Introduction
Non-small cell lung cancer (NSCLC) is among the most
malignant types of tumor, with the highest incidence and mortality
rates of any cancer variety worldwide. It has been reported that
there were 239,320 new cases of lung cancer and 161,250 cases of
mortality from lung cancer in the USA in 2010 (1). Although surgical excision,
chemotherapy, radiation and targeted therapy have been applied to
the treatment of lung cancer, the five-year survival rate remains
at ~15.6%; thus, improved therapies for the treatment of NSCLC are
urgently required (1,2). Traditional Chinese herbs are considered
to be a good source for the identification of novel anti-cancer
agents (3).
Fangchinoline (Fan) is a bioactive compound isolated
from the Stephania tetrandra S. Moore (Fen Fang Ji) Chinese
herb. Various studies have demonstrated that Fan possesses a wide
range of biological activities, including: Blood pressure lowering
activity (4), histamine release
inhibition (4), aortic vascular
smooth muscle cell proliferation suppression (5), anti-oxidative stress (6) and antihypertensive activity (7). Furthermore, the anti-cancer activity of
Fan has been indicated in various tumor cell models, including in
cancer of the prostate (8), breast
(9,10) and liver (11), as well as leukemia (12). The molecular mechanisms of its
anti-cancer activity include the induction of apoptosis, autophagy
and cell cycle arrest; however, there is little information
regarding the effect of Fan on NSCLC cells. In the present study,
the antitumor effects of Fan and the associated molecular
mechanisms were explored in NSCLC cells. Treatment with Fan
stimulated cell cycle arrest at the G0/G1
phase in SPC-A-1 NSCLC cells via downregulation of cyclin-dependent
kinase 4 (CDK4), CDK6 and cyclin D1, which subsequently repressed
the expression of phosphorylated retinoblastoma protein (pRB) and
E2F transcription factor-1 (E2F-1) . Therefore, the results of the
present study suggest that Fan may potentially be useful in the
prevention and treatment of NSCLC.
Materials and methods
Cell culture and agents
Human SPC-A-1 lung cancer cells (Cell Bank of the
Chinese Academy of Sciences, Shanghai, China) were cultured in
Dulbecco's modified Eagle medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin (Sangon Biotech
Co., Ltd., Shanghai, China). Cells were maintained in a humidified
atmosphere of 5% CO2 at 37°C. Fan (purity, >98.0%;
Nature Standard Ltd., Shanghai, China) was prepared as a 50 mM
stock solution in dimethyl sulfoxide (DMSO), prior to
supplementation into the medium at various concentrations, for 48
or 72 h.
Cell Counting Kit-8 (CCK-8) assay
Cells were grown in 96-well culture plates and
treated with various dosages of Fan (1.25, 2.5, 5, 10, 20 and 40
µM), as required, prior to incubation with 10 µl CCK-8 for 2 h.
Following this, a Model 550 microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was used to measure the
optical density (OD) of the samples at a wavelength of 450 nm. The
cell inhibitory rate (IR) was calculated, as follows: IR = [1 -
(ODexperiment - ODblank) /
(ODcontrol - OD blank)] × 100%.
Cell imaging
Following treatment with Fan, phase contrast imaging
and Giemsa staining assays were used to analyze the proliferation
of SPC-A-1 lung cancer cells. SPC-A-1 cells were treated with
various concentrations of Fan (0, 2.5, 5 and 10 µM) and, after 48
h, the cells were visualized under an inverted microscope (CKX41;
Olympus, Tokyo, Japan) prior to staining with a Giemsa assay
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China),
according to the manufacturer's instructions. In brief, the cells
were fixed with the included solution I for 1 min and then solution
II was added to stain the cells for another 5 min. Subsequently,
the solution was removed and the images of cells were obtained
using the Olympus CKX41 microscope.
Flow cytometry analysis
SPC-A-1 cells were cultivated in a 6-well plate for
24 h, prior to treatment with Fan (0, 2.5, 5 or 10 µM) or equal
volumes of DMSO. Following 48 h incubation, the cells were
collected, fixed in 70% ice-cold ethanol (Sangon Biotech Co., Ltd.)
and maintained at 4°C overnight. Cells were then washed in
phosphate-buffered saline and the resultant pellet was re-suspended
in 200 µg/ml RNase (Sangon Biotech, Co. Ltd.) for 1 h at 37°C.
