Introduction
Alzheimer's disease (AD) is an age-associated,
progressive neurodegenerative disorder, which is characterized by
memory impairment and cognitive decline (1). Pathologically, AD is characterized by
an abnormal deposition of β-amyloid (Aβ) protein, which initiates a
series of events, including the formation of senile plaques,
neurofibrillary tangles and neuropil threads, and glial cell
activation and neuronal cell loss (2–4). In
addition, the number of cholinergic neurons, and the levels of
acetylcholine, has been shown to decrease with concurrent decreases
in the levels of the enzyme choline acetyltransferase (ChAT)
(5). These alterations have
previously been suggested to occur due to dysfunctions in the
serotonergic regulation of cholinergic neuronal cell activity
(5).
Ovarian reproductive hormones are potent regulators
of neuronal cell survival in the central nervous system (CNS)and of
various biological processes, including development and neural
injury (6–7). In particular, 17 β-estradiol (E2) and
progesterone (P4) have been demonstrated to exert effects on
cognitive functions (5,7–9), and P4
was reported to have promoted neuron survival by protecting
neuronal cells against damage following brain injury (10). However, controversy exists regarding
the beneficial effects of ovarian hormones on cognition during
aging (11,12). Previous studies have suggested that
hormone treatment may enhance memory in menopausal women; however,
neither beneficial nor detrimental effects have been detected in
other studies (12,13).
The present study aimed to investigate the
mechanisms underlying the effects of ovarian hormones on learning
and memory in a rat model of AD. The rat model was induced via
central injection of aggregated Aβ1–40, which has been used in
previous in vivo studies (1,2). An
ovariectomized (OVX) rat model of AD was established in order to
eliminate complications associated with endogenously produced E2
(14,15). The Morris water maze test (16) was used in order to assess the effects
of E2 and P4 on spatial learning and memory functions in the rat
model of AD. In addition, the apoptosis of neuronal cells and the
protein expression levels of 5-hydroxytryptamine (5-H2, serotonin)
receptor 2A (5-HT2A), glial fibrillary acidic protein (GFAP) and
ChAT, were assessed in the hippocampus of the rats, in order to
establish whether improvements in memory were associated with
neuroprotective mechanisms.
Materials and methods
Rats and agents
A total of 40 female Sprague-Dawley rats, aged 10
weeks and weighing, 250–300 g, were used (Zhejiang University
Laboratory Animal Breeding and Research Center, Hangzhou, China).
The rats were maintained under a 12 h light/dark cycle, with ad
libitum access to food and water. Experiments were carried out
in accordance with the National Institutes of Health Guidelines for
the Care and Use of Laboratory Animals (1), and with approval from the Animal
Subjects Committee at Zhejiang University (Zhejiang, China). Aβ1–40
protein, which was purchased from Sigma-Aldrich (St. Louis, MO,
USA), was dissolved in 0.9% (w/v) saline (final concentration, 5
mg/ml) and incubated at 37°C for 1 week in order to induce
aggregation (1,2).
Surgery and treatment with agents
Rats were anesthetized using an intraperitoneal
injection of 40 mg/kg Nembutal (Merck Millipore, Darmstadt,
Germany), and placed into a stereotaxic frame (Stoelting, Co., Wood
Dale, IL, USA). A sham-operated group (n=8) received a bilateral
injection (coordinates: −3.0 mm anteroposterior, −2.0 mm lateral to
the bregma, and −3.3 mm ventral to the skull surface) of saline (2
µl, over 5 min), whereas ovariectomized rats (n=8)received 1 µl
aggregated Aβ1–40 (10 µg/µl, over 5 min) into the hippocampus
(17). After 1 day, the rats were
anaesthetized using halothane (3% induction followed by 1%
maintenance) (Sigma-Aldrich), an abdominal incision was made
through the skin of the flank of the rats, and the left and right
ovaries were isolated by ligation of the most proximal portion of
the oviduct. Both ovaries and the ovarian fat were removed in
ovariectomized rats. The abdominal incision was performed on the
sham rats; however, the wound was closed without removal of the
ovaries.
Four days following surgery, the Aβ1–40
aggregate-injected ovariectomized animals were randomly assigned
into four groups (n=8/group): i) OVX + vehicle (vehicle-treated),
ii) OVX + E2 (E2-treated), iii) OVX + P4 (P4-treated), and iv) OVX
+ E2 + P4 (P4 + E2-treated). The E2-treated group received daily
subcutaneous injections of E2 (1 mg/kg) for 25 days (15). The P4-treated group received a total
of four subcutaneous injections of P4 (5 mg/kg), every 7 days. The
P4 + E2-treated group received both treatments, and the vehicle
group received daily subcutaneous injections of sterile sesame oil
for 25 days.
Morris water maze test
The effects of the ovarian hormones on the spatial
cognitive performance of the AD rats was investigated using the
Morris water maze test (16), in
which the time taken for the rats to locate a submerged platform in
a water maze (the escape latency) was used as an indication of
spatial memory. In addition, alterations to the escape latency over
consecutive days were used to assess learning with respect to
long-term memory. Briefly, a circular polyester tank (diameter, 140
cm; height, 50 cm) was filled with water to a depth of 30 cm and
maintained at 20±1°C. The maze was divided geographically into four
equal quadrants and release points in each quadrant were designated
as North, East, South, or West. An escape platform measuring 15×15
cm was submerged 2 cm below the water surface in the center of one
quadrant of the pool throughout the training and memory trials.
Testing began 3 weeks after the rats were treated with Aβ1–40 or
vehicle. All rats underwent initial training sessions consisting of
6 trials per day for 4 consecutive days. In each trial, the rats
were placed at one of the four starting points and allowed to swim
freely until they located the platform. The four starting points
were ordered in a random manner between trials. Probe tasks were
performed on the 5th day following training. The rats
were allowed to swim for 120 sec to locate the platform, on which
they were allowed to rest for 20 sec. Those rats unable to locate
the platform were guided to it. Swim paths were recorded using a
digital video camera (STC-TB33USB-AS; Sentech Co., Ltd., Japan) and
analyzed using EthoVision 3.1 software (Noldus Information
Technology, Leesburg, VA, USA).
Tissue preparation
Four rats per group were given a lethal dose of
Nembutal (60 mg/kg body weight) 30 days post-Aβ1–40 injection.
Following sacrifice, the rats were perfused intracardially with
saline and then with 4% paraformaldehyde in 0.1 M phosphate buffer
(pH 7.4). Subsequently, whole brains were carefully removed and
dissected. Brain tissue was post-fixed at 4°C in 4%
paraformaldehyde overnight and then incubated with 30% (w/v)
sucrose in phosphate-buffered saline (PBS) until the tissue dropped
to the bottom of the container. Each brain was dissected into five
sets of serial coronal sections through the hippocampal area using
a cryostat at −20°C. Each fifth section was collected for free
floating immunohistochemical labeling and terminal deoxynucleotidyl
transferase dUTP nick end labeling (TUNEL) assay. Tissue sections
(20 µm) were mounted onto gel-coated slides, upon which coverslips
were mounted using Permount™ (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
An additional four rats per group were sacrificed by
decapitation, after which the brains were carefully harvested, the
hippocampus was dissected, and was immediately frozen in liquid
nitrogen for western blotting.
Immunohistochemical labeling
All labeling experiments were performed in 24-well
culture plates at room temperature. The sections were incubated in
3% H2O2/10% methanol/0.01 M PBS for 5 min in
order to quench endogenous peroxidase, and were then blocked in 5%
normal goat serum supplemented with 0.01 M PBS. Subsequently, the
tissue sections were incubated with polyclonal rabbit primary
anti-ChAT (1:250; AB144P; Chemicon International, Inc., Temecula,
CA, USA), anti-5HT2A (1:500; sc-15073; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), anti-GFAP (1:200; 18–0063; Thermo Fisher
Scientific, Inc.) and anti-caspase-3 (1:500; 160745; Cayman
Chemical, Ann Arbor, MI, USA) overnight at 4°C. After a thorough
wash in PBS, the sections were incubated with biotinylated goat
anti-rabbit immunoglobulin (Ig) G (1:400; Vector Laboratories,
Inc., Burlingame, CA, USA) for 1 h at room temperature, followed by
incubation with an avidin-biotin peroxidase complex (Vectastain
Elite ABC Kit; 1:300; Vector Laboratories, Inc.) for 1.5 h at room
temperature. The reaction product was developed with 0.025%
3,3-diaminobenzidine (DAB) and 0.0033% H2O2
in 0.2 M Tris-HCl (pH 7.6), after which the reaction was quenched
by rinsing the slides with 0.2 M Tris-HCl.
