Introduction
Giant cell tumor of bone (GCT) is a rare and
commonly benign tumor that accounts for ~5% of primary bone tumors
and 15–20% of all benign bone tumors in adults (1,2). GCT
also exhibits a slight increase in prevalence in females and
usually occurs in the third and fourth decades of life (1). Surgical curettage is a preferred
treatment; however, it may be associated with a high local
recurrence rate (18–50%) and occasionally lung metastasis (3,4).
Recently, less-aggressive surgical resection, followed by extended
intralesional curettage and cementation (or bone graft) with or
without the use of adjuvant therapies, such as physical methods
(blurring, hypothermic or hyperthermic reagents), chemical methods
(phenol or hydrogen peroxide) and biologic modalities
(bisphosphonates, interferon or denosumab) have been used to
eliminate tumor remnants (1,3,5,6).
GCTs have been found to include three major cell
types: Multinucleated giant cells that express calcitonin
receptors, tartrate-resistant acid phosphatase activity and other
phenotypic osteoclast markers; a CD68-positive monocyte or
macrophage population; and mononuclear fibroblast-like stromal
cells that are able to proliferate in cell culture. Stromal cells
are likely to be the neoplastic components of this tumor and
regulate the formation of osteoclast-like giant cells in the
neoplasm (7).
Bone morphogenetic proteins (BMPs), members of the
transforming growth factor-β superfamily, were originally studied
as inducers of bone and cartilage formation and are also regulators
of human carcinoma cell, differentiation, proliferation,
morphogenesis and apoptosis (8–10). BMP-2
exhibits potent activity in the induction of cartilage and bone
formation in vivo and in vitro (11,12).
BMP-2 also plays key roles in cell proliferation, chemotaxis,
angiogenesis, apoptosis and differentiation (13–17). The
effects of BMP-2 are mediated via serine-threonine kinase
receptors: BMP receptor type 1A (BMPR1A), BMPR1B and BMPR2. When
BMPR2 is activated by binding to BMP-2, this induces the
phosphorylation of BMPR1A and the recruitment of downstream
signaling Smad1, Smad5 and Smad8 (receptor-regulated Smads), which
then form heteromeric complexes with Smad4 (common-mediator Smad),
and translocate to the nucleus to regulate the transcription of
target genes (18,19). In addition, non-Smad
mitogen-activated protein kinase (MAPK) pathways including p38,
c-jun-N-terminal kinase (JNK) and extracellular signal-regulated
kinase (ERK1/2) pathways, which are also important in cell
proliferation and differentiation, may be activated by BMP
(20–22).
Since its approval by the US Food and Drug
Administration (FDA) in 2002, recombinant human bone morphogenetic
protein-2 (rhBMP-2) has become one of the most commonly used bone
graft substitutes. A previous study revealed that GCT stromal cells
(GCTSCs) might have the ability to differentiate into osteoblasts
that are responsive to BMP-2 (23).
However, the role of BMP-2 in GCTSCs remains unclear. The
inhibitory effect of BMP-2 on GCTSC proliferation has been
investigated in only a few in vitro studies (23,24), and
no in vivo studies. Therefore, preclinical studies are
required to evaluate the effect of BMP-2 on tumor growth. The
purpose of the present study was to assess whether rhBMP-2 promotes
or suppresses GCT growth in vitro. The results may provide
background data useful in the evaluation of the potential of
rhBMP-2 as an adjuvant therapy for patients following the removal
of GCT by surgery.
Materials and methods
Specimens
This study was conducted in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of Guangzhou Liu Hua Qiao Hospital (Guangzhou, China). Written
informed consent was obtained from all participants. Nine GCT
specimens were freshly harvested and then used for primary cell
culture. None of the patients had taken any medication prior to the
surgery. Clinical information for each patient is not shown. The
initial diagnosis was established via frozen section in the
operating room and was later confirmed by permanent histological
examination. A board-certified pathologist reviewed each sample to
confirm the viability (>80% by nuclei counts of hematoxylin and
eosin-stained sections) and tumor content (>90%) of each sample.
Planned analyses were performed on each specimen as sample size
allowed.
Cell cultures
In brief, freshly obtained GCT tissues were chopped
up in Dulbecco's minimum essential medium (DMEM) containing 10%
fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml
streptomycin. The resultant cell suspension together with small
pieces of tissues was transferred to culture flasks and cultured at
37°C in a humidified atmosphere of 5% CO2 and 95% air.