Cells were subsequently stained with 50 µg/ml propidium iodide, and
analyzed using a flow cytometer (FACSCalibur; Beckman Coulter,
Inc., Fullerton, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
SPC-A-1 cells were treated with various
concentrations of Fan for 48 h and the mRNA expression levels of
genes that regulate the cell cycle were examined. Cells were
collected and total RNA was extracted using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). cDNA synthesis was
performed using a RevertAid™ First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.) with 3 µg total RNA, random
hexamers (Fermentas; Thermo Fisher Scientific, Inc.) and specific
oligonucleotide primers to detect the expression levels of cyclin
D1, CDK4 and CDK6 mRNA. The sequences of the primer pairs were as
follows: Cyclin D1, forward 5′-ATGCTGGAGGTCTGCGAGGA-3′ and reverse
5′-TTCGATCTGCTCCTGGCAGG-3′; CDK4, forward
5′-TGGCTTTACTGAGGCGACTG-3′ and reverse 5′-ACGGGTGTAGTGCCATCTG-3′;
CDK6, forward 5′-GGAGTGCCCACTGAAACCAT-3′ and reverse
5′-GTGAGACAGGGCACTGTAGG-3′; and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH), forward 5′-GAGAAGGCTGGGGCTCATTT-3′ and
reverse 5′-GTCAGGTCCACCACTGACAC-3′. GAPDH was used as an internal
control. PCR was performed at a final reaction volume of 25 µl,
containing 1 µl cDNA, 1.5 mM MgCl2, 1 U Taq DNA
polymerase, 0.2 mM dNTP and 20 pM of each gene-specific
oligonucleotide primer. The PCR reaction conditions were as
follows: Denaturation at 94°C for 30 sec, annealing at 52–56°C for
30 sec, and extension at 72°C for 45 sec. The amplified products
were run on 1.5% agarose gel and documented using a Gel Doc XR+
system (Bio-Rad Laboratories, Inc.). The densitometric analysis of
the RT-qPCR results was performed using Quantity One software,
version 4.6.0 (Bio-Rad Laboratories, Inc.) using GAPDH for
normalization.
Western blot analysis
SPC-A-1 cells were cultivated in 6-well plates for
24 h, prior to treatment with Fan (0, 2.5, 5 and 10 µM) or DMSO
(0.02%) for 48 h. Protein expression was detected using 10%
SDS-PAGE at 250 V for 90 min. Subsequently, 20–30 µg total protein
was transferred to polyvinylidene difluoride membranes and the
membranes were blocked for 60 min with freshly prepared 5% non-fat
milk in Tris-buffered saline and Tween-20 (TBST). Following this,
the membranes were incubated with rabbit monoclonal pRb (1:1,500;
#8180), polyclonal E2F-1 (1:2,000; #3742) and GAPDH (1:4,000;
#5174; Cell Signaling Technology, Inc., Danvers, MA, USA) primary
antibodies, washed three times with TBST, and incubated with goat
anti-mouse or goat anti-rabbit IgG-horseradish
peroxidase-conjugated antibodies (1:4,000; #32260; Invitrogen;
Thermo Fisher Scientific, Inc.) for 1 h. Protein bands were
revealed using a ECL Plus Western Blotting Detection System kit (GE
Healthcare Life Sciences, Roosendaal, The Netherlands), with GAPDH
used as a loading control. Densitometric analysis of the western
blot was performed using Quantity One software, version 4.6.0
(Bio-Rad, Laboratories, Inc., USA), with GAPDH used for
normalization.
