Introduction
Antigen-presenting cells (or immune accessory cells)
present antigens to the T and B-cells, and include various cell
types, such as dendritic cells and macrophages. Dendritic cells are
part of the non-lymphocytic type and are also known as reticular
cells. They are classified into four major groups based on their
morphology and immunophenotype, as follows: Langerhans cells,
interdigitating dendritic cells (IDCs), follicular dendritic cells
(FDCs) and fibroblastic reticular cells (FBRCs) (1,2). FBRCs
are commonly located in the capsule, hilar and mesenchymal areas of
the lymph nodes, while other sites include the parafollicular zone
of the spleen and tonsils (3). FBRCs
are considered to form the reticular network, which may facilitate
the migration of lymphocytes and the transport of cytokines and
other modulatory factors (3).
Primary extranodal FBRC tumors (FRCTs) rarely occur and, to the
best of our knowledge, only 19 cases have been reported in the
literature thus far (4–15). However, none of these FRCT cases were
located in the breast tissue.
The present study is the first to report a case of
primary FRCT of the breast in a 57-year-old woman. In addition, the
clinical, cytological, histological and immunophenotypical features
of this tumor were discussed in detail.
Case report
A 57-year-old woman presented at the Ninghai
Maternity and Child Care Hospital (Ninghai, China) complaining of a
pinching sensation in the right breast for ~2 weeks in December
2013. The patient had previously undergone drainage for the
management of mastitis, which was diagnosed 30 years before, in the
same breast. Upon physical examination, a firm, painless mass with
a size of ~3.5×2.5 cm was observed in the right breast. Ultrasound
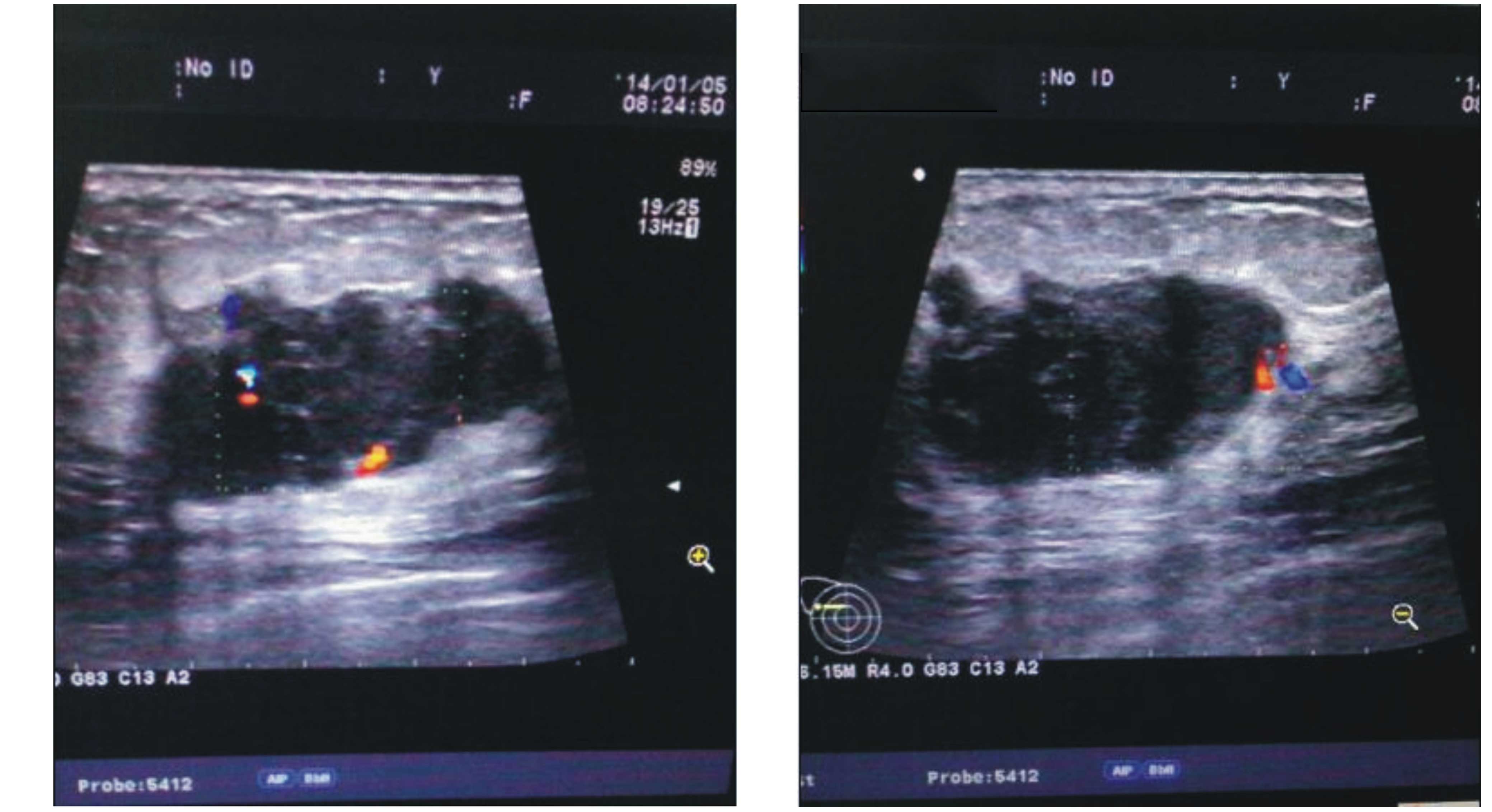
examination showed a non-homogeneous and hypoechoic mass with a
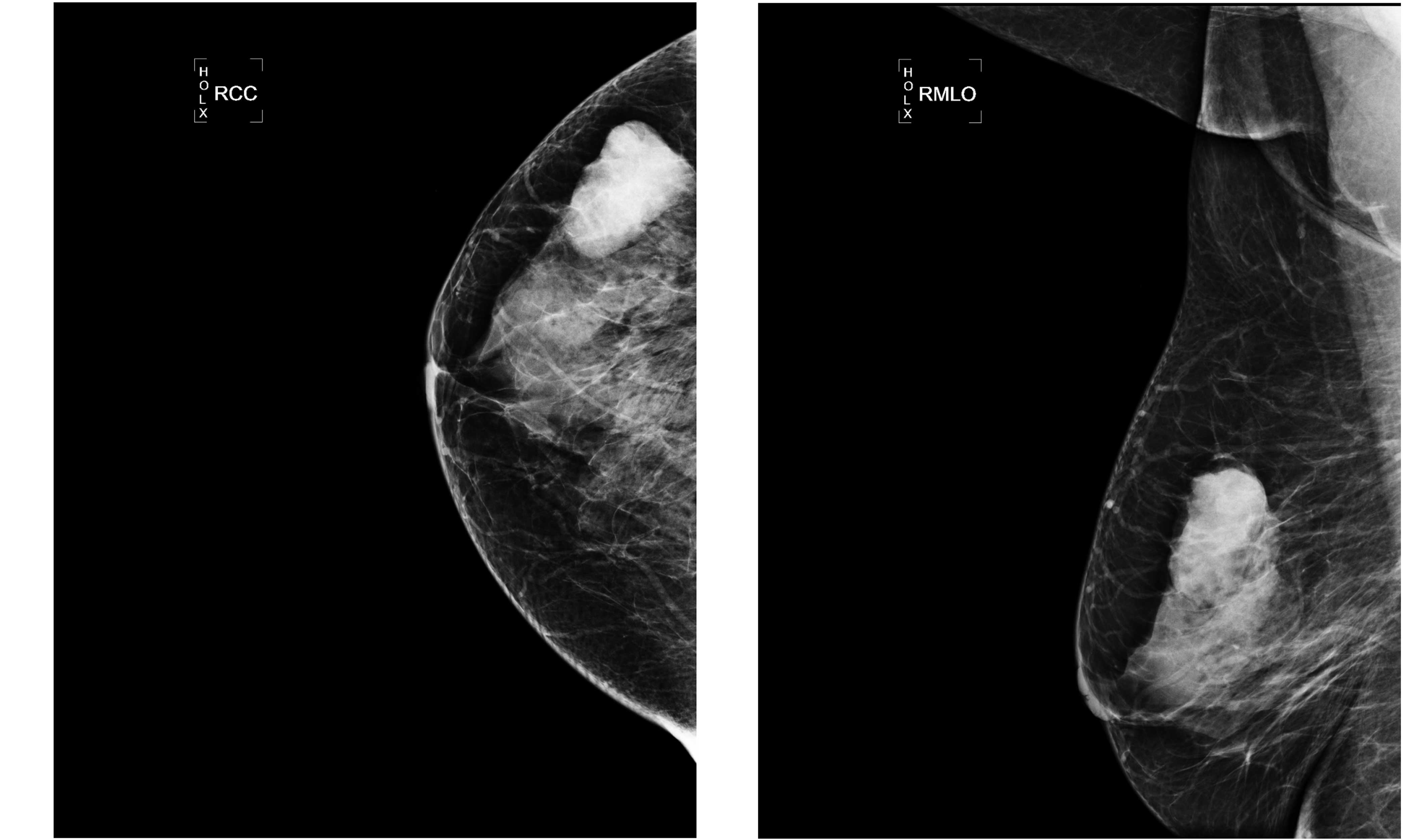
resistance index of ~67% and a size of ~3.3×2.6 cm (Fig. 1). Mammography scans revealed a high
density node with a clear boundary (Fig.
