Introduction
Neoadjuvant chemotherapy is an important part of the
treatment of breast cancer. It not only greatly improves the
effectiveness of surgical treatment and thereby increases the
success rate of breast conservation, but also suppresses systemic
subclinical metastases to a certain degree, and may improve the
survival rate of patients (1).
However, a variety of issues associated with neoadjuvant
chemotherapy require further investigation, such as the
identification of molecular biological markers to predict the
efficacy of neoadjuvant chemotherapy. In particular, S100A4 has
attracted a significant amount of attention from researchers
(2).
S100A4 is a member of the S100 family of proteins;
its expression is associated with the movement, invasion,
metastasis, apoptosis and prognosis of various tumors (3,4). Studies
have demonstrated that pathophysiological progression is associated
with S100A4 in breast cancer (5,6), ovarian
cancer (7), colon cancer (8), bladder cancer (9) and melanoma (10), which is closely associated with tumor
incidence and metastasis. Currently, there are few studies
concerning the correlation between the efficacy of neoadjuvant
chemotherapy and the expression and changes of S100A4 (11–13), and
the conclusions are inconsistent. In the present study, tumor
tissues were collected after double coarse-needle biopsies; and the
levels of S100A4 protein were detected, in order to explore the
potential of S100A4 as molecular biological indicator for
predicting the effect of neoadjuvant chemotherapy and guiding
individual treatment.
Materials and methods
Patients and samples
A group of 65 female patients admitted to the
department of breast and thyroid surgery of the People's Hospital
of Liaocheng (Liaocheng, China) between October 2012 and December
2013 were included in the study. These patients, who were 22–63
years (mean, 42 years) old, were investigated using color
ultrasound-guided coarse needle biopsy and diagnosed as having
invasive breast cancer. Breast lumps were palpable in the clinical
examination. The selected patients, who did not receive any
pre-treatment, were treated with 4 cycles of TAC neoadjuvant
chemotherapy prior to surgery. This study was approved by the
ethics committee of the People's Hospital of Liaocheng. Informed
consent was obtained from all patients.
Time and method of specimen
collection
Hollow needle biopsy was performed respectively
prior to neoadjuvant chemotherapy and following 2 cycles of
neoadjuvant chemotherapy; surgical resection was performed
following 4 cycles of neoadjuvant chemotherapy.
Neoadjuvant chemotherapy
A TAC regimen was administered, with each cycle
comprising 75 mg/m2 d1 docetaxel, 50 mg/m2 d1
doxorubicin and 500 mg/m2 d1 cyclophosphamide for 21
days (d1 indicates that a single dose of the treatment was
administered on day 1 of the treatment cycle).
Efficacy evaluation of neoadjuvant
chemotherapy
The diameter and size of the tumor were measured
with calipers and breast high frequency color Doppler ultrasound
prior to neoadjuvant chemotherapy; the maximum diameters of the
tumors of all patients were measured prior to and following
neoadjuvant chemotherapy by the same operator with the same method
of measurement; the results were recorded every 2 cycles.
Clinical efficacy was assessed using the RECIST
evaluation criteria of Solid Tumors (14), and the results were divided into:
Complete remission (CR), all target lesions disappeared and no new
lesions appeared, tumor markers were normal, and were maintained
for ≥4 weeks; partial remission (PR), the maximum diameter sum of
the target lesions decreased by >30%, and was maintained for ≥4
weeks; stable disease (SD), the maximum diameter sum of the target
lesions decreased less than that for PR, or increased less than
that for disease progression (PD); PD, the maximum diameter sum of
the target lesions increased ≥20%, and the absolute value increased
by ≥5 mm; the emergence of new lesions was also considered as PD.
If only one of the longest diameters of the target lesions
increased by ≥20%, and the sum of the longest diameters of all
recorded target lesions increased by <20%, this was not
evaluated as PD. No residual cancer or only in situ
carcinoma in samples after surgery was evaluated as pathological
complete response (pCR). CR + PR were considered effective; SD + PD
were considered invalid.
Pathologic evaluation of neoadjuvant chemotherapy
was conducted using the Miller and Payne (MP) grading standards
(15): Grade I, tumor lesions almost
unchanged; grade II, a small number of tumor cells disappeared
(≤30%); grade III, most of the tumor tissues disappeared (30–90%);
grade IV, most tumors disappeared (>90%); grade V, no residual
invasive carcinoma. Grades III–V represented effective
chemotherapy; grades I and II represented ineffective chemotherapy;
and grade V represented a pathological complete response (pCR).
Immunohistochemistry
Dewaxed and hydrated tissue sections were placed in
a pressure cooker containing citric tissue antigen retrieval
solution for 3 min heat reparation; S100 A4 rabbit anti-human
polyclonal antibody (1:200; ZA-0257; Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China) was added as the
primary antibody, and then reagents 1 and 2 of the secondary
antibody PV9000 detection system (ZSBIO, Beijing, China) were
dropped in order. The tissues were then stained with DAB solution
and sealed. Positive control samples underwent identical tonsil
slice detection, tonsil lymphocytes appear in the cytoplasm as
yellow or brown coloration, while stromal cells and vascular
endothelial cells exhibited no yellow or brown coloring. Negative
control samples were incubated with phosphate-buffered saline
solution (blank reagent) instead of rabbit anti-human S100A4
antibody.