Half of the culture medium was changed every 2–3 days. Upon
reaching confluence, primary cultures were then subcultured. GCTSCs
in cultures obtained after the 9th passage (Fig. 1) were studied.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay of cell proliferation
GCTSCs (1×105 cells/well) were plated and
cultured in 96-well plates with rhBMP-2 (R&D Systems, Inc.,
Minneapolis, MN, USA) at concentrations of 0, 10, 100 and 300 ng/ml
in DMEM containing 10% FBS for 1, 3, 5 or 7 days. MTT
(Sigma-Aldrich, St. Louis, MO, USA) was added to the culture medium
according to the manufacturer's instructions, and the culture was
continued for another 4 h. Dimethylsulfoxide (100 µl/well) was
added to each well to dissolve the formazan crystals. The optical
density of the resulting product was measured at 490 nm using a
microplate reader (Multiskan GO; Thermo Fisher Scientific, Waltham,
MA, USA).
Flow cytometric analysis
Cell cycle conditions were determined by
fluorescence-activated cell sorting (FACS) analysis using propidium
iodide (PI) staining. GCTSCs in the logarithmic phase of growth
were incubated in the presence or absence of rhBMP-2 (10 ng/ml) in
10% FBS DMEM for 48 h. The cells were then harvested and washed in
cold phosphate-buffered saline (PBS; pH 7.4). The cell pellets were
fixed in 70% cold alcohol for >24 h at 4°C, then washed in cold
PBS and stained with PI solution at 4°C in the dark for 30 min.
Apoptosis induced by rhBMP-2 in GCTSCs was determined by flow
cytometry using the Guava Nexin Reagent kit (EMD Millipore,
Billerica, MA, USA), according to the manufacturer's instructions.
Briefly, treated or untreated cells were collected, washed in cold
PBS and centrifuged at 200 × g for 5 min. The cell pellets were
resuspended in 100 ml DMEM supplemented with 1% FBS, and then
incubated with 100 ml Annexin V-PE and 7-aminoactinomycin D
labeling solution for 20 min at room temperature. Cells were
finally analyzed with a Guava EasyCyte 5HT flow cytometer (EMD
Millipore). The data were analyzed using Guava Nexin Software,
version 2.2.2 (EMD Millipore).
Western blot analysis
Cells were seeded in 6-well plates at a density of
1×106 cells/well. After being allowed to adhere
overnight, the cultures were cultured in the presence or absence of
rhBMP-2 (10 ng/ml) in 10% FBS DMEM for 72 h. After the treatment
period, cells were washed with PBS and then resuspended in lysis
buffer [1% NP-40, 1 mmol/l phenylmethylsulfonyl fluoride, 40 mmol/l
Tris-HCl (pH 8.0), 150 mmol/l NaCl, 100 mmol/l
Na3VO4, 1 mmol/l NaF] at 4°C for 15 min.
Protein concentrations were determined by means of a Bio-Rad
protein assay (Bio-Rad, Richmond, CA, USA). The proteins were
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to polyvinylidene
difluoride (PVDF) membranes (EMD Millipore). After blocking with 5%
non-fat dry milk, each blot was probed with a primary antibody
(1:1,000) directed against ERK1/2 (rabbit monoclonal; cat. no.
4695), p38 MAPK (rabbit monoclonal; cat. no. 8690), JNK (rabbit
monoclonal; cat. no. 9252), phospho-Erk1/2 (rabbit monoclonal; cat.
no. 4094), phospho-p38 MAPK (rabbit monoclonal; cat. no. 4511) or
phospho-JNK (mouse monoclonal; cat. no. 9255) (Cell Signaling
Technology, Danvers, MA, USA) at 4°C overnight. Subsequently, the
membranes were washed three times (5 min/wash) with Tris-buffered
saline containing 0.05% Tween-20 (TBST). The membranes were then
incubated for 30 min at room temperature with a
peroxidase-conjugated AffiniPure goat anti-rabbit IgG secondary
antibody (1:3,000; cat. no. 111-035-003; Jackson Immunoresearch
Laboratories, Inc., West Grove, PA, USA). The membranes were washed
a further three times with TBST and incubated with Super Signal
Enhanced Chemiluminescence substrate (Detection Reagents 1 and 2 at
a 1:1 ratio; Pierce Biotechnology, Inc., Rockford, IL, USA) for 1
min at 25°C. After removing the excess mixture, the blots were
wrapped in a clean piece of plastic wrap, ensuring no bubbles were
present between the blot and wrap. The blots were then exposed for
30–300 sec to X-ray film (Eastman Kodak, Rochester, NY, USA). Band
intensities were quantified using Quantity One software (v. 4.4.0;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) by two observers who
were blind to the experimental groups.