The ChAT, 5HT2A and caspase-3 labeled sections were
counterstained with hematoxylin, and then mounted onto 0.02%
poly-L-lysine-coated slides and allowed to dry at room temperature.
Subsequently, the tissue sections were dehydrated using a graded
series of alcohol, cleared in xylene and cover-slipped. Primary
antibody omission controls were used in order to confirm the
specificity of the immunohistochemical labeling. Five sections from
each animal were selected at random and images were captured under
200× using a Nikon E600 microscope (Nikon Corporation)
magnification in three visual fields/per section (Olympus
Corporation, Tokyo, Japan). The ChAT, 5HT2A, GFAP and caspase-3
immunoreactive cells in each hippocampal region were counted.
Western blot analysis
Prior to total protein extraction, the brain tissue
was homogenized in homogenization buffer containing 10 mM Tris (pH
7.4), 150 mM NaCl, 1% Triton X-100, 2 mM phenylmethylsulfonyl
fluoride, 1 ml ice-cold radioimmunoprecipitation assay buffer
(EDTA-free; both Thermo Fisher Scientific, Inc.) 1 mg/ml pepstatin
A, 1 mg/ml aprotinin and 1 mg/ml leupeptin (Roche Diagnostics,
Basel, Switzerland), using a Polytron® PTA 10S homogenizer
(Kinematica, Inc., CA, USA). Protein concentrations were determined
using the Bradford Protein assay (Beyotime Institute of
Biotechnology, Haimen, China). Proteins were separated by 12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and were
transferred to polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA) at 70 V for 1.5 h at 4°C using Bio-Rad
TransBlot® Turbo™ apparatus (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Subsequently, the membranes were blocked for 1
h at room temperature with 5% (w/v) nonfat milk in Tris-buffered
saline (TBS) supplemented with 0.05% (v/v) Tween 20 (50 mM Tris
base, 200 mM NaCl, 0.05% Tween 20), and then incubated for 12 h
with polyclonal rabbit anti-ChAT (1:500; Chemicon International,
Inc.), anti-5HT2A (1:800; Santa Cruz Biotechnology, Inc.), and
anti-GFAP (1:500; Thermo Fisher Scientific, Inc.) primary
antibodies. The membranes were then washed three times for 10 min
using TBS supplemented with 0.05% Tween 20, and subsequently
incubated for 1 h with secondary horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (1:5,000; Santa Cruz
Biotechnology, Inc.), prior to further washing. Proteins were
detected using enhanced chemiluminescence (ECL reagents; GE
Healthcare Life Sciences, Chalfont, UK) and exposed to radiographic
film (Hyperfilm ECL; GE Healthcare Life Sciences), according to the
manufacturer's protocol.
The densitometry of the immunoreactive bands of
interest was determined via digital images of the radiographic film
using Quantity One 1-D software (Bio-Rad Laboratories, Inc.). In
order to normalize protein bands to a gel loading control, the
membranes were washed in TBS-Tween 20 and probed with rabbit
anti-β-actin (1:5,000; Abcam, Cambridge, MA, USA), followed by
incubation with HRP-conjugated goat anti-rabbit (1:5,000; Santa
Cruz Biotechnology, Inc.) and ECL detection. For the negative
control, the primary antibody was omitted.
Apoptosis assay
TUNEL assays were performed according to the
manufacturer's protocol (Promega Corporation, Madison, WI, USA).
Briefly, tissue sections from each group were fixed with 1%
paraformaldehyde in PBS for 30 min, permeabilized with 0.2% Triton
X-100 in PBS for 5 min, and then rinsed with PBS and incubated with
TUNEL reaction mixture (enzyme, nucleotides) in a humidified
atmosphere at 37°C for 1 h. Staining was then performed using
DAB/H2O2 at 37°C for 5 min. Finally, the
sections were counterstained with methyl green (Santa Cruz
Biotechnology, Inc.) and analyzed under 400× magnification using a
Nikon E600 microscope (Nikon Corporation) in 5 vision fields/per
section. Labeled cells in the hippocampus were counted.
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical significance was analyzed using one-way analysis of
variance with post-hoc Tukey t-tests. P<0.05 was considered to
indicate a statistically significant difference. All statistical
analyses and graphs were performed or generated using GraphPad
Prism Version 4.0 software (GraphPad Software, Inc., La Jolla, CA,
USA).
Results
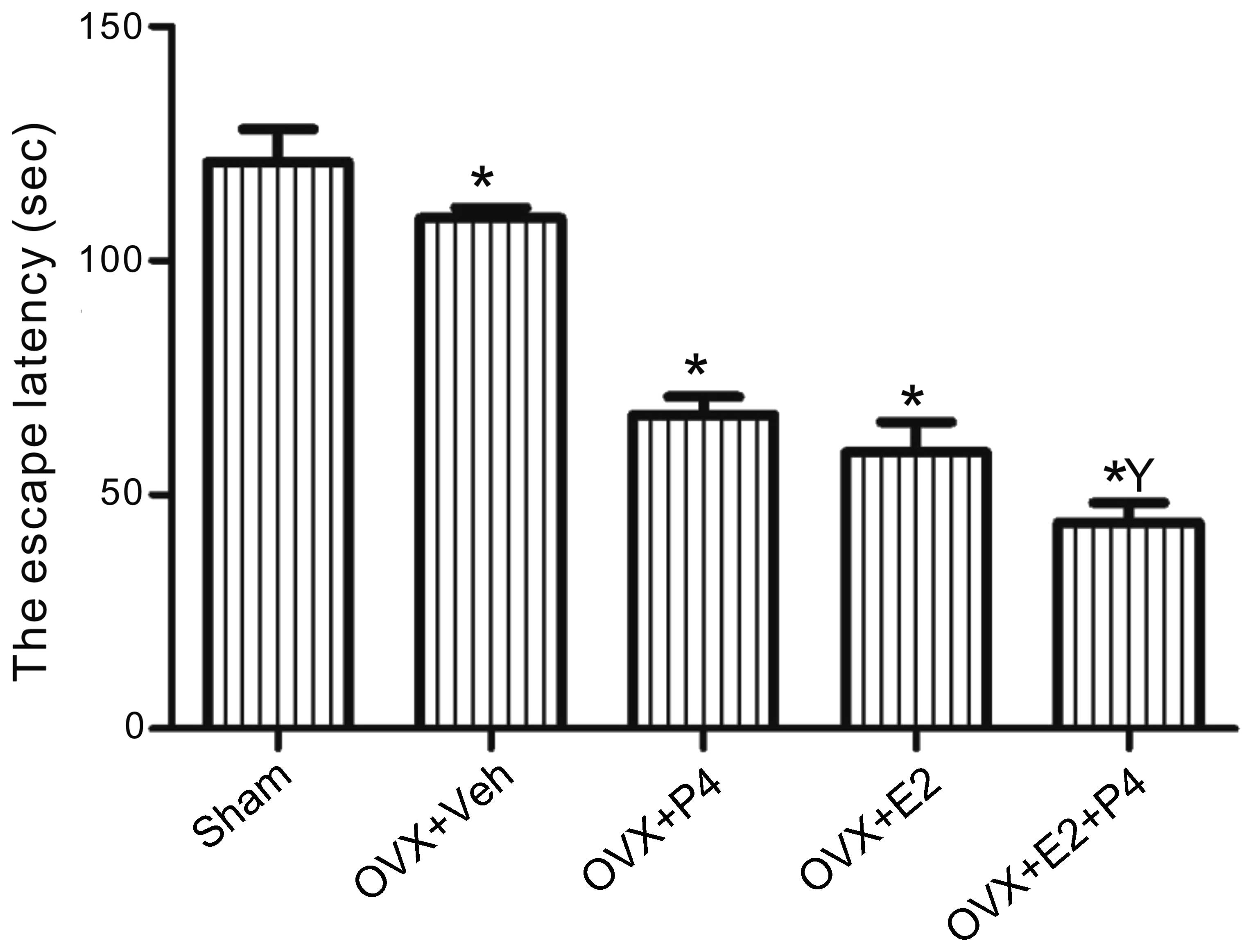
E2 and P4 improve spatial memory in a
rat model of AD
Morris water maze tests were conducted in order to
investigate the effects of ovarian hormones on the spatial and
learning memory functions of a rat model of AD. Sham rats, which
had received bilateral injections of Aβ into the hippocampus but
had not undergone an ovariectomy, exhibited escape latencies of
~120 sec, whereas the escape latencies of the ovariectomized
vehicle-treated rats were ~108 sec. Treatment with E2 and/or P4
significantly decreased the escape latencies of the ovariectomized
rats (Fig. 1), as compared with the
sham-operated and vehicle-treated rats, and this effect was most
significant for the E2 + P4-treated group (P<0.01; Fig. 1).