2). Surgical resection of the mass was performed on December
25, 2013 and the preliminary pathological diagnosis was uncertain
according to the analysis of a frozen section. Further
immunohistochemical analysis rendered the diagnosis of FRCT, based
on the unusual morphological expression and the immunophenotypical
results, which indicated positive lymph nodes.
The resected tumor was fixed in 10% neutral
formalin, dehydrated with a graded alcohol series, and then
embedded in paraffin. Next, 3-µm paraffin sections were stained
with hematoxylin and eosin. Immunohistochemical studies were
performed on the sections (Fig. 3)
using the avidin-biotin peroxidase complex method, as previously
demonstrated (16). The following
primary antibodies were used in the analyses: CD1a (clone 010;
1:50), CD68/KP1 (clone PG-M1; 1:200), desmin (clone D33; 1:50),
HER2/neu (clone CB11; 1:50) and epidermal growth factor receptor
(clone EP38Y; 1:30) that were purchased from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA); CD3 (clone PS1; 1:100), CD21
(clone 2G9; 1:20), CD30 (clone Ber.H2; 1:10), CD35 (clone E11;
1:100), vimentin (clone V9; 1:100), cytokeratin (clone AE1/AE;
1:100), keratin 7 (clone Ov-TL 12/30; 1:100), keratin 19 (clone
RCK108; 1:50), epithelial membrane antigen (clone E29; 1:100),
smooth muscle actin (clone 1A4; 1:100) and Ki-67 (clone GM001;
1:200) that were purchased from Dako (Glostrup, Denmark); CD23
(clone SP23; 1:80) was obtained from Novacastra (Leica Biosystems,
Wetzlar, Germany); CD31 (1:300; clone JC70A), CD45 (1:300; clone
2B11 + PD7/26/16), S-100 protein (1:3,000; 790–2523), estrogen
receptors (1:200; clone SP1) and progesterone receptors (1:500;
clone SP2) that were purchased from Ventana Medical Systems, Inc.
(Tucson, AZ, USA).
The surgical tumor specimen contained a white-grey
cross section with clear boundaries and it measured ~3.5 cm in
diameter. Tumor cells were mainly composed of oval and spindle
cells, and were infiltrated with lymphocytes and plasma cells. The
immunohistochemical analysis results showed that the tumor cells
were only positive for vimentin, while Ki-67 was ~60%
immunoreactive (Fig. 3). These
results rendered the diagnosis of fibroblastic reticular cell
tumor. Subsequently, a right modified radical mastectomy was
performed on January 15, 2015 followed by the initiation of four
cycles of mesna, doxorubicin, ifosfamide and dacarbazine regimen
chemotherapy on February 11, 2014, which cycled every 21 days. The
regimen was as follows: 70 mg adriamycin day 1 (Pfizer, Inc., New
York City, NY, USA); 2.0 g ifosfamided days 1–3 (Baxter Healthcare
Corporation, Deerfield, IL, USA); 0.4 g dacarbazine day 1–3
(Fresenius Kabi, Bad Homburg, Germany). Pathological report showed
that six axillary lymph nodes had been involved, and the
immunohistochemical results were similar to those of the primary
mass. There was no evidence of disease detected at other sites.
Discussion
Dendritic cell tumors are extremely rare, and common
types include FDC sarcoma (FDCS), IDC sarcoma (IDCS) and FRCT,
according to the World Health Organization classification (17). FRCTs may be further subdivided into
cytokeratin-negative and cytokeratin-positive based on their
cytokeratin expression (18).
Achieving an accurate diagnosis of dendritic cell sarcoma (DCS) is
difficult, particularly in extra-nodal sites. In addition, these
neoplasms must be differentiated from other more common tumors,
including carcinomas and soft tissue sarcomas.