Statistical analysis
The results are expressed as the mean ± standard
deviation. The statistical analyses were conducted using SPSS 19.0
(IBM SPSS, Armonk, NY, USA) statistical software. The photometric
values obtained in MTT assays were analyzed by one-way analysis of
variance, with post-hoc multiple comparisons made between groups
using a least significant difference test. The comparisons between
cell cycle alterations, cell apoptosis, and the expression of
Erk1/2, p38 and JNK in the rhBMP2 (10 ng/ml) and control groups
were conducted using independent sample t-tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
MTT assay
As shown in Fig. 2,
growth of the GCTSCs was significantly inhibited by the addition of
10 or 100 ng/ml rhBMP-2 (P<0.01) or 300 ng/ml rhBMP-2
(P<0.05) for 5 days compared with the control. The growth of
GCTSCs was significantly inhibited compared with the control when
treated with all three concentrations of rhBMP-2 for 7 days
(P<0.001), whereas no growth inhibition of GCTSCs compared with
the control was observed following the addition of 10, 100 or 300
ng/ml rhBMP-2 for 1 or 3 days. Treatment of GCTSCs with 100 or 300
ng/ml rhBMP-2 for 1 day induced a slight stimulation of cell
growth, but not significantly.
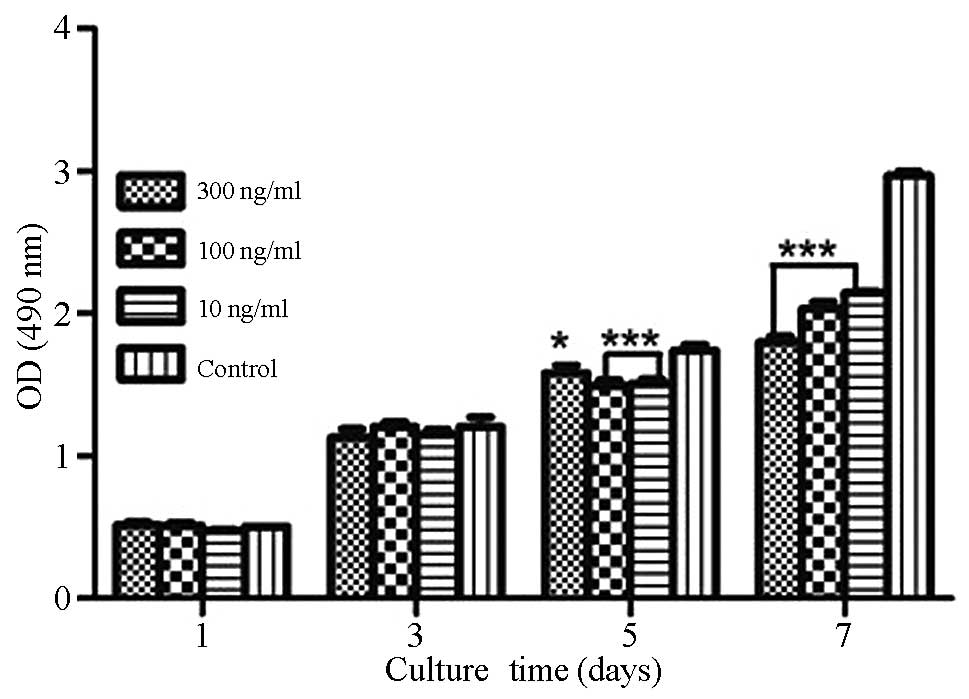 | Figure 2.Effects of different concentrations of
rhBMP-2 on GCTSC proliferation as evaluated using an MTT assay.
