which
is produced by inflammatory cells that have infiltrated the lung
tissue, may accelerate calcium mobilization and influx via specific
channels in the membranes of PASMCs (10). Furthermore, increased levels of
calcium in the cytoplasm may activate the proliferation of SMCs via
the CaN/NFAT pathway (9). Therefore,
inhibiting the production of TNF-α may be a potential treatment for
PH as it may interrupt the activation of the CaN/NFAT pathway and
thus prevent PASMCs hyperproliferation.
As a subset of stromal stem cells, MSCs are widely
distributed around the body and share the same biological
characteristics, including the potential of multi-lineage
differentiation and self-renewal. Previous studies have reported
that MSCs have been harvested from the bone marrow (22), umbilical cord blood (23), the umbilical cord (17), the placenta (24), and adipose tissue (25) thus far. Umbilical cord-derived MSCs
are particularly superior in terms of non-invasiveness, reduced
donor harm/pain, and greater expansion capability (17,26),
which warrant the promising application of them in the future.
However, in spite of the low immunogenicity and low potential for
graft rejection of MSCs, as the traditional culture system is
supplemented with FBS, the potential for zoonotic infection still
exists with clinical application. Therefore, in the present study,
the traditional culture medium for MSCs was exchanged for
serum-free medium on the third passage, in order to decrease the
effects of serum as much as possible. The results of the present
study indicated that, under serum-free culture conditions, MSCs
maintain their phenotype and similar growth characteristics. Thus,
as MSCs retain the potential for multi-lineage differentiation,
this could ensure the reliability of subsequent studies.
Successful localization to the injured lung tissue
is the crucial first step in the treatment of PH with MSCs.
Therefore, in the present study, CM-Dil was used to observe the
distribution of MSCs in the lung tissue; the results of which
demonstrated that, 2 days after the transplantation of the MSCs, an
abundance of CM-Dil positive cells were localized in the lungs.
This may suggest that MSCs have a protective effect in situ.
The hypothesis that MSCs may differentiate into various lung tissue
cell types after transplantation into a host with pulmonary
diseases has been questioned; this is mainly due to a lack of a
niche for MSCs during injury, which may be an obstacle to their
engraftment or differentiation (27). A more acceptable hypothesis is that
MSCs may have important cytoprotective effects, predominantly via
various paracrine mechanisms (27).
The authors of the present study have previously demonstrated that
the ability of CD4+ T cells to produce TNF-α was
decreased when co-cultured with MSCs, and the immunosuppressive
activity of MSCs is highly associated with their ability to secrete
immunoregulatory cytokines and PGE2 (12). The present study has also
demonstrated that in vivo exogenous transplantation of MSCs
decreased the levels of TNF-α not only in the lung tissue but also
in plasma by its immunosuppressive activity. Furthermore, the
expression levels of CaN and NFATc2 in pulmonary arteries were
downregulated; hence, the medial hypertrophy of the pulmonary
muscular arterioles was improved, in addition to an alleviation of
pulmonary artery pressure.
In conclusion, the present study demonstrates for
the first time, to the best of our knowledge, that human umbilical
cord-derived MSCs cultured in serum-free medium may suppress the
hyperproliferation of PASMCs associated with PH. Furthermore, the
regulating effect was accomplished by the immunosuppressive
activity of MSCs, particularly via suppression of TNF-α, and the
subsequent inhibition of the CaN/NFAT pathway in PASMCs. However,
the prospective efficacy and potential side-effects of these
regulatory effects require confirmation in future studies.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 30560159).
References
|
1
|
Frid MG, Brunetti JA, Burke DL, Carpenter
TC, Davie NJ, Reeves JT, Roedersheimer MT, van Rooijen N and
Stenmark KR: Hypoxia-induced pulmonary vascular remodeling requires
recruitment of circulating mesenchymal precursors of a
monocyte/macrophage lineage. Am J Pathol. 168:659–669. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hall S, Brogan P, Haworth SG and Klein N:
Contribution of inflammation to the pathology of idiopathic
pulmonary arterial hypertension in children. Thorax. 64:778–783.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pinto RF, Higuchi ML and Aiello VD:
Decreased numbers of T-lymphocytes and predominance of recently
recruited macrophages in the walls of peripheral pulmonary arteries
from 26 patients with pulmonary hypertension secondary to
congenital cardiac shunts. Cardiovasc Pathol. 13:268–275. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heath D and Edwards JE: The pathology of
hypertensive pulmonary vascular disease; a description of six
grades of structural changes in the pulmonary arteries with special
reference to congenital cardiac septal defects. Circulation.
18:533–547. 1958. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perros F, Dorfmüller P, Montani D, Hammad
H, Waelput W, Girerd B, Raymond N, Mercier O, Mussot S,
Cohen-Kaminsky S, et al: Pulmonary lymphoid neogenesis in
idiopathic pulmonary arterial hypertension. Am J Respir Crit Care
Med. 185:311–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soon E, Holmes AM, Treacy CM, Doughty NJ,
Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin
P, et al: Elevated levels of inflammatory cytokines predict
survival in idiopathic and familial pulmonary arterial
hypertension. Circulation. 122:920–927. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Frutos S, Spangler R, Alò D and Bosc
LV: NFATc3 mediates chronic hypoxia-induced pulmonary arterial
remodeling with alpha-actin up-regulation. J Biol Chem.