To the best of our knowledge, the current study
presented the first case of FRCT originating from the breast. The
first documented FRCT case was detected in the thoracic lymph nodes
and was reported by Gould et al in 1990 (5) and, to date, only 19 cases of FRCT have
been reported in the literature (1).
The involved organs in previous cases included the lymph nodes,
liver, lung, spleen, soft tissue and bone. However, the data on
FRCT is limited due to its rare incidence, and no etiology
associated with this disease has been confirmed. The patient of the
present study experienced breast abscess and received drainage
therapy ~30 years prior to the FRCT diagnosis. Although the
occurrence site of the abscess was almost identical as that of the
FRCT, no association between the two conditions can be inferred due
to the long time interval between their occurrence.
FDCS has the highest incidence when compared to
other dendritic cell tumor types (1). Certain FDCS cases were found to express
CD21 protein, which is a receptor for Epstein-Barr virus, and that
may be a pathogenesis cause (19,20).
IDCS may be originated from the hematopoietic or solid organ, and
only 2 cases of IDCS originating from the breast have been reported
(21,22). The malignant transformation and
transdifferentiation of B cells may be a possible cause of IDCS
formation (23).
For the accurate diagnosis of DCS, a combination of
observations from macroscopic, immunohistochemical and electron
microscopy examinations is required. Macroscopically, FRCT cells
present as whorls, fascicles or a storiform pattern, and their
shape may be a spindle, circle or ovoid. Lymphoplasmacytic
infiltration and epithelioid cells were observed between tumor
cells (4). FRCT cells have been
demonstrated to have certain myofibroblastic-like features with
immunoreactivity for vimentin, smooth muscle actin and desmin,
whereas they were negative for CD21, CD35 and S-100 protein
(9). Differentiating between FBRC
subtypes that express cytokeratins and other epithelial markers
from carcinoma is challenging (24,25).
Electron microscopy can be used to observe evident signs of smooth
muscle differentiation in tumor cells, however, these properties
are not observed in all cases. The morphology of FDCs and IDCs is
similar to that of FBRCs; however, FDCs are immunoreactive for
CD21, CD35, Ki-FDRC1p and Ki-M4p (21,26),
whereas IDCs are immunoreactive for S-100 protein and variably
immunoreactive for CD1a and histiocytic markers (27). Furthermore, FDCs are found to have a
fluffy cytoplasm bulge and marked desmosome upon electron
microscopy observation, while IDCs cells have a slender cytoplasm
bulge and no desmosome. Notably, Jones et al (7) demonstrated that a differentiation
intermediate exists between FDCs and FBRCs, which suggests there
may be an association between the FDCs and FBRCs (7).
The accepted strategies for the treatment of FRCTs
remain controversial. Due to the small number of cases with various
treatment modalities, no conclusion can be made from previous
studies. Commonly, surgical resection is performed as a primary
treatment modality for FDCs and FRCT. However, in IDCs, the surgery
was not found to have an effect on the overall survival of patients
(18). The role of adjuvant therapy,
such as radiotherapy and chemotherapy, in these tumors remains
uncertain. In early disease, adjuvant therapies are considered to
have no beneficial effect on the prognosis of sarcomas (28). Localized FRCT cases have been treated
with radiotherapy more frequently than chemotherapy (18). The current patient received modified
radical mastectomy as the primary treatment, and a pathological
report showed that six axillary lymph nodes had been involved.
Subsequent to the surgery, the patient received chemotherapy. The
patient was followed-up for 20 months following the chemotherapy
and the recovery was uneventful.
In conclusion, the present study reported the first
case of primary breast FBRC tumor. FBRC tumors are rarely observed
and are easily misdiagnosed. The diagnosis, treatment and prognosis
details reported for the current patient will assist in improving
the knowledge on the characteristics of this disease.
Acknowledgements
The authors would like to thank Dr Ping Gong from
New York Presbyterian Hospital (New York City, NY, USA) and
Professor Xiaoqiu Li from Fudan University Shanghai Cancer Center
(Shanghai, China) for their precise advice on the diagnosis in the
present study.