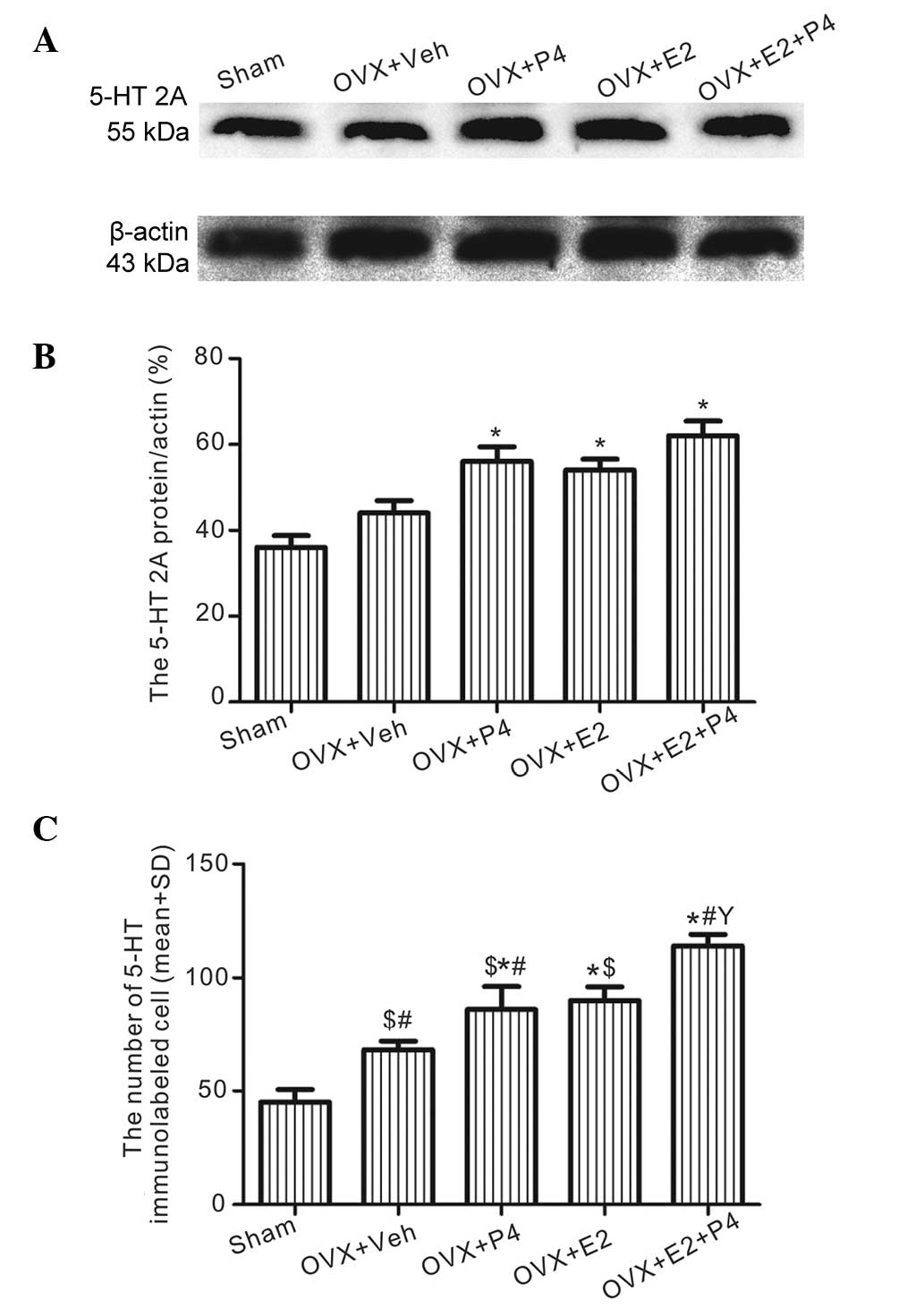
E2 and P4 increase the protein expression levels of
5-HT2A and ChAT in the hippocampus. Western blot analysis
demonstrated that the protein expression levels of 5-HT2A in the
hippocampus of the P4 and/or E2-treated groups were significantly
increased, as compared with those in the sham and vehicle-treated
rats (P<0.01; Fig. 2A and B).
These results were consistent with immunostaining (Fig. 2C), which detected that, as compared
with the sham and vehicle-treated groups (Fig. 3A and B), 5-HT2A was upregulated in
the P4-, E2-, and P4 + E2-treated groups (Fig.3C–E). In addition, the P4 + E2-treated
group demonstrated significantly increased 5-HT2A expression
levels, as compared with the rats treated with E2 or P4 alone
(P<0.05; Fig. 2C; Fig. 3C and D).