Evaluation of S100A4 expression
S100A4 positive expression was observed as brown
cytoplasm, which was determined by a semi-quantitative scoring
method in accordance with the cytoplasmic color intensity and
number of positive cells; five visual fields of each slice were
randomly selected under a microscope at a magnification of ×200
(100 tumor cells per visual field) and the percentage of positive
cells was recorded according to the degree of staining (A) and the
proportion of stained cells (B) as follows: (A) degree of staining:
no staining, 0 points; pale yellow, 1 point; brown, 2 points; and
tan, 3 points. (B) proportion of stained cells: <5%, 0 points; 5
to 25%, 1 point; 26–50%, 2 points; 51–75%, 3 points; >75%, 4
points. An integral A+B ≥2 was considered to be S100A4 positive.
Specifically: 0–1 points was negative (−); 2 or 3 points was weakly
positive (1+); 4 or 5 points represented moderately positive (2+);
and 6 or 7 points represented strongly positive (3+).
Statistical analysis
The data were statistically analyzed using the SPSS
software package, version 17.0 (SPSS, Inc., Chicago, IL, USA).
Changes of the expression of S100A4 before, during and after
neoadjuvant chemotherapy were analyzed by Wilcoxon signed rank sum
test; the correlation between the expression intensity of S100A4
and the effect of neoadjuvant chemotherapy was analyzed by
χ2 and Spearman's rank correlation tests; P<0.05 was
considered to indicate a statistically significant result.
Results
Efficacy evaluation of neoadjuvant
chemotherapy
Clinical evaluation of neoadjuvant
chemotherapy
Following 2 cycles of neoadjuvant chemotherapy there
were 47 cases of effective chemotherapy, with an overall response
rate of 47/65 (72.31%); 8 cases had clinical CR (12.31%). Following
4 cycles of neoadjuvant chemotherapy, there were 49 cases of
effective chemotherapy, with an overall effective rate of 49/65
(75.38%), 18 cases had clinical CR (27.69%), and 16 patients
(24.62%) were not sensitive to neoadjuvant chemotherapy. Clinical
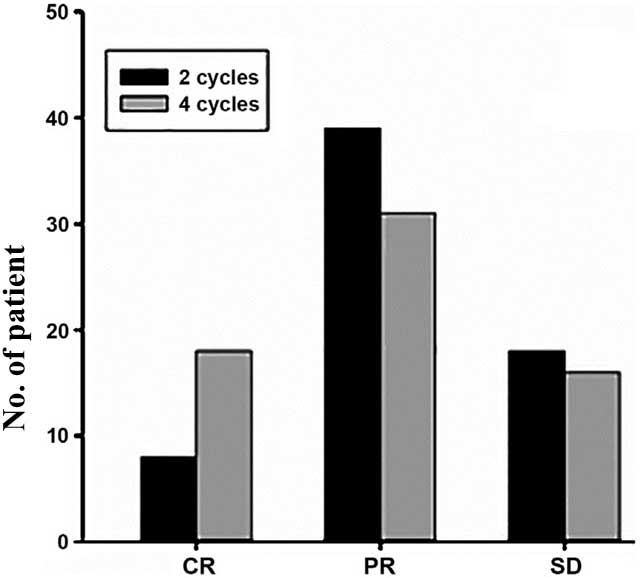
evaluation results are shown in Table
I and Fig. 1.
 | Table I.Clinical evaluation after neoadjuvant
chemotherapy. |
Table I.
Clinical evaluation after neoadjuvant
chemotherapy.
| Efficacy | After 2 cycles, n
(%) | After 4 cycles, n
(%) |
|---|
| CR | 8
(12.31) | 18 (27.69) |
| PR | 39 (60.00) | 31 (47.69) |
| SD | 18 (27.69) | 16 (24.62) |
| PD | 0 | 0 |
Pathologic evaluation of neoadjuvant
chemotherapy
Following 2 cycles of neoadjuvant chemotherapy, MP
pathological grading of the 65 patients was performed. There were 9
cases of grade I, 13 cases of grade II, 37 cases of grade III, 6
cases of grade IV and 0 cases of grade V; the chemotherapy response
rate was 43/65 (66.15%) and no pCR was observed. Following 4 cycles
of neoadjuvant chemotherapy, there were 7 cases of grade I, 12
cases of grade II, 19 cases of grade III, 13 cases of grade IV and
14 cases of grade V; the chemotherapy response rate was 46/65
(70.77%), and the proportion of patients achieving pCR was 14/65
(21.54%). There were 7 patients who continued to have no
sensitivity to neoadjuvant chemotherapy.