Statistical analysis
All cellular experiments were performed at least
three times. Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using SPSS 14.0 for Windows
(SPSS, Inc., Chicago, IL, USA). Experimental and control groups
were compared using the unpaired Student's t-test and one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Fan inhibits the proliferation of
SPC-A-1 lung cancer cells
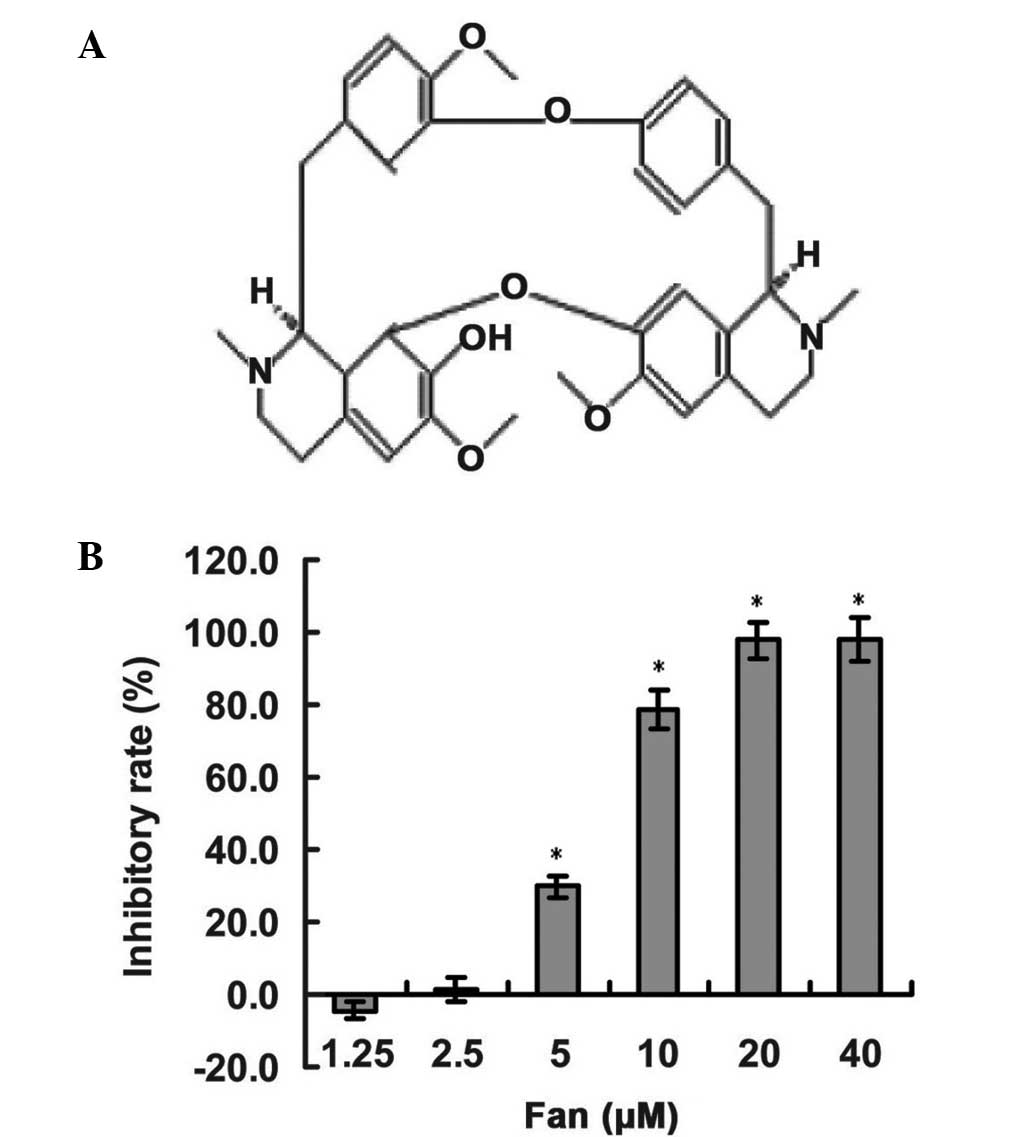
To assess the inhibitory effect of Fan (Fig. 1A) on the growth and survival of lung
cancer cells, human SPC-A-1 lung cancer cells were treated with Fan
at concentrations of 1.25, 2.5, 5, 10, 20 and 40 µM for 72 h, using
a CCK-8 assay. As shown in Fig. 1B,
the proliferative inhibitory effect of Fan was observed in a
concentration-dependent manner, with statistical significance
(P<0.01 for 5–40 µm). The half-maximal inhibitory concentration