GCTSCs were incubated with different concentrations of rhBMP-2 in
DMEM containing 10% FBS for 1, 3, 5 or 7 days. Data are presented
as the mean ± standard deviation (n=3). *P<0.05, ***P<0.01
vs. control. rhBMP-2, recombinant human bone morphogenetic
protein-2; GCTSC, giant cell tumor of bone stromal cell; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; DMEM,
Dulbecco's minimum essential medium; FBS, fetal bovine serum; OD,
optical density. |
Effects of rhBMP-2 on GCTSC apoptosis
and cell cycle distribution
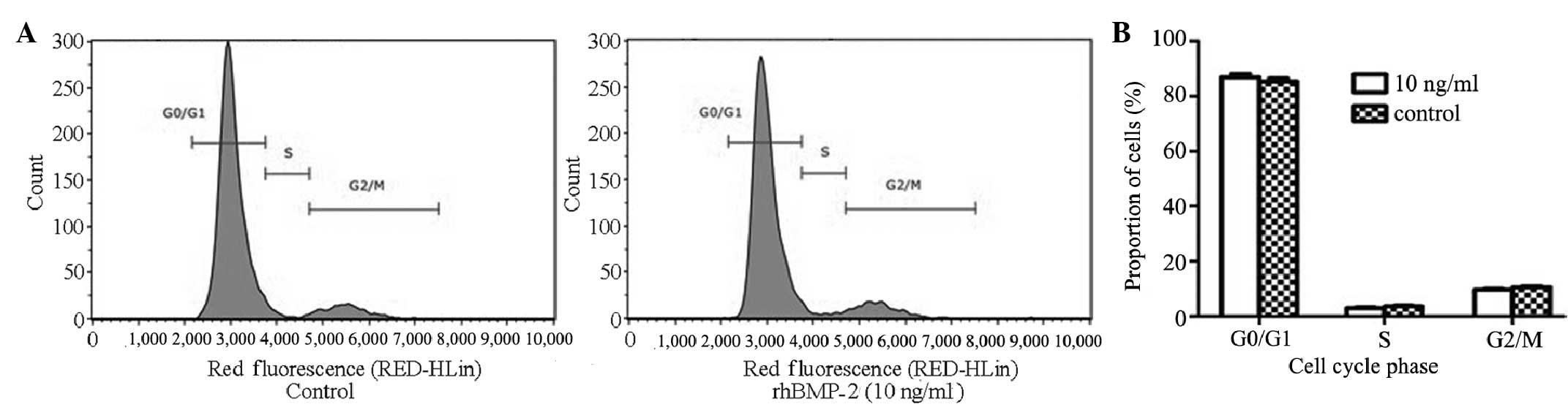
To determine whether BMP-2 affected the cell cycle
distribution of GCTSCs, the GCTSCs were incubated with rhBMP-2 (10
ng/ml) for 48 h and analyzed using flow cytometry (Fig. 3). The cell cycle kinetics
demonstrated that there was no significant difference between the
control and rhBMP-2 (10 ng/ml) treatment groups in the percentage
of cells in the G0/G1, S and G2/M phases. The inhibition of
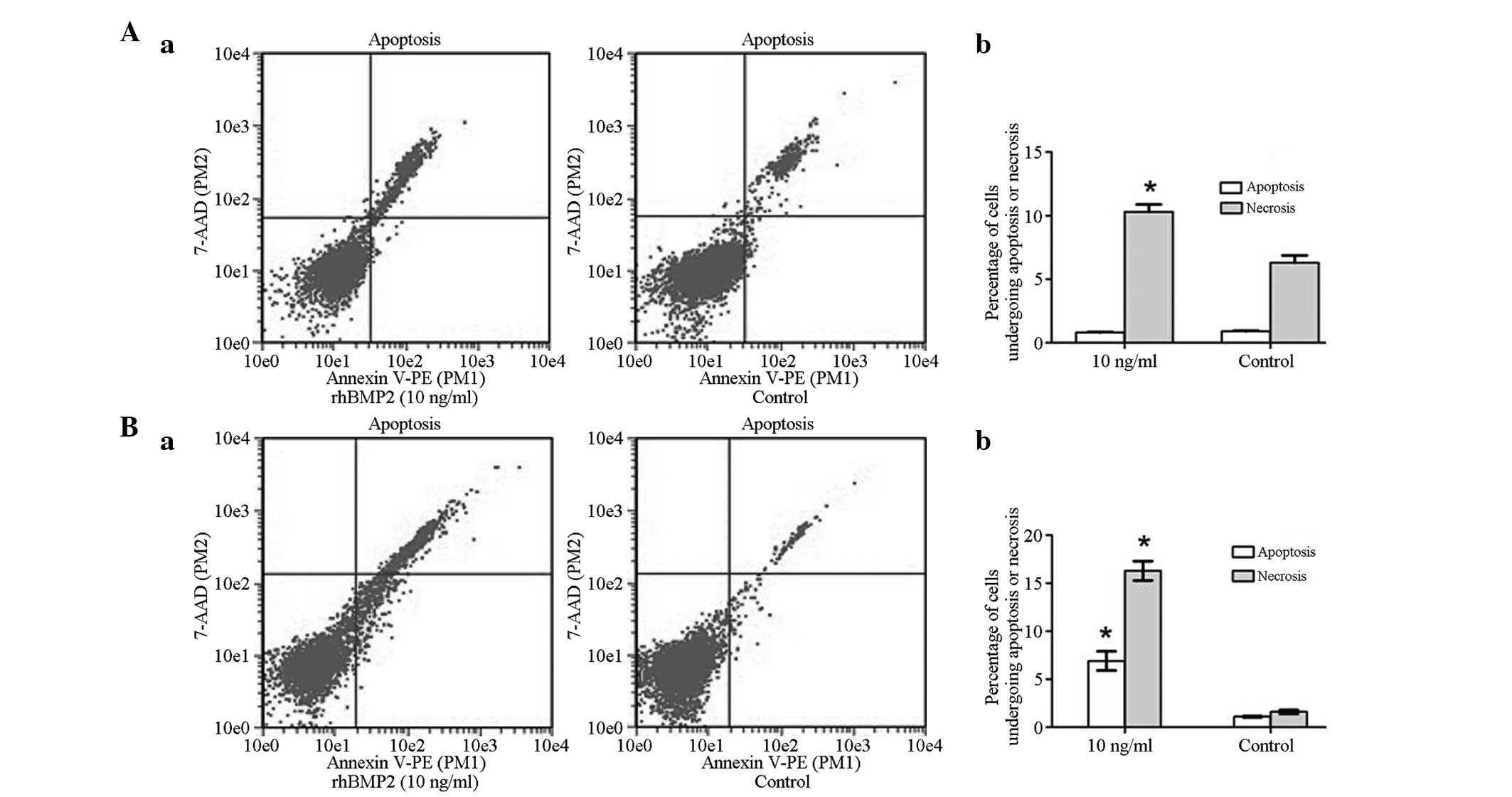
apoptosis is a critical factor for tumor progression. Therefore,
Annexin V-PE/PI staining was evaluated by flow cytometry. Following
culture of the GCTSCs with rhBMP-2 (10 ng/ml) for 48 h, the
percentage of apoptotic cells was very similar to that in the
untreated control group, indicating that rhBMP-2 did not change the
incidence of apoptosis over the 48-h treatment period. However, the
percentage of necrotic cells markedly increased in the BMP-2 group
compared with the control group (*P<0.05; Fig. 4A). Notably, after 72 h of rhBMP-2
treatment, the percentage of apoptotic and necrotic cells was
significantly increased in the BMP-2 group compared with the
control group (**P<0.01; Fig.