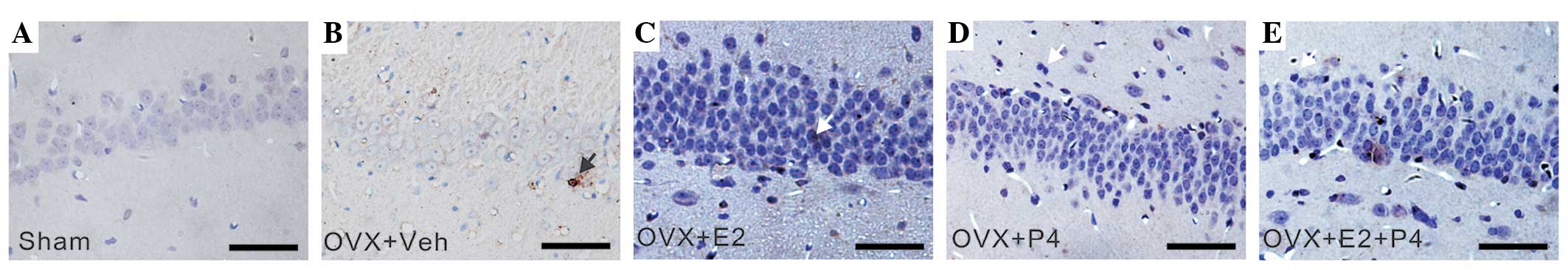
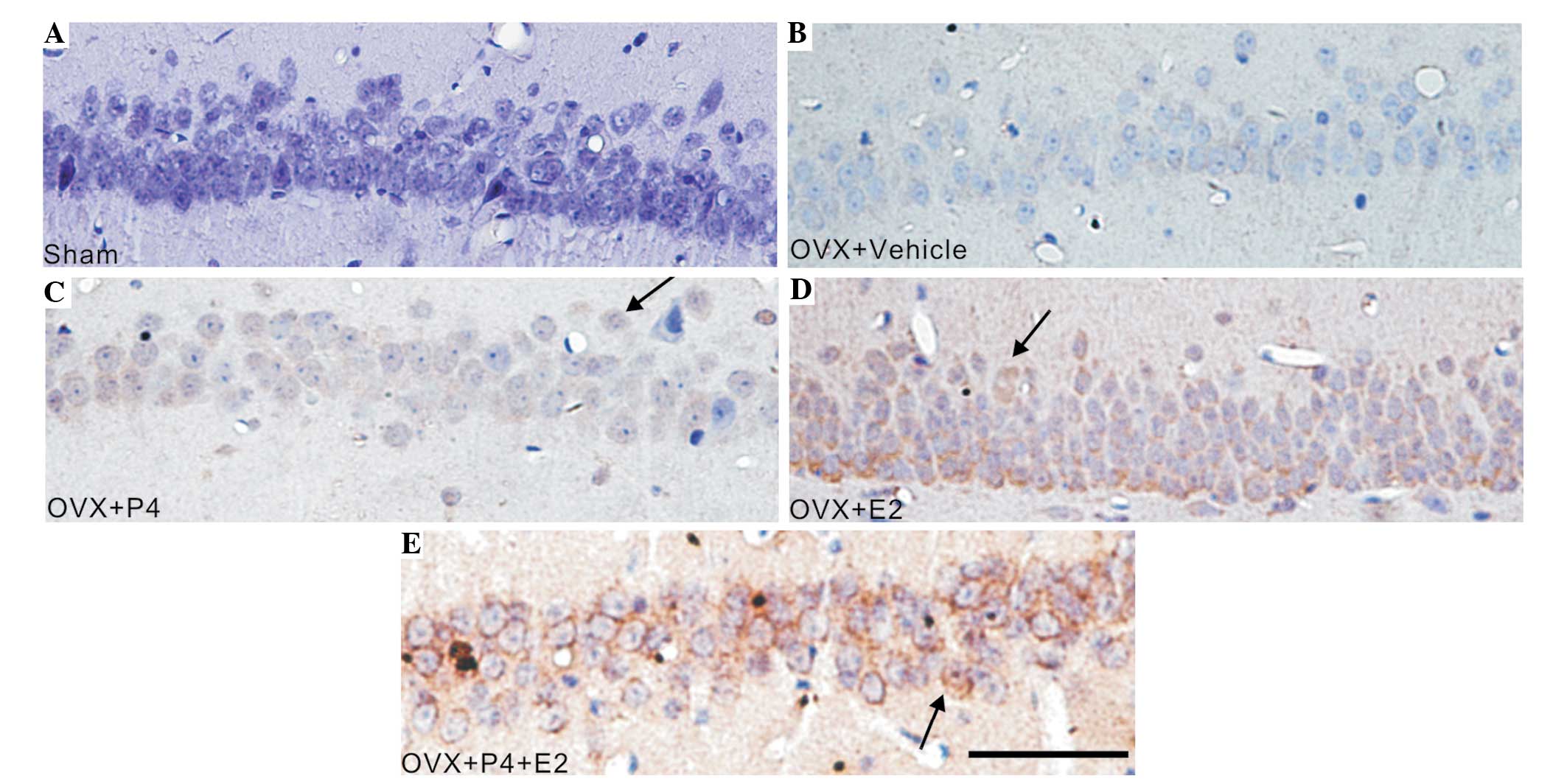 | Figure 3.E2 and P4 treatment increased the
5-HT2A immunoreactivity of astrocytes in the hippocampus of a rat
model of AD. The tissue sections were developed using
3,3-diaminobenzidine (brown) chromogen and counterstained with
hematoxylin. Arrows indicate 5-HT2A-positive cells. (A) Sham group,
(B) vehicle-treated group, (C) P4-treated group, (D) E2-treated
group and (E) P4+ E2-treated group (scale bar=100 µm). E2, 17
β-estradiol; P4, progesterone; OVX, ovariectomized; veh, vehicle;
5-HT2A, 5-hydroxytryptamine (serotonin) receptor 2A; AD,
Alzheimer's disease. |
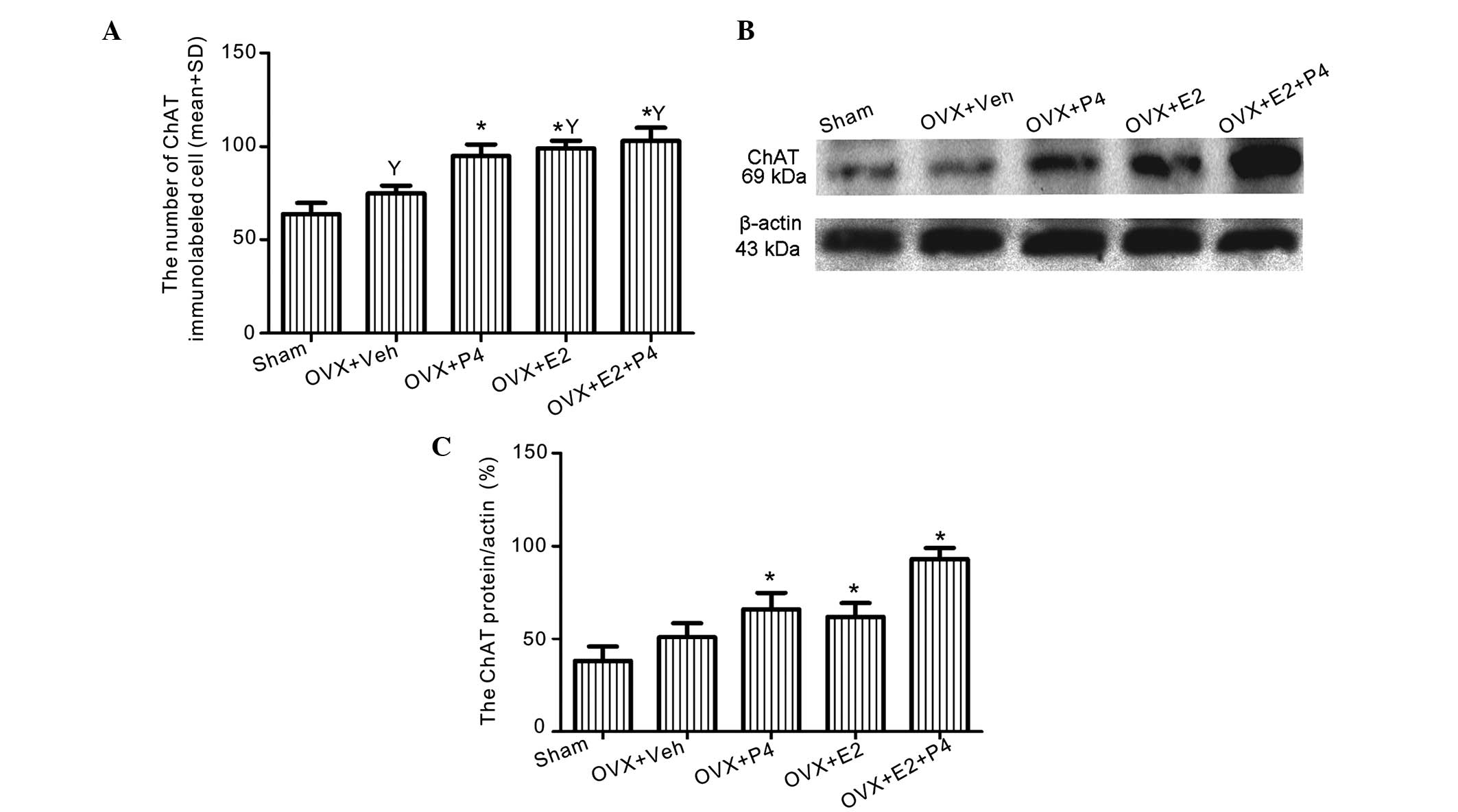
The protein expression levels of ChAT were increased
in the ovarian hormone-treated groups, particularly in the E2- and
E2 + P4-treated groups, as compared with the sham and
vehicle-treated rats (P<0.01; Figs.
4 and 5A), as demonstrated using
immunolabeling. Western blotting detected a significant increase in
the expression levels of ChAT in the P4 and E2-treated groups
(P<0.05; Fig. 5B and C).