value of Fan in SPC-A-1 cells at 72 h was 7.19 µM. Furthermore,
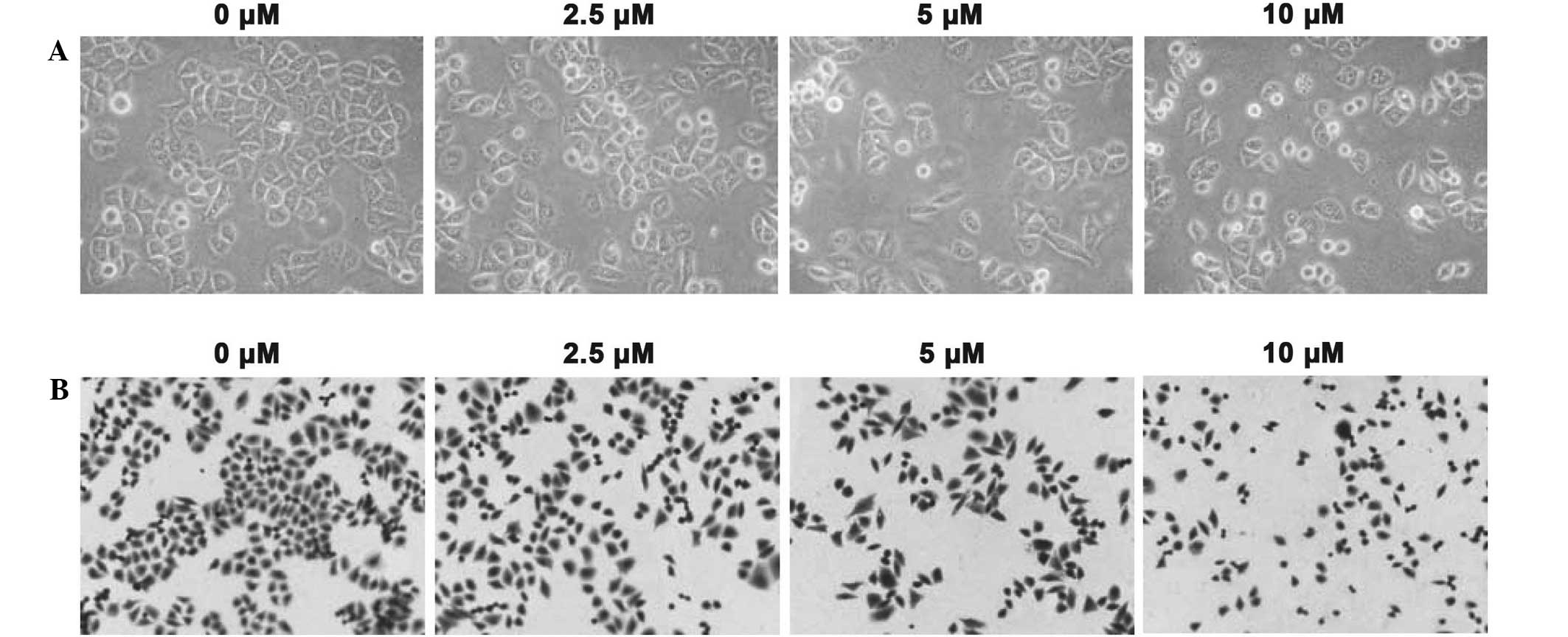
phase contrast imaging and Giemsa staining assays were also
performed to measure the inhibitory function of Fan treatment
(Fig. 2A and B, respectively).
Following treatment with 2.5, 5 or 10 µM Fan for 48 h, the total
cell number and cell volume of the SPC-A-1 cells decreased in a
dose-dependent manner, and morphological changes, such as membrane
blebbing, were detected. Thus, Fan appears to inhibit the
proliferation of SPC-A-1 lung cancer cells.
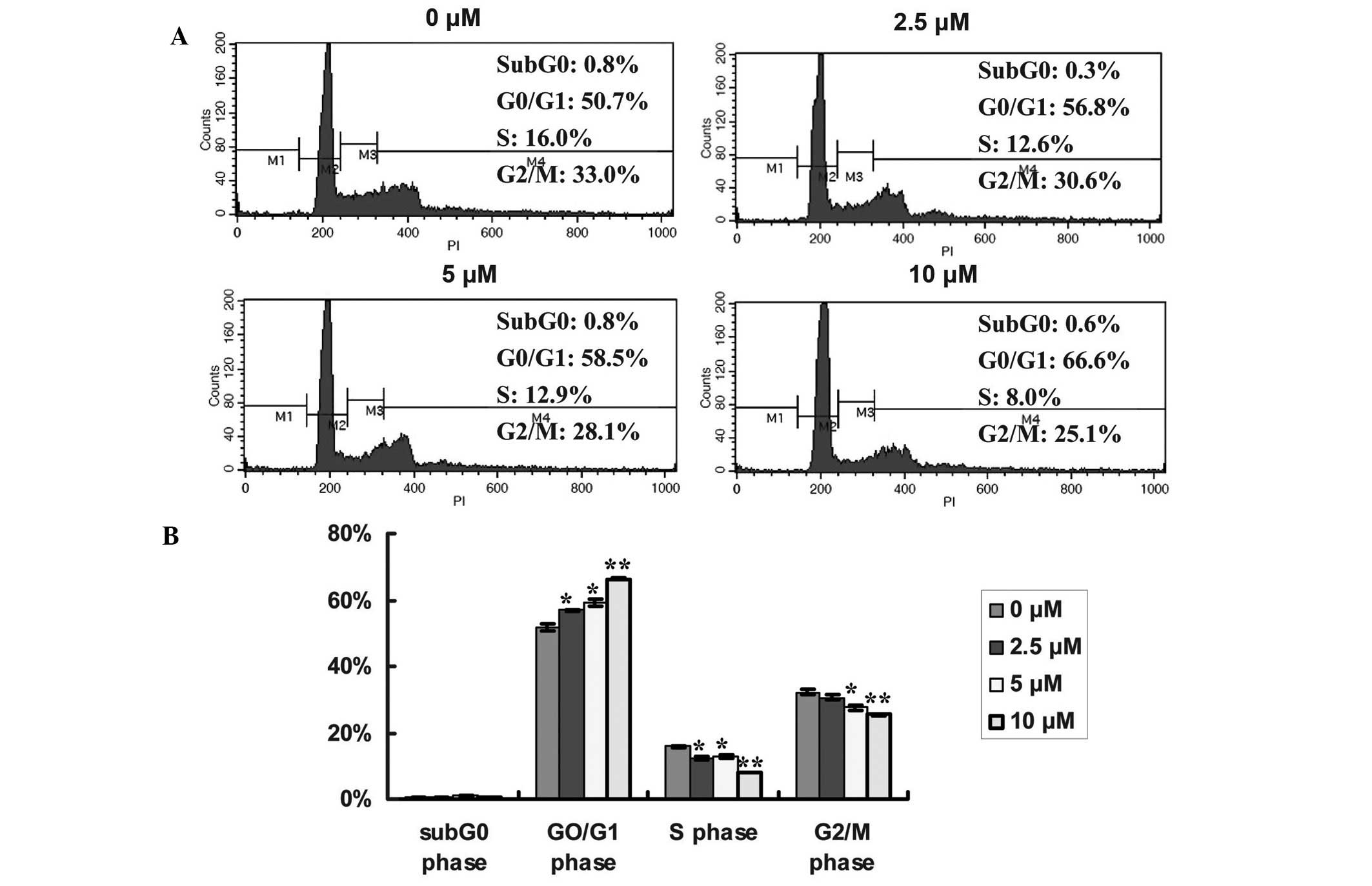
Fan induces cell cycle arrest of
SPC-A-1 cells at the G0/G1 phase
To determine whether Fan-induced suppression of cell
proliferation was associated with an alteration in cell cycle
distribution, the dose-dependent effects of Fan on the cell cycle
distribution of lung cancer cells were measured (Fig.3A and B). Following treatment with 2.5,
5 or 10 µM Fan for 48 h, the proportion of SPC-A-1 cells in the
G0/G1 phase (56.86±0.19, P<0.05;
59.12±1.00, P<0.05; and 66.22±0.32%, P<0.01; respectively)
significantly increased, compared with the control (51.84±1.06%);
whereas the percentage of cells in the S phase significantly
decreased from 15.78±0.17% (control), to 12.42±0.52 (P<0.05),
12.83±0.65 (P<0.05) and 7.96±0.05% (P<0.01), respectively.
Furthermore, the proportion of SPC-A-1 cells in the G2/M
phase decreased in a dose-dependent manner from 32.16±0.81%
(control) to 30.67±0.70 (P<0.05), 27.52±0.60 (P<0.05) and
25.38±0.29% (P<0.01), respectively. These results indicated that
Fan-induced inhibition of SPC-A-1 cell proliferation is cell
cycle-dependent, and may result in the enhanced accumulation of
cells in the G0/G1 phase. A representative profile of
the cell cycle distribution is outlined in Fig. 3.