The pathological evaluation results of the coarse
needle specimens following 2 cycles of neoadjuvant chemotherapy and
surgical specimens after 4 cycles of neoadjuvant chemotherapy were
compared using the χ2 test, which showed no
statistically significant difference (χ2=0.32,
P>0.05), indicating that for patients that were determined to
have grade I or II disease by pathologic evaluation after 2 cycles
of neoadjuvant chemotherapy, alternative chemotherapy regimens
should be considered. The grading and effectiveness data are shown
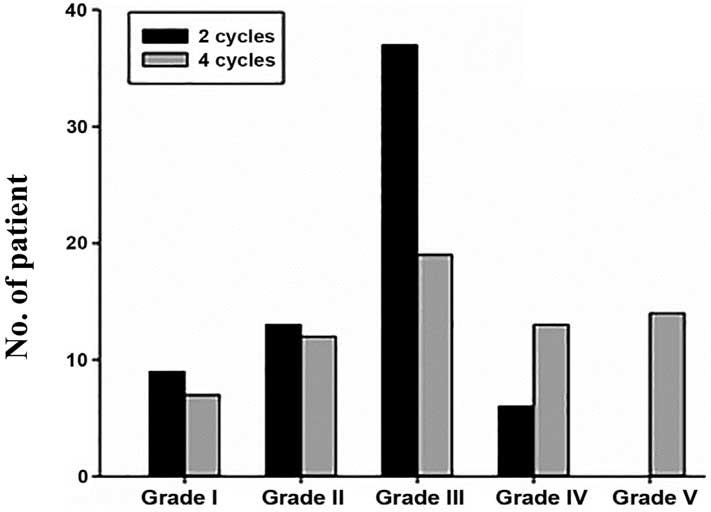
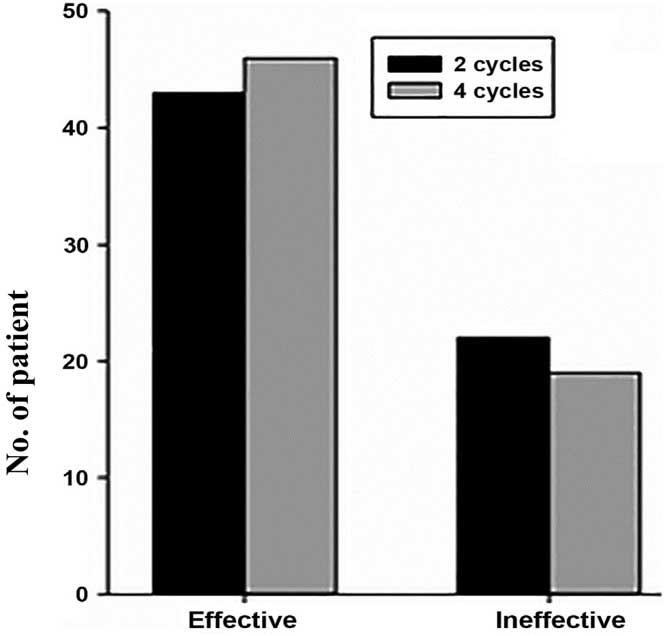
in Tables II and III and Figs.
2 and 3.
 | Table II.Pathological grading after neoadjuvant
chemotherapy. |
Table II.
Pathological grading after neoadjuvant
chemotherapy.
| Grade | After 2 cycles, n
(%) | After 4 cycles, n
(%) |
|---|
| I | 9
(13.85) | 7
(10.77) |
| II | 13 (20.00) | 12 (18.46) |
| III | 37 (56.92) | 19 (29.23) |
| IV | 6
(9.23) | 13 (20.00) |
| V |
0 | 14 (21.54) |
 | Table III.Pathological grading of samples after
2 and 4 cycles of neoadjuvant chemotherapy. |
Table III.
Pathological grading of samples after
2 and 4 cycles of neoadjuvant chemotherapy.