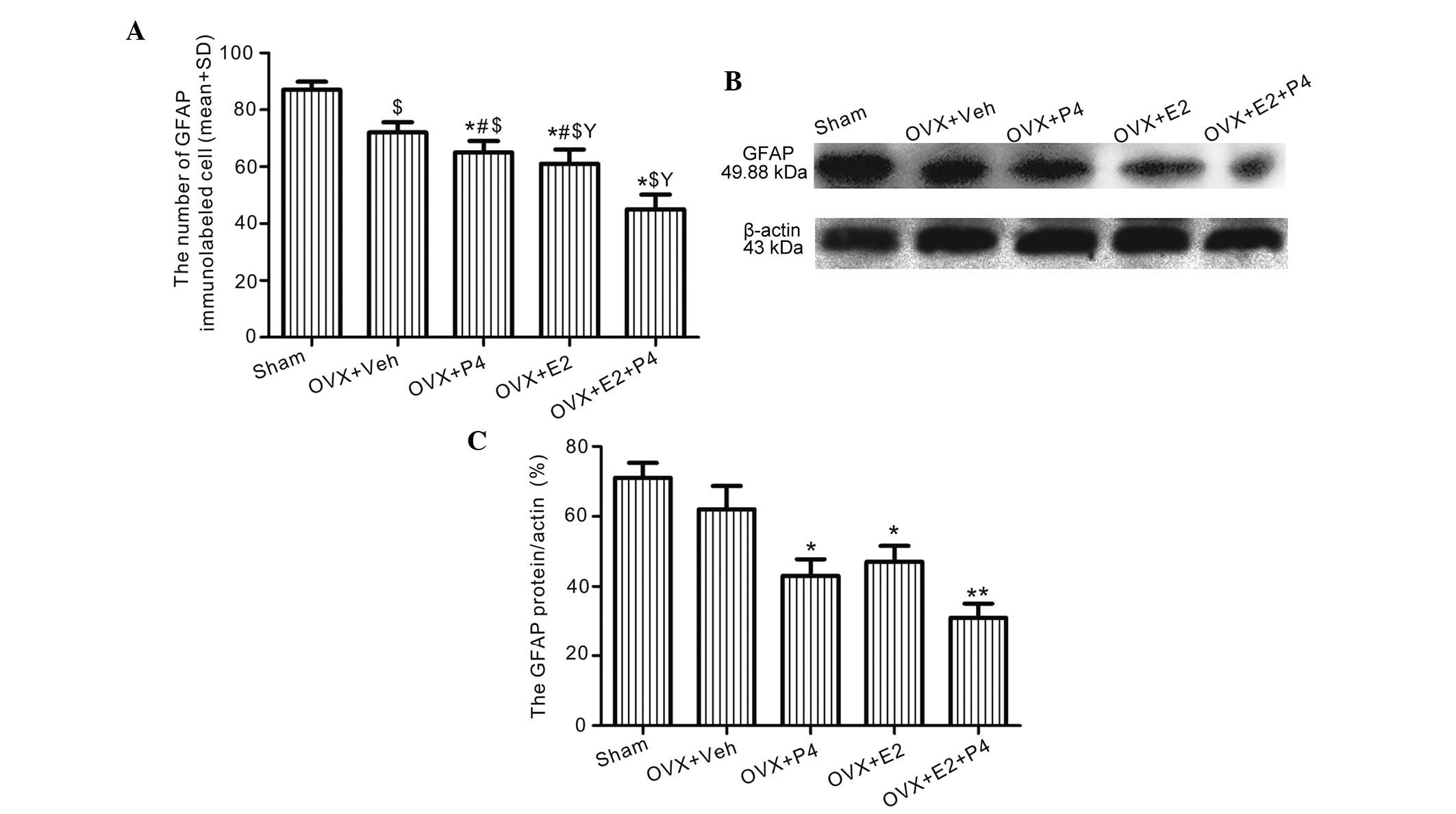
E2 and P4 decrease the protein
expression levels of GFAP in the hippocampus
Immunolabeling detected significantly decreased
levels of GFAP in the astrocytes of the ovarian hormone-treated
rats, as compared with the vehicle-treated rats (P<0.01;
Figs. 6 and 7A), and this effect was most significant
for the E2-treated group, as compared with the P4- and E2 +
P4-treated groups (P<0.05; Figs.
6 and 7A). In addition, western
blotting detected significantly decreased GFAP protein expression
levels in the hippocampus of the E2-, P4-, and E2 + P4-treated
groups, as compared with the vehicle-treated rats (P<0.01;
Fig. 7B and C).
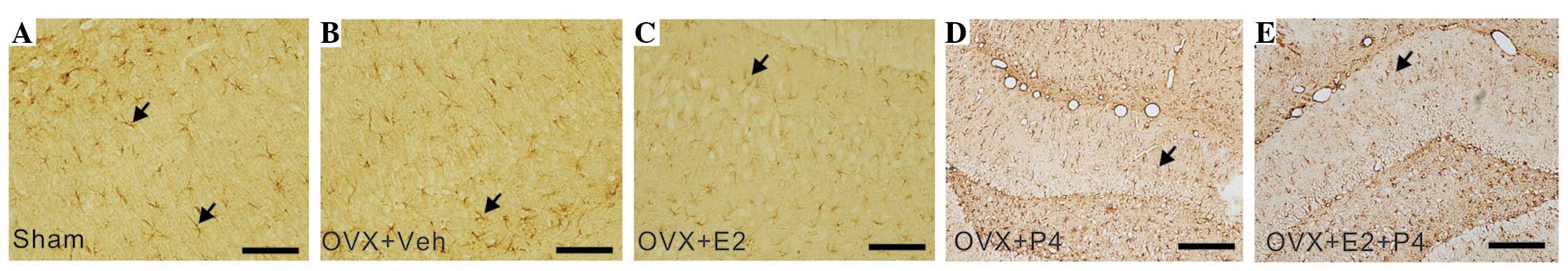 | Figure 6.E2 and P4 treatment decreased the
protein expression levels of GFAP in the hippocampus of a rat model
of AD, as detected by GFAP immunoreactivity staining. Antibody-GFAP
complexes were visualized via 3,3-diaminobenzidine staining and
hematoxylin counterstaining. Arrows denote GFAP-positive cells. (A)
Sham group, (B) vehicle-treated group, (C) E2-treated group, (D)
P4-treated group and (E) P4 + E2-treated group (scale bar=100 µm).