4B). Indeed, these data indicate that rhBMP-2 increased the
susceptibility of GCTSCs to apoptosis, which corresponds with the
results of the MTT assay.
Western blot analysis
To clarify the mechanisms underlying the effects of
BMP-2 on GCTSCs, the expression of non-Smad MAPK pathway-associated
proteins, including p38, JNK and ERK1/2 were examined. As shown in
Fig. 5, following the addition of
rhBMP-2 (10 ng/ml) to GCTSCs for 72 h, the levels of p38, JNK and
ERK1/2 detected in exponentially growing GCTSCs were similar to
those in the control group, but the expression levels of
phospho-p38, phospho-ERK1/2 and phospho-JNK were significantly
increased in the rhBMP-2-treated GCTSCs compared with those in the
control group.
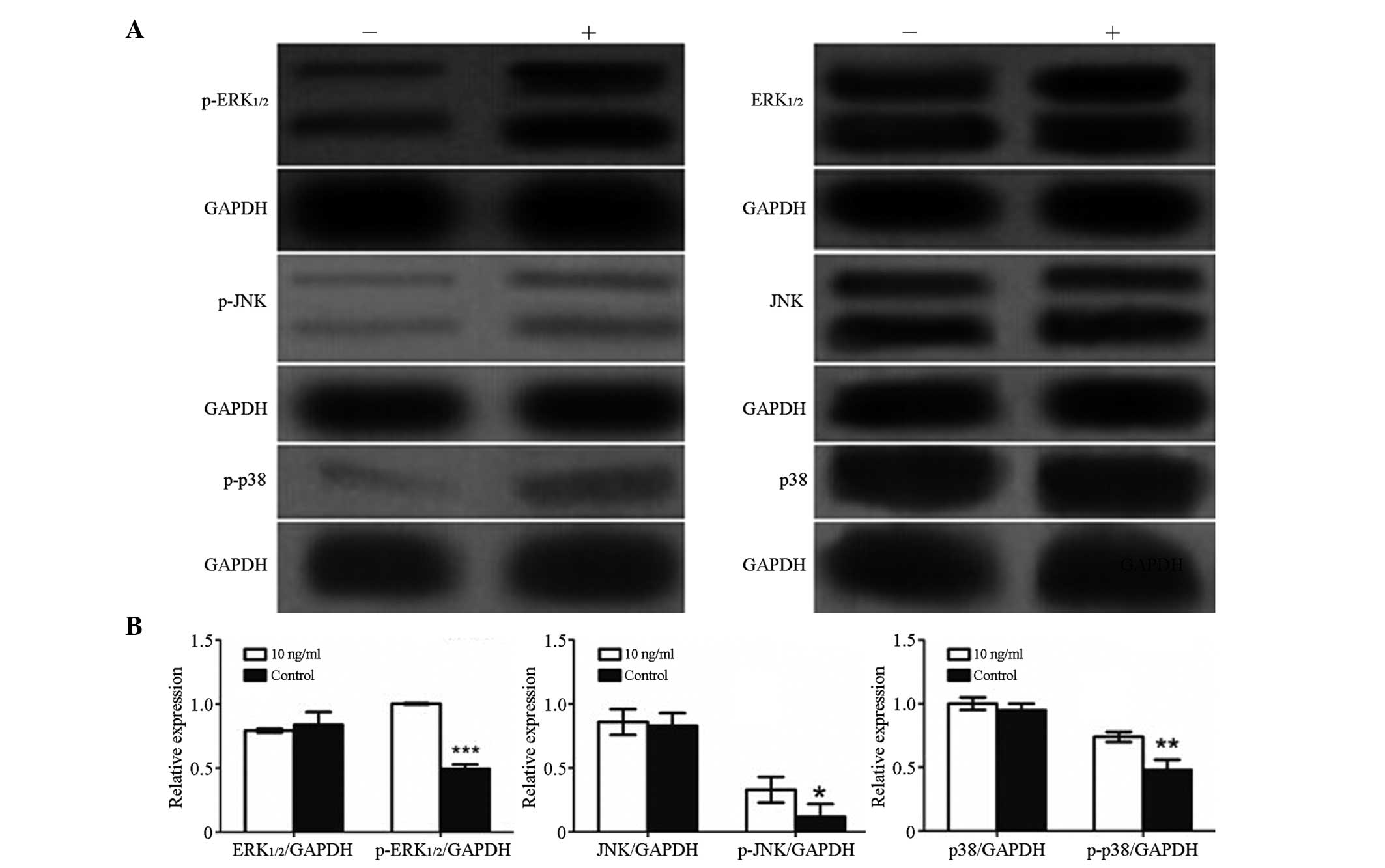 | Figure 5.Effects of the treatment of GCTSCs
with rhBMP-2 (10 ng/ml) for 72 h on BMP signaling pathways. (A)
Western blot analysis of the activation of the signaling pathways
in GCTSCs. GAPDH served as the internal control; + indicates
treated with rhBMP-2 (10 ng/ml) and - indicates control. (B) Bar
plots indicating the relative expression of protein in GCTSCs
between the control and rhBMP-2-treated groups. Expression levels
of p-ERK1/2, p-p38 and p-JNK increased significantly following
treatment with rhBMP-2, but the levels of ERK1/2, p38 and JNK were
unchanged. Results are means ± SD of three independent experiments.
*P<0.05, **P<0.01, ***P<0.001 vs. the control group.
rhBMP-2, recombinant human bone morphogenetic protein-2; GCTSC,
giant cell tumor of bone stromal cell; p, phospho; ERK,
extracellular signal-regulated kinase; JNK, c-Jun-N-terminal
kinase. |
Discussion
GCT is a common benign tumor of bone in adults. The
use of bone cement with or without phenol or other toxic substances