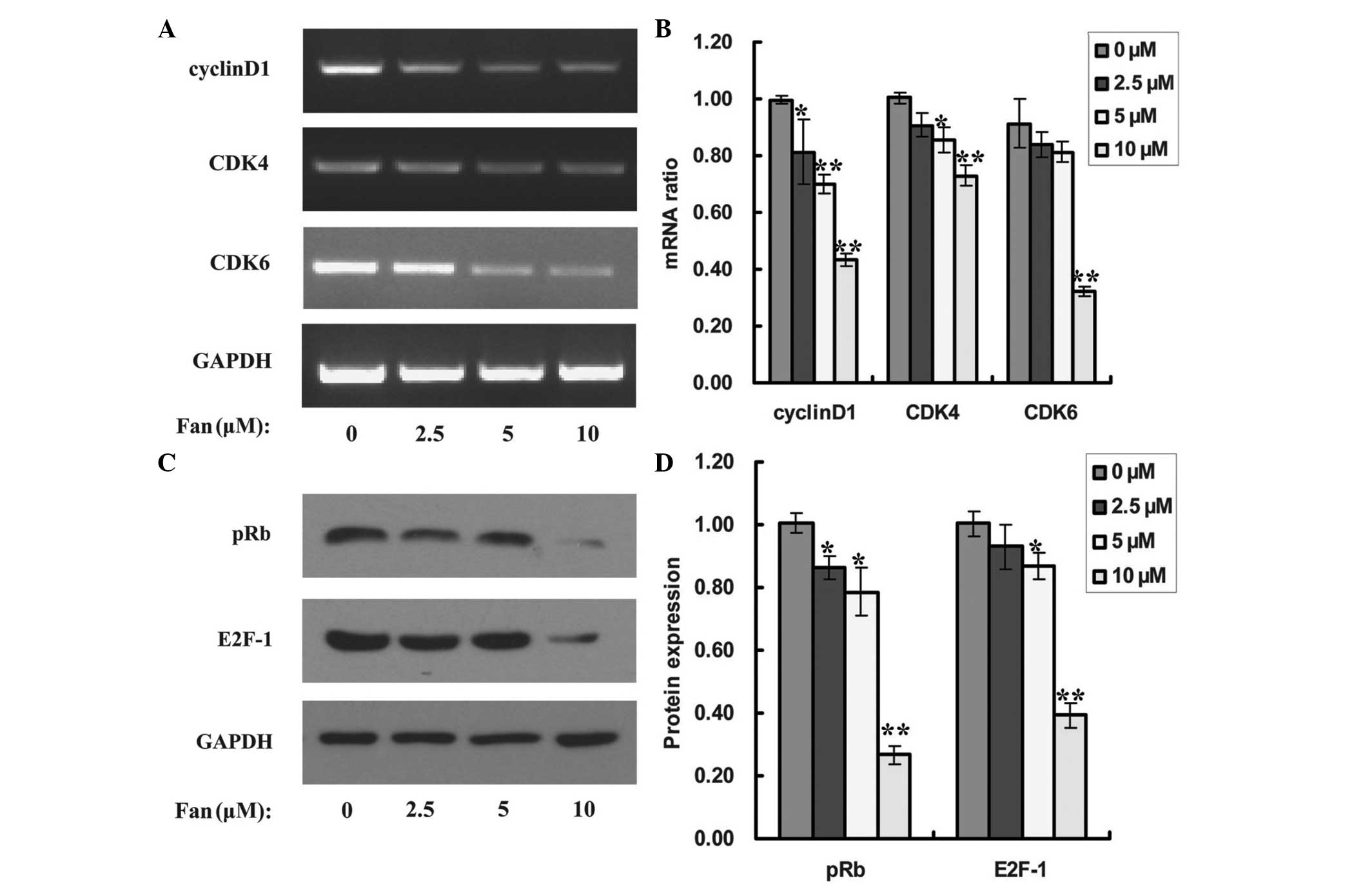
Fan affects cell cycle-related gene
and protein expression in SPC-A-1 cells
D-type cyclins, such as cyclin D1, and its partner
kinases CDK4 and CDK6, are central mediators of the G1 phase
transition (13). To examine whether
the enhancement of G0/G1 phase arrest in
Fan-treated SPC-A-1 cells was a result of the dysregulation of cell
cycle-related genes, the mRNA expression levels of cyclin D1, CDK4
and CDK6 were analyzed. The administration of Fan repressed the
expression of cyclin D1, CDK4 and CDK6 mRNAs (Fig. 4A). Fan concentrations of 2.5, 5 and
10 µM significantly inhibited cyclin D1 levels by 19 (P<0.05),
30 (P<0.01) and 57% (P<0.01), respectively, compared with no
treatment (Fig. 4B); whereas CDK4
expression levels were inhibited by 9 (P>0.05), 14 (P<0.05)
and 27% (P<0.01), respectively (Fig.
4B), and CDK6 expression levels were inhibited by 16
(P>0.05), 19 (P>0.05) and 68% (P<0.01), respectively
(Fig. 4B).