E2, 17 β-estradiol; P4, progesterone; OVX, ovariectomized; veh,
vehicle; GFAP, glial fibrillary acidic protein; AD, Alzheimer's
disease. |
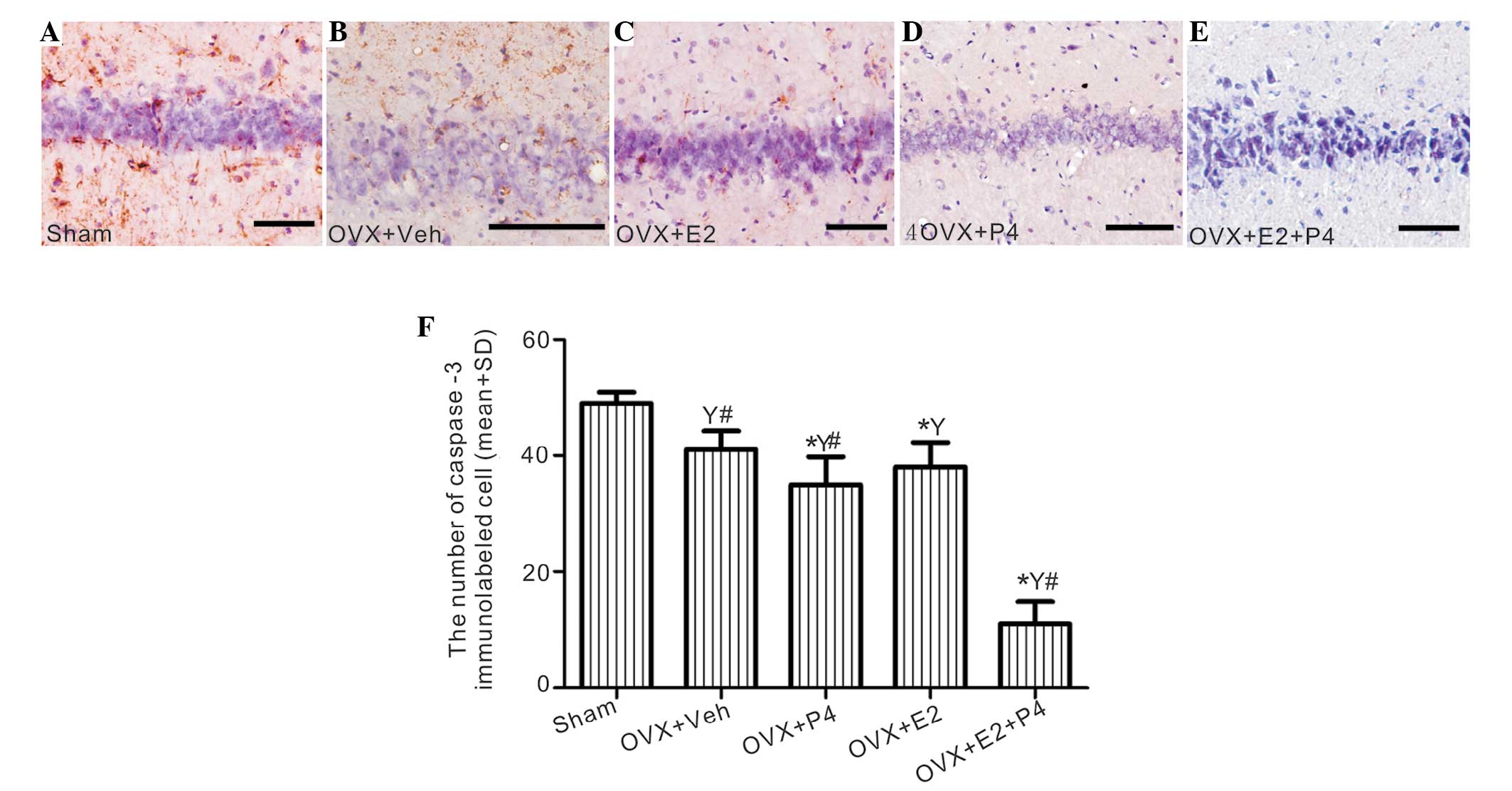
E2 and P4 suppress apoptotic signals
and inhibit cell apoptosis
A significant decrease in caspase-3 immunoreactivity
of the neuronal cells was detected in the hippocampus of the P4-
and/or E2-treated group, and this was associated with a visible
decline in the levels of apoptosis, as demonstrated by the TUNEL
assay, as compared with the sham rats (P<0.01; Fig. 8 and 9). This effect was most significant for the
P4 + E2-treated group, as compared with the rats treated with P4 or
E2 alone (P<0.05; Fig. 8 and
9); thus suggesting that the
combined treatment is more effective than either ovarian hormone
alone.
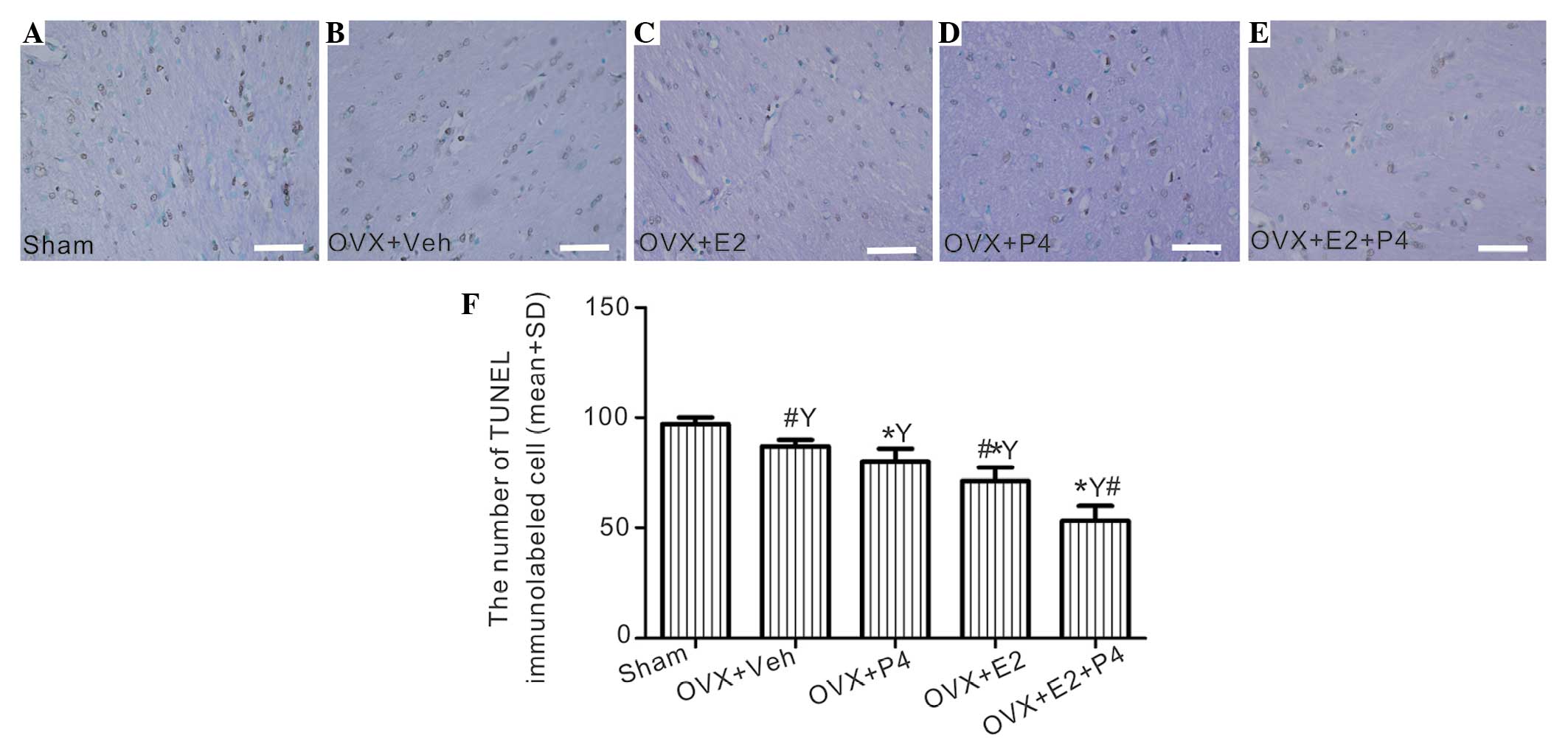 | Figure 9.The effects of E2 and P4 treatment on
apoptotic cells in the hippocampus of a rat model of AD. (A-E)
Apoptotic cells in the tissue sections were detected by terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
analysis, and were counterstained using methyl green. (A) Sham
group, (B) vehicle-treated group, (C) E2-treated group, (D)
P4-treated group and (E) P4 + E2-treated group (scale bar=100 µm).
(F) The number of TUNEL-labeled cells in each group was calculated.
*P<0.05 vs. the sham and vehicle-treated rats;
#P<0.05 vs. the P4-treated group;
YP<0.01 vs. the sham group. E2, 17 β-estradiol; P4,
progesterone; OVX, ovariectomized; veh, vehicle; GFAP, glial
fibrillary acidic protein; AD, Alzheimer's disease. |
Discussion
Various steroid hormones have been shown to have a
role in regulating the production of Aβ protein; thus suggesting
that selective lowering of Aβ levels using steroids may be
considered a potential strategy in the prevention and treatment of
AD (18). E2 and P4, which are
ovarian hormones involved in reproduction, have previously been
demonstrated to exert additional effects on the CNS (7,19).
Previous studies have suggested that there may be an increased risk
of neuronal dysfunction following menopause, or an ovariectomy;
thus suggesting that reduced levels of ovarian hormones may
increase the risk of AD (19,20). In
addition, E2 and P4 have previously been associated with anxiety
and mood (21–23), and E2 replacement therapy has been
suggested as a potential strategy for restoring the deficits that
occur following an ovariectomy, in order to improve mood, cognitive
function and the overall quality of life (5,8,11,12). The
effects of hormonal treatment on cognition during aging are
controversial (12). Previous
studies have suggested that P4 replacement therapy may reduce the
susceptibility of neuronal cells to toxic insult in a manner that
is relevant to aging and AD (7,19).