|
| Pathological
evaluation |
|
|
|---|
|
|
|
|
|
|---|
| Time of sampling | Effective | Ineffective | χ2 | P-value |
|---|
| After 2 cycles | 43 | 22 | 0.32 | >0.05 |
| After 4 cycles | 46 | 19 |
|
|
S100A4 expression prior to neoadjuvant
chemotherapy, and after 2 or 4 cycles of neoadjuvant
chemotherapy
Among the 65 patients, prior to neoadjuvant
chemotherapy, there were 20 patients who tested negative for S100A4
expression (30.77%), 16 patients with weakly positive S100A4
expression (24.62%), 15 patients with moderately positive S100A4
expression (23.08%) and 14 patients with strongly positive S100A4
expression (21.53%). Following 2 cycles of neoadjuvant
chemotherapy, there were 22 cases negative for expression of S100A4
(33.85%), 17 cases with weakly positive expression of S100A4
(26.15%), 15 cases with moderately positive expression of S100A4
(23.08%) and 11 cases with strongly positive expression of S100A4
(16.92%). After 4 cycles of neoadjuvant chemotherapy, there were 23
cases negative for expression of S100A4 (45.10%), 15 cases with
weakly positive expression of S100A4 (29.41%), 8 cases with
moderately positive expression of S100A4 (15.69%) and 5 cases with
strongly positive expression of S100A4 (9.80%). The remaining 14
patients achieved pathological complete remission after 4 cycles of
neoadjuvant chemotherapy, and so it was not possible to determine
S100A4 expression postoperatively. A comparison of 100A4 expression
prior to chemotherapy and after 4 cycles of chemotherapy showed
that expression was unchanged in 19 cases (37.26%), increased in 15
cases (29.41%) and decreased in 17 cases (33.33%). Wilcoxon signed
rank sum test showed that: There were significant differences in
S100A4 expression between the time-points prior to neoadjuvant
chemotherapy and following 4 cycles of neoadjuvant chemotherapy
(P=0.032); there was no significant difference between
pre-neoadjuvant chemotherapy and 2 cycles post-neoadjuvant
chemotherapy (P>0.05); and neoadjuvant chemotherapy may lead to
a reduction in the expression of S100A4 (Tables IV–VI).
 | Table IV.S100A4 expression prior to and after 4
cycles of breast cancer neoadjuvant chemotherapy. |
Table IV.
S100A4 expression prior to and after 4
cycles of breast cancer neoadjuvant chemotherapy.
|
| S100A4 expression
after chemotherapy |
|
|---|
|
|
|
|
|---|
| S100A4 expression
prior to chemotherapy | − | + | ++ | +++ | P-value |
|---|
| − | 11 | 5 | 2 | 1 | 0.032 |
| + | 3 | 6 | 4 | 1 |
|
| ++ | 6 | 2 | 1 | 2 |
|
| +++ | 3 | 2 | 1 | 1 |
|
 | Table VI.S100A4 expression prior to and after
2 cycles of breast cancer neoadjuvant chemotherapy. |
Table VI.
S100A4 expression prior to and after
2 cycles of breast cancer neoadjuvant chemotherapy.
|
| S100A4 expression
after chemotherapy |
|
|---|
|
|
|
|
|---|
| S100A4 expression
prior to chemotherapy | − | + | ++ | +++ | P-value |
|---|
| − | 14 | 5 | 1 | 0 | 0.214 |
| + | 4 | 8 | 4 | 0 |
|
| ++ | 4 | 2 | 8 | 1 |
|
| +++ | 0 | 2 | 2 | 10 |
|
Correlation between S100A4 expression
and the efficacy of neoadjuvant chemotherapy
All 65 patients with breast cancer received
neoadjuvant chemotherapy, and after 4 cycles of TAC neoadjuvant
chemotherapy, no disease progression was observed. Routine MP
grading evaluations and the expression levels of S100A4 protein for
each grade as follows: Grade I, 7 cases (10.77%); S100A4 expression
was negative in 3 cases (42.86%); weakly positive in 2 cases
(28.57%); moderately positive in 1 case (14.29%); and strongly
positive in 1 case (14.29%); Grade II, 12 cases (18.46%); S100A4
expression was negative in 8 cases (66.67%); weakly positive in 3
cases (25.00%); and moderately positive in 1 case (8.33%); Grade
III, 19 patients (29.23%); S100A4 expression was negative in 6
cases (31.58%); weakly positive in 7 cases (36.84%); moderately
positive in 3 cases (15.79%); and strongly positive in 3 cases
(15.79%); Grade IV, 13 cases (20.00%); S100A4 expression was
negative in 2 cases (15.38%); weakly positive in 2 cases (15.38%);
moderately positive in 6 cases (46.15%), and strongly positive in 3
cases (23.09%); Grade V, 14 (21.54%); S100A4 expression was
negative in 1 case (7.14%); weakly positive in 2 cases (14.28%);
moderately positive in 4 cases (28.57%); and strongly positive in 7
cases (50.00%).
The results were analyzed by χ2 test and
Spearman's rank correlation test, which showed that the efficacy of
neoadjuvant chemotherapy was positively correlated with S100A4
expression (χ2=7.46, P<0.01); the higher the S100A4
expression, the better the efficacy of neoadjuvant chemotherapy
(r=0.259, P<0.05), as shown in Tables VII and VIII.
 | Table VII.Correlation between S100A4 expression
prior to chemotherapy and the efficacy of neoadjuvant chemotherapy
in breast cancer. |
Table VII.
Correlation between S100A4 expression
prior to chemotherapy and the efficacy of neoadjuvant chemotherapy
in breast cancer.
|
|
| S100A4 expression
before chemotherapy |
|
|---|
|
|
|
|
|
|---|
| Chemotherapy
efficacy | n | − | + | ++ | +++ | P-value |
|---|
| Grade I | 7 | 3 | 2 | 1 | 1 | <0.05 |
| Grade II | 12 | 8 | 3 | 1 | 0 |
|
| Grade III | 19 | 6 | 7 | 3 | 3 |
|
| Grade IV | 13 | 2 | 2 | 6 | 3 |
|
| Grade V | 14 | 1 | 2 | 4 | 7 |
|
 | Table VIII.Correlation between S100A4 expression
prior to chemotherapy and the efficacy of neoadjuvant chemotherapy
in breast cancer. |
Table VIII.