has been widely used as effective adjuvant therapy following the
surgical curettage of GCT (3,5,6,25–28).
rhBMP-2 is an osteoinductive growth factor that can promote bone
formation, and is widely used in fracture nonunions and spine
fusion for the treatment of degenerative spinal disorders. Numerous
previous studies have shown that rhBMP-2 has a dual role in tumor
biology: it functions as a tumor promoter or a tumor suppressor,
depending on the type of cell or tissue, the BMP-2 dosage and the
presence of other factors that are not yet defined in the
microenvironment (10,13–17,29,30).
This behavior suggests that in different types of tumor, it acts on
a type of cellular homeostatic mechanism through as yet unknown
regulatory signaling pathways, such as non-Smad pathways (19–22). For
these reasons, surgeons may hesitate to use BMP-2 on their patients
for fracture healing or spine fusion. Therefore, further
preclinical studies are required to evaluate the effect of BMP-2 on
the growth of GCTs to guide its use in patients.
The data from the present study provide the first
evidence that BMP-2 has a significant inhibitory effect on
tumorigenic GCTSC proliferation at lower concentrations (10 and 100
ng/ml) of rhBMP-2, as compared with a higher concentration (300
ng/ml), for 5 days in vitro. Following treatment for 7 days,
the different concentrations of rhBMP-2 exerted similar inhibitory
effects on tumorigenic GCTSC proliferation compared with that in
the control group. This result is consistent with previous studies
that have shown an inhibitory effect of BMP-2 on cancer cell
growth, including prostate, breast, myeloma, gastric and colon
cancers (13,14,16,17,31–34).