The cyclin D1-CDK4/6 complexes formed during the G1
phase may phosphorylate Rb protein and activate a transcriptional
factor, E2F-1 (14). Therefore, to
determine whether Fan suppressed the expression of cyclin D1, CDK4
and CDK6 via inhibition of the pRB/E2F-1 signaling pathway, the
expression levels of pRB and E2F-1 in Fan-treated SPC-A-1 cells
were examined, using a western blot assay. As demonstrated in
Fig. 4C, treatment with Fan
significantly inhibited the expression of pRB protein, and at 2.5,
5 and 10 µM, the suppression rates were 14 (P<0.05), 21
(P<0.05) and 73% (P<0.01), respectively (Fig. 4D). Furthermore, Fan also
significantly repressed the expression of E2F-1 protein, and the
suppression rates were determined to be 7, 13 (P<0.05) and 61%
(P<0.01) at 2.5, 5 and 10 µM, respectively (Fig. 4C and D).
Discussion
Previous studies have demonstrated that Fan is
associated with various functions, including: Blood pressure
lowering activity (4), the
inhibition of histamine release (4),
anti-oxidative stress (6) and
anti-cancer activity (9–11). However, little is known about the
effect of Fan on cell cycle arrest in cancerous cells. Various
studies have shown that Fan induces cell cycle arrest at the
G0/G1 phase in breast cancer and leukemia
cells, by decreasing the expression levels of CDK4 and cyclin D1
(8,10,12). The
present study demonstrated that, in SPC-A-1 lung cancer cells, Fan
stimulated cell cycle arrest at the G0/G1
phase by downregulating the cellular levels of CDK4, CDK6 and
cyclin D1, leading to the hypophosphorylation of Rb and the
subsequent suppression of E2F-1 activity.
Cell proliferation is dependent on the progression
of the cell cycle, which is composed of the G1, S,
G2 and M phases. The transition from the G1 to S phase
is critical, as it controls the subsequent progress of the cell
cycle. In the present study, Fan inhibited the proliferation of
SPC-A-1 lung cancer cells in a dose-dependent manner, with
G0/G1 phase accumulation, and a decrease in S
and G2/M phase, demonstrating that Fan may have
suppressed SPC-A-1 cell cycle initiation and blocked DNA synthesis.
The G1 to S phase transition is tightly regulated by the activation
of CDKs, which act consecutively in G1 to initiate the S phase, and
in the G2 phase to initiate mitosis (15,16).
Therefore, it is unsurprising that the G1 checkpoint is the most
conspicuous target for various anti-cancer agents. D-type cyclins,
cyclin E and CDK4/6, CDK inhibitors and pRB are the central players
of G1 phase transition (15,17). Upon mitogenic stimulation, D-type
cyclins, such as cyclin D1, are induced, and subsequently bind to
and activate CDK4 and CDK6. These cyclin D-dependent kinases then
initiate the phosphorylation of Rb, relieving the inhibition of
E2F-1 and allowing for the expression of specific E2F-1 target
genes (18). In the present study,
Fan suppressed the expression of cyclin D1, CDK4 and CDK6,
suggesting that Fan successfully blocked the cell cycle progression
of SPC-A-1 lung cancer cells. Considering that previous studies
have determined that the CDK4/6 complex phosphorylates Rb protein
(18–20), it is logical that the administration
of Fan may also have suppressed the phosphorylation of Rb. As a
tumor suppressor protein, Rb may inhibit cancer cell proliferation
via cell cycle arrest, as it is the hyperphosphorylation of Rb that
induces Rb to dissociate from E2F-1 and subsequently promotes the
G1 to S phase transition (19,20). In
the present study, Fan inhibited the phosphorylation of Rb protein
and E2F-1, which may have resulted from the Fan-induced inhibition
of CDK4, CDK6 and cyclin D1.
In conclusion, the present study suggested that Fan
promotes the cell cycle arrest of SPC-A-1 lung cancer cells at the
G0/G1 phase by downregulating the cellular
levels of CDK4, CDK6 and cyclin D1, leading to hypophosphorylation
of Rb and subsequent suppression of the E2F-1 activity. Thus, the
present results suggest that Fan may be a potential drug candidate
for the prevention of lung cancer and have clinical applications in
the future, and E2F-1 may be an effective target for consideration
in anti-lung cancer drugs.
Acknowledgements
The work of the present study was supported by
funding from the Jiangsu Province Health Department (grant no.
J201410) and the Yangzhou Vocational College of Environment and
Resources.
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mutlu H, Buyukcelik A, Aksahin A, Kibar M,
Cihan YB, Kaya E, Seyrek E, Yavuz S, Erden A, Calikusu Z, Aslan T
and Akca Z: Does sunlight exposure improve survival in patients
with non-small cell lung cancer? Asian Pac J Cancer Prev.