Correlation between S100A4 expression
prior to chemotherapy and the efficacy of neoadjuvant chemotherapy
in breast cancer.
|
| Efficacy, n |
|
|
|---|
|
|
|
|
|
|---|
| S100A4
expression | Effective | Ineffective | P-value |
|---|
| Low | 20 | 26 | <0.01 |
| High | 16 | 3 |
|
Discussion
Since 1970s, it has become widely accepted that
breast disease is a systemic disease that can be hematogenous in
the early stages. Therefore, treatment of breast cancer by surgery
has gradually developed into a comprehensive treatment of the whole
body. Chemotherapy and endocrine therapy, in particular, have
greatly improved survival and quality of life and become the main
methods used for the treatment of breast cancer, particularly for
invasive breast cancer. Currently, neoadjuvant chemotherapy is
recognized as the standard treatment for locally advanced breast
cancer and inflammatory breast cancer by the medical profession.
Its advantage is that it reduces local breast tumor size and
controlling cancer invasion, thereby reducing tumor grade (16). It also provides data to support in
vivo sensitivity testing in individuals to determine the
effectiveness of a chemotherapy treatment plan. pCR, overall
survival (OS) and disease-free survival (DFS) have improved
significantly. Since the beginning of the 1980s, numerous
neoadjuvant clinical studies of chemotherapy have been conducted.
The National Surgical Adjuvant Breast and Bowel Project (NSABP)
B-18 and B-27 protocols (1) and
other experimental studies, revealed that postoperative
chemotherapy and neoadjuvant chemotherapy in patients with breast
cancer had no significant difference in OS and DFS during long-term
follow-up (median follow-up, 16 years). However, if the patients
reached pCR following neoadjuvant chemotherapy, both OS and DFS
improved significantly.
At present, the evaluation of neoadjuvant
chemotherapy is reliant upon clinical examination and pathological
testing. The clinical evaluation includes specialist examination,
breast ultrasound, mammography and magnetic resonance imaging
(MRI). Among the currently advocated types of imaging examination,
breast MRI is the primary option. Pathological evaluation, which
involves the observation of postoperative specimens under a
microscope to detect the apoptosis, degeneration and disappearance
of tumor cells, is a more intuitive method with high reliability.
Studies have shown that survival is significantly improved in
patients with a pCR prognosis (1,17).
Therefore, pathological assessment is an important method for the
evaluation of neoadjuvant chemotherapy. However, both neoadjuvant
chemotherapy clinical assessment and pathological assessment have
defects. The identification of predictors of the efficacy of
neoadjuvant chemotherapy is important for reducing the suffering
and economic burden of chemotherapy, enabling other effective
treatment methods to be sought, and improving patient survival and
quality of life.
Since the advent of neoadjuvant chemotherapy,
considerable research has been conducted to predict its efficacy.
This is important for preventing chemotherapy-insensitive patients
from undergoing neoadjuvant chemotherapy-induced progression. Early
studies of neoadjuvant chemotherapy predictors mainly concerned
clinical indicators. For example, the NSABP B-18 study (18) found that the histological grading of
breast cancer was predictive for the efficacy of neoadjuvant
chemotherapy. With the development of molecular biology, increasing
attention has been focused on biological factors in breast cancer
for predicting the efficacy of neoadjuvant chemotherapy. However,
at present, that has been no consensus on any of the predictors
that have been proposed. However, they have laid the foundation for
further studies on predictive methods and predictors of neoadjuvant
chemotherapy efficacy.
S100A4, also known as Mts1, pEL298, 18A2, 42A, p9Ka,
calvasculin, CAPL and FSP1, has a molecular weight of
11.5×103, and is located in the long arm of chromosome
zone 2 band 1 (1q21) (19). This
zone is unstable; therefore chromosomal absence, translocation,
duplication and other changes can easily occur, which are closely
associated with the incidence, development and invasion of tumors.
The S100A4 gene encodes a calcium-binding protein with a double EF
helix, and is a member of the S100 calcium-binding protein
superfamily. It is a metastasis-associated protein. A previous
study found that S100A4 was capable of regulating the cell cycle
and promoting invasion and metastasis (20). In addition, it is also associated
with calcium signaling pathways for the regulation of the
expression of genes associated with cell motility, adhesion,
proliferation, differentiation, apoptosis and other
pathophysiological processes. Albertazzi et al (21) found that in breast cancer, the
expression level of S100A4 protein correlated with metastasis.