Whilst the results from older animal models have provided a strong
rationale for the use of hormone replacement therapy to prevent
dementia and AD, the results from clinical trials have been
conflicting (12,24). For example, numerous clinical trials
have reported an increased risk of dementia and cognitive decline
in women treated with E2 alone or in combination with a progestin
(13,25–27).
In the present study, Aβ1–40 was injected into the
hippocampus of OVX rats in order to generate a model of AD, and the
therapeutic effects of E2 and P4 were investigated. In particular,
the spatial and learning memory functions of all rats were assessed
using the Morris water maze test. The spatial memory of the Aβ1–40-
and vehicle-treated rats declined, as compared with the
sham-operated rats, whereas treatment with E2 and P4 was shown to
exert protective effects. These results suggested that E2 and P4
treatment may have improved learning and memory functions in a rat
model of AD. The increased ChAT and 5-HT2A immunoreactivity of the
neurons in the ovarian hormone-treated rats, as well as the
observed amelioration of astrogliosis (indicated by upregulation of
GFAP expression) in the hippocampus of the rats, provided further
evidence for potential therapeutic effects of E2 and P4. However,
there were various limitations associated with the rat model of AD
used in the present study, including that the experimental rats
were of a young age.
In a previous study, ChAT activity in the frontal
cortex and hippocampus decreased in an amyloid precursor protein
695 transgenic mouse model, which also had learning and memory
deficits (28). The hippocampus is
necessary for various types of learning and memory formation in
rats and other mammals (29), and
the loss of cholinergic neurons is considered a key event in the
pathogenesis of dementia (30).
Therefore, the decline of ChAT-positive neurons detected in the
present study may have contributed to the age-associated learning
and memory deficits observed. However, the reduction in the number
of ChAT-positive neurons in the hippocampus of the rat model of AD
was attenuated by treatment with E2 and/or P4, and this may have
ensured that normal levels of acetylcholine were available for
memory formation and consolidation.
5-HT is another neurotransmitter, which has
previously been associated with behavior and cognition (31). 5-HT2C receptors are distributed
throughout the human CNS, including within the prefrontal cortex
and hippocampus (32), and drugs
with a high affinity for 5-HT2A receptors have been shown to
modulate memory formation in rats (33). In addition, previous studies have
suggested that 5-HT dysfunction may have important functional
consequences in patients with AD (32,34). In
a study analyzing retrospective data, low densities of 5-HT2A
binding sites were associated with aggressive progression of AD
(35).
In the present study, 5-HT2A expression levels were
decreased in the rat model of AD, and this was attenuated by
exogenous ovarian hormone replacement therapy. In particular, P4
was shown to have the greatest protective effects. As well as the
direct effects of steroidal hormones on cholinergic neurons,
previous studies have reported indirect effects for E2 on
serotonergic neurons, which facilitated acetylcholine release
(5). Notably, the present study
demonstrated an association between ChAT and 5-HT2A
immunoreactivity in the hippocampus following E2 and P4 treatment,
which was consistent with the hypothesis that serotonin is able to
modulate cholinergic systems involved in cognition.
Astrogliosis, which involves an augmentation and
increase in size of GFAP-immunoreactive astrocytes, is frequently
detected in scenarios of brain damage (36,37).
Upregulation of GFAP in astrocytes has been reported for various
neurodegenerative disorders and in the autogenic
senescence-accelerated mouse model (36–38).
Increased astrocyte-neuron contact may restrict or reduce synaptic
plasticity by decreasing potential interneuron contacts (39). The results of the present study
demonstrated that GFAP-positive astrocytes increased with age in
the hippocampus of an Aβ-induced rat model of AD; thus suggesting
that there were age-associated pathological changes occurring in
the CNS, and that an increase in astrocyte volume may lead to a
corresponding decrease in neuronal cell volume (36).
In the present study, less severe astrogliosis was
detected in the brains of the E2- and P4-treated rats, as compared
with the sham and vehicle-treated rats; this was particularly
evident in the E2-treated group. Furthermore, the marked decrease
in the expression levels of GFAP in the E2- and/or P4-treated rats
was associated with increased 5-HT2A immunoreactivity and ChAT
expression levels in these rats. The results of the present study
suggested that the increased astrogliosis in the rat model of AD
may have contributed to specific functional deficits, including
impairment of learning ability and memory, and alterations in
cholinergic and serotonergic function, whereas E2 and P4 treatment
was able to alleviate the astrogliosis.
In the present study, the effects of E2 and P4 on
apoptosis in the rat model of AD were also investigated. Increased
caspase-3 expression levels and a higher number of TUNEL-positive
cells were detected in the vehicle-treated rats, as compared with
the sham rats. Treatment with E2 and P4 significantly downregulated
caspase-3 expression levels and decreased the number of
TUNEL-positive cells, thus suggesting that they were able to
suppress apoptosis. In addition, P4 treatment was associated with
the most significant neuroprotective effects, which is consistent
with previous studies in which P4 was shown to protect neurons
against glutamate toxicity (40,41) and
to inhibit caspase-3 activation (42). In the present study, it is possible
that the anti-apoptotic effects of P4 and E2 resulted in a better
performance in the spatial memory task.
The ability of E2 and P4 to reduce cholinergic
deficits, apoptotic signaling, astrogliosis and 5-HT2A
immunoreactivity, may have protected against memory impairment in
the rat model of AD. In general, E2 was better able to protect the
cholinergic neurons suppressing reactive gliosis, as compared with
P4, whereas P4 was more able to protect the 5-HT2A system and to
prevent apoptotic mechanisms. Notably, when the two drugs were
combined, the effects were not additive, thus suggesting that E2
and P4 exerted neuroprotective effects which had partially
overlapping mechanisms, or that they antagonized each others
actions using unidentified mechanisms. Therefore, in order to fully
elucidate the mechanisms involved, further studies are required,
which is particularly important when considering hormone
replacement therapy for treatment of the menopause or of patients
with AD.
In conclusion, the present study investigated the
effects of ovarian hormones on spatial and learning memory in an
ovariectomized rat model of AD, which was generated by
stereotaxically injecting Aβ(1–40)
bilaterally into the hippocampus of ovariectomized rats. The
results of the present study demonstrated that treatment with
ovarian hormones significantly reduced the expression levels of
GFAP, and increased the expression levels of ChAT and 5-HT2A in the
hippocampus, alleviated neuronal apoptosis and improved learning
and memory functions in the rats. These results suggested that the
ovarian hormones, E2 and P4, may confer protection against
Aβ-induced toxicity in vivo.
Acknowledgements
The authors of the present study would like to thank
Dr Henry Davis (Zhejiang University) for the critical reading of
this paper. The present study was supported by the National Natural
Science Foundation of China (grant no. 81371493 and 81171240).
References
|
1
|
Han M, Liu Y, Tan Q, Zhang B, Wang W, Liu
J, Zhang XJ, Wang YY and Zhang JM: Therapeutic efficacy of
stemazole in a beta-amyloid injection rat model of Alzheimer's
disease. Eur J Pharmacol. 657:104–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fang M, Wang J, Han S, Hu Z, Zhan JB, Ling
S, Rudd JA and Geng Y: Protective effects of ω-conotoxin on
amyloid-β-induced damage in PC12 cells. Toxicol Lett. 206:325–338.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He FQ, Qiu BY, Zhang XH, Li TK, Xie Q, Cui
DJ, Huang XL and Gan HT: Tetrandrine attenuates spatial memory
impairment and hippocampal neuroinflammation via inhibiting NF-κB
activation in a rat model of Alzheimer's disease induced by
amyloid-β(1–42). Brain Res. 1384:89–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ling S, Zhou J, Rudd JA, Hu Z and Fang M:
The recent updates of therapeutic approaches against aβ for the
treatment of Alzheimer's disease. Anat Rec (Hoboken).