However, the present study confirmed in vitro that rhBMP-2
inhibits GCTSC proliferation in a non-dose- and time-dependent
manner. In contrast with previous studies which indicated through
flow cytometric analysis that the inhibitory effect of BMP-2 on
cell growth was due to G1 phase arrest (13,14), the
present study showed that there was no difference in the percentage
of cells in each phase of the cell cycle between the untreated
GCTSCs and those treated with rhBMP-2 for 48 h.
Therefore, the present study confirmed that BMP-2
inhibits cell growth in vitro by inducing apoptosis in
GCTSCs. The observed increase in cell apoptosis may be associated
with the upregulation of phospho-p38, phospho-ERK1/2 and
phospho-JNK, and the stimulation of MAPK signaling pathways.
In conclusion, BMP-2 inhibited GCTSC proliferation
through the induction of apoptosis. The results demonstrate that
rhBMP-2 is suitable for use as an antineoplastic therapeutic agent
for the treatment of GCT.
Acknowledgements
This study was sponsored by the National Nature
Science Foundation of China (grant no. 81172012) and Guangdong
Natural Science Foundation (grant no. S2011010001062).
References
|
1
|
Mendenhall WM, Zlotecki RA, Scarborough
MT, Gibbs CP and Mendenhall NP: Giant cell tumor of bone. Am J Clin
Oncol. 29:96–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gamberi G, Serra M, Ragazzini P, Magagnoli
G, Pazzaglia L, Ponticelli F, Ferrari C, Zanasi M, Bertoni F, Picci
P and Benassi MS: Identification of markers of possible prognostic
value in 57 giant cell tumors of bone. Oncol Rep. 10:351–356.
2003.PubMed/NCBI
|
|
3
|
Trieb K, Bitzan P, Lang S, Dominkus M and
Kotz R: Recurrence of curetted and bone-grafted giant-cell tumours
with and without adjuvant phenol therapy. Eur J Surg Oncol.
27:200–202. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Campanacci M, Baldini N, Boriani S and
Sudanese A: Giant-cell tumor of bone. J Bone Joint Surg Am.
69:106–114. 1987.PubMed/NCBI
|
|
5
|
Tse LF, Wong KC, Kumta SM, Huang L, Chow
TC and Griffith JF: Bisphosphonates reduce local recurrence in
extremity giant cell tumor of bone: A case-control study. Bone.
42:68–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gibbs CP, Lewis VO and Peabody T: Beyond
bone grafting: Techniques in the surgical management of benign bone
tumors. Instr Course Lect. 54:497–503. 2005.PubMed/NCBI
|
|
7
|
Zheng MH, Robbins P, Xu J, Huang L, Wood
DJ and Papadimitriou JM: The histogenesis of giant cell tumour of
bone: A model of interaction between neoplastic cells and
osteoclasts. Histol Histopathol. 16:297–307. 2001.PubMed/NCBI
|
|
8
|
Hopkins DR, Keles S and Greenspan DS: The
bone morphogenetic protein 1/Tolloid-like metalloproteinases.
Matrix Biol. 26:508–523. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Senta H, Park H, Bergeron E, Drevelle O,
Fong D, Leblanc E, Cabana F, Roux S, Grenier G and Faucheux N: Cell
responses to bone morphogenetic proteins and peptides derived from
them: Biomedical applications and limitations. Cytokine Growth
Factor Rev. 20:213–222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Singh A and Morris RJ: The Yin and Yang of
bone morphogenetic proteins in cancer. Cytokine Growth Factor Rev.
21:299–313. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barboza E, Caúla A and Machado F:
Potential of recombinant human bone morphogenetic protein-2 in bone
regeneration. Implant Dent. 8:360–367. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hogan BL: Bone morphogenetic proteins:
Multifunctional regulators of vertebrate development. Genes Dev.
10:1580–1594. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen A, Wang D, Liu X, He S, Yu Z and Wang
J: Inhibitory effect of BMP-2 on the proliferation of breast cancer
cells. Mol Med Rep. 6:615–620. 2012.PubMed/NCBI
|
|
14
|
Ye S, Park BH, Song KJ, Kim JR, Jang KY,
Park HS, Bae JS, Brochmann EJ, Wang JC, Murray SS and Lee KB: In
vivo inhibition of bone morphogenetic protein-2 on breast cancer
cell growth. Spine (Phila Pa 1976). 38:E143–E150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Langenfeld EM, Kong Y and Langenfeld J:
Bone morphogenetic protein 2 stimulation of tumor growth involves
the activation of Smad-1/5. Oncogene. 25:685–692. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kokorina NA, Lewis JS Jr, Zakharkin SO,
Krebsbach PH and Nussenbaum B: rhBMP-2 has adverse effects on human
oral carcinoma cell lines in vivo. Laryngoscope. 122:95–102. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Molina CA, Sarabia-Estrada R, Gokaslan ZL,
Witham TF, Bydon A, Wolinsky JP and Sciubba DM: Delayed onset of
paralysis and slowed tumor growth following in situ placement of
recombinant human bone morphogenetic protein 2 within spine tumors
in a rat model of metastatic breast cancer. J Neurosurg Spine.