Although studies have not shown directly that S100A4 plays an
active role, liprin B1, methionine amino peptidase, P53 and certain
proteins involved in cytoskeletal rearrangement and cell motility
have been found to interact with the S100A4 protein, increasing the
ability of tumor cells to become invasive and metastatic (3). Wang et al (22) detected the S100A4 protein by
immunohistochemistry in colorectal cancer, adjacent normal mucosa,
lymph node metastasis, liver cancer and colorectal adenomas. They
found that its expression level in colorectal cancer was
significantly higher than that in adjacent normal mucosa and
adenoma (P<0.05). They also found that its expression levels in
patients with lymph node metastasis and liver metastasis and at
relatively-advanced Duke stage were significantly higher than those
in patients without lymph node metastasis and liver metastasis and
at an early Duke stage in colorectal cancer (P<0.05). Through
study of the S100 family, Jin et al (23) found that the expression level of
S100A4 protein was only associated with chemotherapy during
chemotherapy (P<0.05). Ambartsumian et al (24) observed that S100A4 played an
important role in promoting angiogenesis directly; it was involved
in cancer development and metastasis throughout most of the
pathophysiological process. Through the investigation of clinically
obtained tissue samples, Rudland et al found that for breast
cancer, the number of lymph node metastases and the scope and
degree of malignancy were closely correlated with the expression
level of S100A4 (6). By
investigating the expression levels of S100A4 in Luminal A type
breast cancer, Luminal B type breast cancer, breast cancer with
HER-2 overexpression, basal-like breast cancer and adjacent breast
carcinoma, Wang et al (25)
found that the positive rate of S100A4 expression in tissues
adjacent to breast cancer was (45.0%; 18/40), significantly lower
than that in normal tissues (62.0%; 67/108) (P<0.05).
Furthermore, in different molecular subtypes of breast tissue, the
positive rate of S100A4 expression in HER-2-overexpressing and
basal-like breast cancer tissues were higher than that in the
Luminal A and Luminal B types (P<0.05). S100A4 exhibited high
expression levels in breast cancer patients with lymph node
metastasis (P<0.05).
Clinical studies (26,27) have
shown that changes in the expression of certain genes could predict
the efficacy of neoadjuvant chemotherapy. By comparing the
expression of the estrogen receptor (ER), progesterone receptor
(PR) and HER-2 in 43 patients receiving TAC neoadjuvant
chemotherapy before chemotherapy and after surgery, Li et al
(28) found that the expression
levels of ER, PR and HER-2 did not significantly change during the
course of chemotherapy (P>0.05), but observed that ER and/or
PR-negative breast cancer patients were more sensitive to
chemotherapy. MacGrogan et al (29) found that in patients with no or low
ER expression and high Ki67 expression, the efficacy of
chemotherapy was likely to be improved. Zhou et al (30) reported that neoadjuvant chemotherapy
is able to reduce the expression of Ki67, but has insignificant
effects on the expression of ER, PR and HER-2. Zhao et al
(31) conducted a retrospective
analysis of the correlation between the expression of ER, PR, p53
and Bcl-2 and the efficacy of neoadjuvant chemotherapy in 98 cases
of breast cancer, and found that the expression of p53 and Bcl-2
changed significantly following chemotherapy (P<0.05); as the
effect of chemotherapy was increased, the expression of p53
decreased, and the expression intensity of Bcl-2 was positively
correlated with chemotherapy efficacy (P<0.05). However, the
expression levels of ER and PR did not significantly change from
their pretreatment levels after chemotherapy. According to these
previous studies, neoadjuvant chemotherapy can reduce the
expression of Ki67 and p53, and enhance the expression of Bcl-2,
but has no significant effect on the expression of ER, PR and
HER-2,.
The present study analyzed the correlation between
the expression of S100A4 protein prior to neoadjuvant chemotherapy
and the efficacy of neoadjuvant chemotherapy. It was observed that
in the 65 patients receiving neoadjuvant chemotherapy, there were
46 cases (70.77%) in which chemotherapy was effective, of which 36
cases expressed S100A4 protein (78.26%) and 10 cases did not
(21.74%). Chemotherapy was ineffective in 19 cases, 8 of whom
tested positive for S100A4 protein expression (42.10%) and 11 of
whom tested negative (57.90%). There were significant differences
in the efficacy of neoadjuvant chemotherapy between positive and
negative S100A4 expression groups (P<0.05). Furthermore, the
efficacy of neoadjuvant chemotherapy was positively correlated with
S100A4 protein expression (r=0.259, P<0.05); the higher the
S100A protein expression, the better the efficacy of neoadjuvant
chemotherapy. In the present study, in the 14 patients with a pCR,
there were 9 cases (64.29%) with strongly positive expression of
S100A4 protein (+++) and 2 cases (14.29%) that were negative for
S100A4 protein expression, a significantly smaller proportion than
those who were positive for S100A4 protein expression. The reasons
for this are hypothesized to be as follows: S100A4 protein
expression is closely associated with the degree of tumor
differentiation and malignancy; with a high degree of
differentiation, low-grade malignant tumor cells are not sensitive
to chemotherapy and have low S100A4 expression. By contrast,
less-differentiated and highly malignant tumor cells highly express
S100A4 protein and are also more sensitive to chemotherapy.