282:15081–15089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bonnet S, Rochefort G, Sutendra G, Archer
SL, Haromy A, Webster L, Hashimoto K, Bonnet SN and Michelakis ED:
The nuclear factor of activated T cells in pulmonary arterial
hypertension can be therapeutically targeted. Proc Natl Acad Sci
USA. 104:11418–11423. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Said SI, Hamidi SA and Bosc L Gonzalez:
Asthma and pulmonary arterial hypertension: Do they share a key
mechanism of pathogenesis? Eur Respir J. 35:730–734. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rowlands DJ, Islam MN, Das SR, Huertas A,
Quadri SK, Horiuchi K, Inamdar N, Emin MT, Lindert J, Ten VS,
Bhattacharya S and Bhattacharya J: Activation of TNFR1 ectodomain
shedding by mitochondrial Ca2+ determines the severity of
inflammation in mouse lung microvessels. J Clin Invest.
121:1986–1999. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu M, Han ZB, Liu JF, Wang YW, Zhang JZ,
Li CT, Xin PL, Han ZC and Zhu XP: Serum-free media and the
immunoregulatory properties of mesenchymal stem cells in vivo and
in vitro. Cell Physiol Biochem. 33:569–580. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang ZX, Han ZB, Ji YR, Wang YW, Liang L,
Chi Y, Yang SG, Li LN, Luo WF, Li JP, et al: CD106 identifies a
subpopulation of mesenchymal stem cells with unique
immunomodulatory properties. PLoS One. 8:e593542013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Le Blanc K, Rasmusson I, Sundberg B,
Gӧtherstrӧm C, Hassan M, Unzel M and Ringdén O: Treatment of severe
acute graft-versus-host disease with third party haploidentical
mesenchymal stem cells. Lancet. 363:1439–1441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zappia E, Casazza S, Pedemonte E,
Benvenuto F, Bonanni I, Gerdoni E, Giunti D, Ceravolo A, Cazzanti
F, Frassoni F, Mancardi G and Uccelli A: Mesenchymal stem cells
ameliorate experimental autoimmune encephalomyelitis inducing
T-cell anergy. Blood. 106:1755–1761. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baber SR, Deng W, Master RG, Bunnell BA,
Taylor BK, Murthy SN, Hyman AL and Kadowitz PJ: Intratracheal
mesenchymal stem cell administration attenuates
monocrotaline-induced pulmonary hypertension and endothelial
dysfunction. Am J Physiol Heart Circ Physiol. 292:H1120–H1128.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luan Y, Zhang X, Kong F, Cheng GH, Qi TG
and Zhang ZH: Mesenchymal stem cell prevention of vascular
remodeling in high flow-induced pulmonary hypertension through a
paracrine mechanism. Int Immunopharmacol. 14:432–437. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X,
Gong W, Han ZB, Xu ZS, Lu YX, Liu D, Chen ZZ and Han ZC: Isolation
and characterization of human umbilical cord mesenchymal stem cells
with hematopoiesis-supportive function and other potentials.
Haematologica. 91:1017–1026. 2006.PubMed/NCBI
|
|
18
|
Matsuda Y, Hoshikawa Y, Ameshima S, Suzuki
S, Okada Y, Tabata T, Sugawara T, Matsumura Y and Kondo T: Effects
of peroxisome proliferator-activated receptor gamma ligands on
monocrotaline-induced pulmonary hypertension in rats. Nihon Kokyuki
Gakkai Zasshi. 43:283–288. 2005.(In Japanese). PubMed/NCBI
|
|
19
|
Maruyama H, Watanabe S, Kimura T, Liang J,
Nagasawa T, Onodera M, Aonuma K and Yamaguchi I: Granulocyte
colony-stimulating factor prevents progression of
monocrotaline-induced pulmonary arterial hypertension in rats. Circ
J. 71:138–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zangiabadi A, De Pasquale CG and Sajkov D:
Pulmonary hypertension and right heart dysfunction in chronic lung
disease. Biomed Res Int. 2014:7396742014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Patel R, Aronow WS, Patel L, Gandhi K,
Desai H, Kaul D and Sahgal SP: Treatment of pulmonary hypertension.
Med Sci Monit. 18:RA31–RA39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL
and Chen TH: Isolation of multipotent mesenchymal stem cells from
umbilical cord blood. Blood. 103:1669–1675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fukuchi Y, Nakajima H, Sugiyama D, Hirose
I, Kitamura T and Tsuji K: Human placenta-derived cells have
mesenchymal stem/progenitor cell potential. Stem Cells. 22:649–658.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell
JW, Katz AJ, Benhaim P, Lorenz HP and Hedrick MH: Multilineage
cells from human adipose tissue: Implications for cell-based
therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baksh D, Yao R and Tuan RS: Comparison of
proliferative and multilineage differentiation potential of human
mesenchymal stem cells derived from umbilical cord and bone marrow.
Stem Cells. 25:1384–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jun D, Garat C, West J, Thorn N, Chow K,
Cleaver T, Sullivan T, Torchia EC, Childs C, Shade T, et al: The
pathology of bleomycin-induced fibrosis is associated with loss of
resident lung mesenchymal stem cells that regulate effector T-cell
proliferation. Stem Cells. 29:725–735. 2011. View Article : Google Scholar : PubMed/NCBI
|