16:365–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haÿ E, Lemonnier J, Fromigué O, Guénou H
and Marie PJ: Bone morphogenetic protein receptor IB signaling
mediates apoptosis independently of differentiation in osteoblastic
cells. J Biol Chem. 279:1650–1658. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kimura N, Matsuo R, Shibuya H, Nakashima K
and Taga T: BMP2-induced apoptosis is mediated by activation of the
TAK1-p38 kinase pathway that is negatively regulated by Smad6. J
Biol Chem. 275:17647–17652. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee KS, Hong SH and Bae SC: Both the Smad
and p38 MAPK pathways play a crucial role in Runx2 expression
following induction by transforming growth factor-beta and bone
morphogenetic protein. Oncogene. 21:7156–7163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moustakas A and Heldin CH: Non-Smad
TGF-beta signals. J Cell Sci. 118:3573–3584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guicheux J, Lemonnier J, Ghayor C, Suzuki
A, Palmer G and Caverzasio J: Activation of p38 mitogen-activated
protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their
implication in the stimulation of osteoblastic cell
differentiation. J Bone Miner Res. 18:2060–2068. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang L, Teng XY, Cheng YY, Lee KM and
Kumta SM: Expression of preosteoblast markers and Cbfa-1 and
Osterix gene transcripts in stromal tumour cells of giant cell
tumour of bone. Bone. 34:393–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kudo N, Ogose A, Ariizumi T, Kawashima H,
Hotta T, Hatano H, Morita T, Nagata M, Siki Y, Kawai A, et al:
Expression of bone morphogenetic proteins in giant cell tumor of
bone. Anticancer Res. 29:2219–2225. 2009.PubMed/NCBI
|
|
25
|
Su YP, Chen WM and Chen TH: Giant-cell
tumors of bone: An analysis of 87 cases. Int Orthop. 28:239–243.
2004.PubMed/NCBI
|
|
26
|
Saiz P, Virkus W, Piasecki P, Templeton A,
Shott S and Gitelis S: Results of giant cell tumor of bone treated
with intralesional excision. Clin Orthop Related Res. 424:221–226.
2004. View Article : Google Scholar
|
|
27
|
Ward WG Sr and Li G: Customized treatment
algorithm for giant cell tumor of bone: Report of a series. Clin
Orthop Relat Res. 397:259–270. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Turcotte RE, Wunder JS, Isler MH, Schachar
N, Masri BA, Moreau G and Davis AM: Canadian Sarcoma Group: Giant
cell tumor of bone. Giant cell tumor of long bone: A Canadian
sarcoma group study. Clin Orthop Relat Res. 397:248–258. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kleeff J, Maruyama H, Ishiwata T, Sawhney
H, Friess H, Büchler MW and Korc M: Bone morphogenetic protein 2
exerts diverse effects on cell growth in vitro and is expressed in
human pancreatic cancer in vivo. Gastroenterology. 116:1202–1216.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ide H, Yoshida T, Matsumoto N, Aoki K,
Osada Y, Sugimura T and Terada M: Growth regulation of human
prostate cancer cells by bone morphogenetic protein-2. Cancer Res.
57:5022–5027. 1997.PubMed/NCBI
|
|
31
|
Beck SE, Jung BH, Fiorino A, Gomez J,
Rosario ED, Cabrera BL, Huang SC, Chow JY and Carethers JM: Bone
morphogenetic protein signaling and growth suppression in colon
cancer. Am J Physiol Gastrointest Liver Physiol. 291:G135–G145.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wen XZ, Miyake S, Akiyama Y and Yuasa Y:
BMP-2 modulates the proliferation and differentiation of normal and
cancerous gastric cells. Biochem Biophys Res Commun. 316:100–106.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brubaker KD, Corey E, Brown LG and
Vessella RL: Bone morphogenetic protein signaling in prostate
cancer cell lines. J Cell Biochem. 91:151–160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pouliot F, Blais A and Labrie C:
Overexpression of a dominant negative type II bone morphogenetic
protein receptor inhibits the growth of human breast cancer cells.
Cancer Res. 63:277–281. 2003.PubMed/NCBI
|