In this study, S100A4 expression in 65 patients with
breast cancer was detected prior to, during (following 2 cycles)
and after (following 4 cycles) of neoadjuvant chemotherapy; it was
observed that there were significant differences in S100A4
expression prior to, during and after neoadjuvant chemotherapy,
particularly between the expression levels prior to and following
neoadjuvant chemotherapy (all P<0.05); and a reduction in S100A4
expression was associated with an enhanced chemotherapeutic effect.
Although the mechanism remains incompletely elucidated, it may
involve the following: i) S100A4 protein is secreted by tumor cells
and tumor-activated stromal cells; when neoadjuvant chemotherapy
was administered, tumor cells and tumor-activated stromal cells
were continually killed, leading to the decreased expression of
S100A4; ii) neoadjuvant chemotherapy destroys the original
structure of DNA so that genetic mutations or gene rearrangements
occur, resulting in the damage of the S100A4 gene, thereby reducing
the expression of S100A4 protein. However, further studies are
required to fully explore the underlying mechanism.
References
|
1
|
Rastogi P, Anderson SJ, Bear HD, Geyer CE,
Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil
SR, et al: Preoperative chemotherapy: Updates of national surgical
adjuvant breast and bowel project protocols B-18 and B-27. J Clin
Oncol. 26:778–785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schmidt-Hansen B, Klingelhöfer J,
Grum-Schwensen B, Christensen A, Andresen S, Kruse C, Hansen T,
Ambartsumian N, Lukanidin E and Grigorian M: Functional
significance of metastasis-inducing S100A4(Mts1) in tumor-stroma
interplay. J Biol Chem. 279:24498–24504. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garrett SC, Varney KM, Weber DJ and
Bresnick AR: S100A4, a mediator of metastasis. J Biol Chem.
281:677–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Emberley ED, Murphy LC and Watson PH: S100
proteins and their influence on pro-survival pathways in cancer.
Biochem Cell Biol. 82:508–515. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee WY, Su WC, Lin PW, Guo HR, Chang TW
and Chen HH: Expression of S100A4 and Met: Potential predictors for
metastasis and survival in early-stage breast cancer. Oncology.
66:429–438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rudland PS, Platt-Higgins A, Renshaw C,
West CR, Winstanley JH, Robertson L and Barraclough R: Prognostic
significance of the metastasis-inducing protein S100A4 (p9Ka) in
human breast cancer. Cancer Res. 60:1595–1603. 2000.PubMed/NCBI
|
|
7
|
Kikuchi N, Horiuchi A, Osada R, Imai T,
Wang C, Chen X and Konishi I: Nuclear expression of S100A4 is
associated with aggressive behavior of epithelial ovarian
carcinoma: An important autocrine/paracrine factor in tumor
progression. Cancer Sci. 97:1061–1069. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim JH, Kim CN, Kim SY, Lee JS, Cho D, Kim
JW and Yoon SY: Enhanced S100A4 protein expression is clinic
pathologically significant to metastatic potential and p53
dysfunction in colorectal cancer. Oncol Rep. 22:41–47. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agerbaek M, Alsner J, Marcussen N,
Lundbeck F and Von der Maase H: Focal S100A4 protein expression is
an independent predictor of development of metastatic disease in
cystectomized bladder cancer patients. Eur Urol. 50:777–785. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andersen K, Nesland JM, Holm R, Flørenes
VA, Fodstad Ø and Maelandsmo GM: Expression of S100A4 combined with
reduced E-cadherin expression predicts patient outcome in malignant
melanoma. Mod Pathol. 17:990–997. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taylor S, Herrington S, Prime W, Rudland
PS and Barraclough R: S100A4(p9Ka) protein in colon carcinoma and
liver metastases: Association with carcinoma cells and
T-lymphocytes. Br J Cancer. 86:409–416. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Komatsu K, Murata K, Kameyama M, Ayaki M,
Mukai M, Ishiguro S, Miyoshi J, Tatsuta M, Inoue M and Nakamura H:
Expression of S100A6 and S100A4 in matched samples of human
colorectal mucosa, primary colorectal adenocarcinomas and liver
metastases. Oncology. 63:192–200. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kriajevska M, Tarabykina S, Bronstein I,
Maitland N, Lomonosov M, Hansen K, Georgiev G and Lukanidin E:
Metastasis-associated Mts1(S100A4) protein modulates protein kinase
C phosphorylation of the heavy chain of nonmuscle myosin. J Biol
Chem. 273:9852–9856. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen ZW and Liao ML: RECIST criteria used
in evaluating the efficacy of cancer therapy. Zhong Guo Ai Zheng.
10:6–8. 2004.(In Chinese).
|
|
15
|
Corben AD, Abi-Raad R, Popa I, Teo CH,
Macklin EA, Koerner FC, Taghian AG and Brachtel EF: Pathologic
response and long-term follow-up in breast cancer patients treated
with neoadjuvant chemotherapy: A comparison between classifications
and their practical application. Arch Pathol Lab Med.