294:1307–1318. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsuda Y, Hirano H and Watanabe Y:
Effects of estrogen on acetylcholine release in frontal cortex of
female rats: Involvement of serotonergic neuronal systems. Brain
Res. 937:58–65. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vest RS and Pike CJ: Gender, sex steroid
hormones, and Alzheimer's disease. Horm Behav. 63:301–307. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu Z, Li Y, Fang M, Wai MS and Yew DT:
Exogenous progesterone: A potential therapeutic candidate in CNS
injury and neurodegeneration. Curr Med Chem. 16:1418–1425. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Birzniece V, Johansson IM, Wang MD,
Bäckström T and Olsson T: Ovarian hormone effects on
5-hydroxytryptamine(2A) and 5-hydroxytryptamine(2C) receptor mRNA
expression in the ventral hippocampus and frontal cortex of female
rats. Neurosci Lett. 319:157–161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McLaughlin KJ, Bimonte-Nelson H,
Neisewander JL and Conrad CD: Assessment of estradiol influence on
spatial tasks and hippocampal CA1 spines: Evidence that the
duration of hormone deprivation after ovariectomy compromises
17beta-estradiol effectiveness in altering CA1 spines. Horm Behav.
54:386–395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Si D, Li J, Liu J, Wang X, Wei Z, Tian Q,
Wang H and Liu G: Progesterone protects blood-brain barrier
function and improves neurological outcome following traumatic
brain injury in rats. Exp Ther Med. 8:1010–1014. 2014.PubMed/NCBI
|
|
11
|
Sandstrom NJ and Williams CL: Spatial
memory retention is enhanced by acute and continuous estradiol
replacement. Horm Behav. 45:128–135. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chisholm NC and Juraska JM: Factors
influencing the cognitive and neural effects of hormone treatment
during aging in a rodent model. Brain Res. 1514:40–49. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang VC, Neese SL, Korol DL and Schantz
SL: Chronic estradiol replacement impairs performance on an operant
delayed spatial alternation task in young, middle-aged, and old
rats. Horm Behav. 56:382–390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meng Y, Wang R, Yang F, Ji ZJ, Fang L and
Sheng SL: Amyloid precursor protein 17-mer peptide ameliorates
hippocampal neurodegeneration in ovariectomized rats. Neurosci
Lett. 468:173–177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mittal G, Carswell H, Brett R, Currie S
and Kumar MN: Development and evaluation of polymer nanoparticles
for oral delivery of estradiol to rat brain in a model of
Alzheimer's pathology. J Control Release. 150:220–228. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hruska Z and Dohanich GP: The effects of
chronic estradiol treatment on working memory deficits induced by
combined infusion of beta-amyloid (1–42) and ibotenic acid. Horm
Behav. 52:297–306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shang XL, Zhao JH, Cao YP and Xue YX:
Effects of synaptic plasticity regulated by 17beta-estradiol on
learning and memory in rats with Alzheimer's disease. Neurosci
Bull. 26:133–139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung JI, Ladd TB, Kukar T, Price AR, Moore
BD, Koo EH, Golde TE and Felsenstein KM: Steroids as γ-secretase
modulators. FASEB J. 27:3775–3785. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singh M and Su C: Progesterone-induced
neuroprotection: Factors that may predict therapeutic efficacy.
Brain Res. 1514:98–106. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh M: Progesterone-induced
neuroprotection. Endocrine. 29:271–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Misra M, Katzman DK, Estella NM, Eddy KT,
Weigel T, Goldstein MA, Miller KK and Klibanski A: Impact of
physiologic estrogen replacement on anxiety symptoms, body shape
perception, and eating attitudes in adolescent girls with anorexia
nervosa: Data from a randomized controlled trial. J Clin
Psychiatry. 74:e765–e771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bristot G, Ascoli B, Gubert C, Panizzutti
B, Kapczinski F and Rosa AR: Progesterone and its metabolites as
therapeutic targets in psychiatric disorders. Expert Opin Ther
Targets. 18:679–690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Henry JF and Sherwin BB: Hormones and
cognitive functioning during late pregnancy and postpartum: A
longitudinal study. Behav Neurosci. 126:73–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bojar I, Gujski M, Raczkiewicz D and
Rothenberg KG: Cognitive functions, apolipoprotein E genotype and
hormonal replacement therapy of postmenopausal women. Neuro
Endocrinol Lett. 34:635–642. 2013.PubMed/NCBI
|
|
25
|
Brown S: IMS updates its recommendations
on the use of HRT. Menopause Int. 19:105–106. 2013.PubMed/NCBI
|
|
26
|
Rocca WA, Grossardt BR and Shuster LT:
Oophorectomy, estrogen, and dementia: A 2014 update. Mol Cell
Endocrinol. 389:7–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rasgon NL, Geist CL, Kenna HA, Wroolie TE,
Williams KE and Silverman DH: Prospective randomized trial to
assess effects of continuing hormone therapy on cerebral function
in postmenopausal women at risk for dementia. PLoS One.
9:e890952014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng Z, Cheng Y and Zhang JT: Long-term
effects of melatonin or 17 beta-estradiol on improving spatial
memory performance in cognitively impaired, ovariectomized adult
rats. J Pineal Res. 37:198–206. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abel T, Nguyen PV, Barad M, Deuel TA,
Kandel ER and Bourtchouladze R: Genetic demonstration of a role for
PKA in the late phase of LTP and in hippocampus-based long-term
memory. Cell. 88:615–626. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Armstrong RA: What causes alzheimer's
disease? Folia Neuropathol. 51:169–188. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu Z, Rudd JA and Fang M: Development of
the human corpus striatum and the presence of nNOS and 5-HT2A
receptors. Anat Rec (Hoboken). 95:127–131. 2012. View Article : Google Scholar
|
|
32
|
de Quervain DJ, Henke K, Aerni A, Coluccia
D, Wollmer MA, Hock C, Nitsch RM and Papassotiropoulos A: A
functional genetic variation of the 5-HT2a receptor affects human
memory. Nat Neurosci. 6:1141–1142. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meneses A: 5-HT systems: Emergent targets
for memory formation and memory alterations. Rev Neurosci.
24:629–664. 2013.PubMed/NCBI
|
|
34
|
Boulougouris V, Glennon JC and Robbins TW:
Dissociable effects of selective 5-HT2A and 5-HT2C receptor
antagonists on serial spatial reversal learning in rats.
Neuropsychopharmacology. 33:2007–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lorke DE, Lu G, Cho E and Yew DT:
Serotonin 5-HT2A and 5-HT6 receptors in the prefrontal cortex of
Alzheimer and normal aging patients. BMC Neurosci. 7:362006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han S, Rudd JA, Hu ZY, Zhang L, Yew DT and
Fang M: Analysis of neuronal nitric oxide synthase expression and
increasing astrogliosis in the brain of
senescence-accelerated-prone 8 mice. Int J Neurosci. 120:602–608.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tomassoni D, Nwankwo IE, Gabrielli MG,
Bhatt S, Muhammad AB, Lokhandwala MF, Tayebati SK and Amenta F:
Astrogliosis in the brain of obese Zucker rat: A model of metabolic
syndrome. Neurosci Lett. 543:136–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu Y, Zhang AQ and Yew DT: Age related
changes of various markers of astrocytes in senescence-accelerated
mice hippocampus. Neurochem Int. 46:565–574. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Finch CE: Neurons, glia, and plasticity in
normal brain aging. Adv Gerontol. 10:35–39. 2002.PubMed/NCBI
|
|
40
|
Kaur P, Jodhka PK, Underwood WA, Bowles
CA, de Fiebre NC, de Fiebre CM and Singh M: Progesterone increases
brain-derived neuroptrophic factor expression and protects against
glutamate toxicity in a mitogen-activated protein kinase- and
phosphoinositide-3 kinase-dependent manner in cerebral cortical
explants. J Neurosci Res. 85:2441–2449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu Z, Cai H, Zhang P, Li H, Liu H and Li
Z: Activation of ERK1/2 and PI3K/Akt by IGF-1 on GAP-43 expression
in DRG neurons with excitotoxicity induced by glutamate in vitro.
Cell Mol Neurobiol. 32:191–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nguyen H and Syed V: Progesterone inhibits
growth and induces apoptosis in cancer cells through modulation of
reactive oxygen species. Gynecol Endocrinol. 27:830–836. 2011.
View Article : Google Scholar : PubMed/NCBI
|