14:6301–6304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li SG, Chen HY, Ou-Yang CS, Wang XX, Yang
ZJ, Tong Y and Cho WC: The efficacy of Chinese herbal medicine as
an adjunctive therapy for advanced non-small cell lung cancer, A
systematic review and meta-analysis. Plos One. 8:e57604–e57615.
2013.
|
|
4
|
Nakamura K, Tsuchiya S, Sugimoto Y,
Sugimura Y and Yamada Y: Histamine release inhibition activity of
bisbenzylisoquinoline alkaloids. Planta Med. 58:505–508. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang YH, Fang LH and Ku BS: Fangchinoline
inhibits rat aortic vascular smooth muscle cell proliferation and
cell cycle progression through inhibition of ERK1/2 activation and
c-fos expression. Biochem Pharmacol. 66:1853–1860. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sekiya N, Hikiami H, Yokoyama K, Kouta K,
Sakakibara I, Shimada Y and Terasawa K: Inhibitory effects of
Stephania tetrandra S. Histopathology. Biol Pharm Bull. 28:667–670.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim HS, Zhang YH, Oh KW and Ahn HY:
Vasodilating and hypotensive effects of fangchinoline and
tetrandrine on the rat aorta and the stroke-prone spontaneously
hypertensive rat. J Ethnopharmacol. 58:117–123. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang CD, Huang JG, Gao X, Li Y, Zhou SY,
Yan X, Zou A, Chang JL, Wang YS, Yang GX and He GY: Fangchinoline
induced G1/S arrest by modulating expression of p27, PCNA, and
cyclin D in human prostate carcinoma cancer PC3 cells and tumor
xenograft. Biosci Biotechnol Biochem. 74:488–493. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xing ZB, Yao L, Zhang GQ, Zhang XY, Zhang
YX and Pang D: Fangchinoline inhibits breast adenocarcinoma
proliferation by inducing apoptosis. Chem Pharm Bull (Tokyo).
59:1476–1480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xing Z, Zhang Y, Zhang X, Yang Y, Ma Y and
Pang D: Fangchinoline induces G1 arrest in breast cancer cells
through cell-cycle regulation. Phytother Res. 27:1790–1794. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang N, Pan W, Zhu M, Zhang M, Hao X,
Liang G and Feng Y: Fangchinoline induces autophagic cell death via
p53/sestrin2/AMPK signalling in human hepatocellular carcinoma
cells. Br J Pharmacol. 164(2b): 731–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Chen J, Wang L, Huang Y, Leng Y
and Wang G: Fangchinoline induces G0/G1 arrest by modulating the
expression of CDKN1A and CCND2 in K562 human chronic myelogenous
leukemia cells. Exp Ther Med. 5:1105–1112. 2013.PubMed/NCBI
|
|
13
|
Chiron D, Martin P, Di Liberto M, Huang X,
Ely S, Lannutti BJ, Leonard JP, Mason CE and Chen-Kiang S:
Induction of prolonged early G1 arrest by CDK4/CDK6 inhibition
reprograms lymphoma cells for durable PI3Kdelta inhibition through
PIK3IP1. Cell Cycle. 12:1892–1900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu X, Zhang J, Han K, Zhang Z, Chen G,
Zhang J, Mao X and Cao B: Natural pesticide dihydrorotenone arrests
human plasma cancer cells at the G0/G1 phase of the cell cycle. J
Biochem Mol Toxicol. 28:232–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lim S and Kaldis P: Cdks cyclins and CKIs:
Roles beyond cell cycle regulation. Development. 140:3079–3093.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Genovese C, Trani D, Caputi M and Claudio
PP: Cell cycle control and beyond, Emerging roles for the
retinoblastoma gene family. Oncogene. 25:5201–5209. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu P, Jiang EJ, Wen SY and Lu DD:
Amentoflavone acts as a radioprotector for irradiated v79 cells by
regulating reactive oxygen species (ROS), cell cycle and
mitochondrial mass. Asian Pac J Cancer Prev. 15:7521–7526. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang YX, Cai H, Jiang G, Zhou TB and Wu H:
Silibinin inhibits proliferation, induces apoptosis and causes cell
cycle arrest in human gastric cancer MGC803 cells via STAT3 pathway
inhibition. Asian Pac J Cancer Prev. 15:6791–6798. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giacinti C and Giordano A: RB and cell
cycle progression. Oncogene. 25:5220–5227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blain SW: Switching cyclin D-Cdk4 kinase
activity on and off Cell Cycle. 7:892–898. 2008.PubMed/NCBI
|