137:1074–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu G and Wang YS: Progress of
breast-conserving surgery after neoadjuvant chemotherapy for breast
cancer. Zhongguo Pu Wai Ji Chu Yu Lin Chuang Za Zhi. 17:1249–1252.
2010.(In Chinese).
|
|
17
|
Ring AE, Smith IE, Ashley S, Fulford LG
and Lakhani SR: Oestrogen receptor status, pathological complete
response and prognosis in patients receiving neoadjuvant
chemotherapy for early breast cancer. Br J Cancer. 91:2012–2017.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fisher ER, Wang J, Bryant J, Fisher B,
Mamounas E and Wolmark N: Pathbiology of preoperative chemotherapy:
Findings from the national surgical adjuvant breast and bowel
(NSABP) protocol B-18. Cancer. 95:681–695. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Zhang DL, Jiao XL and Dong Q:
S100A4 regulates migration and invasion in hepatocellular carcinoma
HepG2 cells via NF-κB-dependent MMP-9 signal. Eur Rev Med Pharmacol
Sci. 17:2372–2382. 2013.PubMed/NCBI
|
|
20
|
Helfman DM, Kim EJ, Lukanidin E and
Grigorian M: The metastasis associated protein S100A4: Role in
tumor progression and metastasis. Br J Cancer. 92:1955–1958. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Albertazzi E, Cajone F, Leone BE, Naguib
RN, Lakshmi MS and Sherbet GV: Expression of metastasis-associated
genes h-mts1 (S100A4) and nm23 in carcinoma of breast is related to
disease progression. DNA Cell Biol. 17:335–342. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang XS, Lin SS, Wang GY, Zou DL, Chen HS
and You Q: Correlation between S100A4 and E-Cad protein expression
and invasion, metastasis and prognosis of colorectal cancer.
Zhongguo Zhong Liu Lin Chuang. 36:690–693. 2009.(In Chinese).
|
|
23
|
Jin L, Shen Q, Ding S, Jiang W, Jiang L
and Zhu X: Immunohistochemical expression of Annexin A2 and S100A
proteins in patients with bulky stage IB-IIA cervical cancer
treated with neoadjuvant chemotherapy. Gynecol Oncol. 126:140–146.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ambartsumian N, Klingelhöfer J, Grigorian
M, Christensen C, Kriajevska M, Tulchinsky E, Georgiev G, Berezin
V, Bock E, Rygaard J, et al: The metastasis-associated Mts1
(S100A4) protein could act as an angiogenic factor. Oncogene.
20:4685–4695. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Q, Yu MH, Wang G and Wang MH:
Expression and clinical significance of DLL4 and S100A4 in
different molecular subtypes of breast carcinoma. Zhongguo Pu Wai
Ji Chu Yu Lin Chuang Za Zhi. 19:957–961. 2012.(In Chinese).
|
|
26
|
Chang JC, Wooten EC, Tsimelzon A,
Hilsenbeck SG, Gutierrez MC, Elledge R, Mohsin S, Osborne CK,
Chamness GC, Allred DC and O'Connell P: Gene expression profiling
for the prediction of therapeutic response to docetaxel in patients
with breast cancer. Lancet. 362:362–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hess KR, Anderson K, Symmans WF, Valero V,
Ibrahim N, Mejia JA, Booser D, Theriault RL, Buzdar AU, Dempsey PJ,
et al: Pharmacogenomic predictor of sensitivity to preoperative
chemotherapy with paclitaxel and fluorouracil, doxorubicin and
cyclpophosphamide in breast cancer. J Clin Oncol. 24:4236–4244.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li SJ, Han B, Fu T, Shi AP, Wu D, Liu GJ
and Fan ZM: The relationship between ER, PR and the expression of
HER-2 and breast cancer neoadjuvant chemotherapy reaction. Zhong
Guo Lao Nian Xue Za Zhi. 29:474–476. 2009.(In Chinese).
|
|
29
|
MacGrogan G, Mauriac L, Durand M, Bonichon
F, Trojani M, de Mascarel I and Coindre JM: Primary chemotherapy in
breast invasive carcinoma: Predictive value of the
immunohistochemical detection of hormonal receptors, p53, c-erbB-2,
MiB1, pS2 and GST pi. Br J Cancer. 74:1458–1465. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou QH, Wu Y, Cai ZR and Zhu JX:
Influence of neoadjuvant chemotherapy on ER, PR, C-erbB-2, Ki-67
expressions of breast cancer patients. Zhong Guo Ai Zheng Za Zhi.
18:139–141. 2008.(In Chinese).
|
|
31
|
Zhao YC, Li Y, Zhu YY and Luo CY:
Significance of expression changes of ER, PR, p53 and Bcl-2 induced
by neoadjuvant chemotherapy (NAC) in breast cancer. Xian Dai Zhong
Liu Yi Xue. 19:2017–2020. 2011.(In Chinese).
|