References
|
1
|
Wu L and Liu YJ: Development of
dendritic-cell lineages. Immunity. 26:741–750. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sato K and Fujita S: Dendritic cells:
Nature and classification. Allergol Int. 56:183–191. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balogh P, Fisi V and Szakal AK:
Fibroblastic reticular cells of the peripheral lymphoid organs,
Unique features of a ubiquitous cell type. Mol Immunol. 46:1–7.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andriko JW, Kaldjian EP, Tsokos M,
Abbondanzo SL and Jaffe ES: Reticulum cell neoplasms of lymph
nodes, A clinicopathologic study of 11 cases with recognition of a
new subtype derived from fibroblastic reticular cells. Am J Surg
Pathol. 22:1048–1058. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gould VE, Warren WH, Faber LP, Kuhn C and
Franke WW: Malignant cells of epithelial phenotype limited to
thoracic lymph nodes. Eur J Cancer. 26:1121–1126. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan ACL, Serrano-Olmo J, Erlandson RA and
Rosai J: Cytokeratin-positive malignant tumors with reticulum cell
morphology, A subtype of fibroblastic reticulum cell neoplasm? Am J
Surg Pathol. 24:107–116. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones D, Amin M, Ordonez NG, Glassman AB,
Hayes KJ and Medeiros LJ: Reticulum cell sarcoma of lymph node with
mixed dendritic and fibroblastic features. Mod Pathol.
14:1059–1067. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lucioni M, Boveri E, Rosso R, Benazzo M,
Necchi V, Danova M, Incardona P, Franco C, Viglio A, Riboni R, et
al: Lymph node reticulum cell neoplasm with progression into
cytokeratin-positive interstitial reticulum cell sarcoma (CIRC): A
case study. Histopathology. 43:583–591. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martel M, Sarli D, Colecchia M, Coppa J,
Romito R, Schiavo M, Mazzaferro V and Rosai J: Fibroblastic
reticular cell tumor of the spleen, Report of a case and review of
the entity. Hum Pathol. 34:954–957. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schuerfeld K, Lazzi S, De Santi MM,
Gozzetti A, Leoncini L and Pileri SA: Cytokeratin-positive
interstitial cell neoplasm, A case report and classification
issues. Histopathology. 43:491–494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mücke R, Reichl B, Micke O, Heyder R,
Büntzel J, Marx A, Müller-Hermelink HK and Ott G: Surgery and
radiotherapy of one rare case with neoplasm derived from
fibroblastic reticulum cells of a cervical lymph node. Acta Oncol.
43:766–768. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dong YC, Wu B, Sheng Z, Wang JD, Zhou HB
and Zhou XJ: Cytokeratin-positive interstitial reticulum cell
tumors of lymph nodes, A case report and review of literature. Chin
Med J (Engl). 121:658–663. 2008.PubMed/NCBI
|
|
13
|
Kwon JE, Yang W-I, Kim HK, Kwon KW, Kwon
TJ, Choi EC and Lee KG: Cytokeratin-positive interstitial reticulum
cell sarcoma: A case report with cytological, immunohistochemical,
and ultrastructural findings. Cytopathology. 20:202–205. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yaman E, Gonul II, Buyukberber S, Ozturk
B, Akyurek N, Coskun U, Kaya AO, Yildiz R, Sare M and Kitapci M:
Metastatic fibroblastic reticulum cell sarcoma of the liver,
Pathological and PET-CT evaluation. Pathology. 41:289–292. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suárez D, Izquierdo FM, Méndez JR, Escobar
J, Cabeza A and Junco P: Tumor of fibroblastic reticular cells of
lymph node coincidental with an undifferentiated endometrial
stromal sarcoma. Histopathology. APMIS. 119:216–220. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Omoto Y, Kurosumi M, Hozumi Y, Oba H,
Kawanowa K, Takei H and Yasuda Y: Immunohistochemical assessment of
primary breast tumors and metachronous brain metastases, with
particular regard to differences in the expression of biological
markers and prognosis. Exp Ther Med. 1:561–567. 2010.PubMed/NCBI
|
|
17
|
Vardiman JW: TheW orld Health Organization
(WHO) classification of tumors of the hematopoietic and lymphoid
tissues: An overview with emphasis on the myeloid neoplasms. Chem
Biol Interact. 184:16–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saygin C, Uzunaslan D, Ozguroglu M,
Senocak M and Tuzuner N: Dendritic cell sarcoma, A pooled analysis
including 462 cases with presentation of our case series. Crit Rev
Oncol Hematol. 88:253–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fingeroth JD, Weis JJ, Tedder TF,
Strominger JL, Biro PA and Fearon DT: Epstein-Barr virus receptor
of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci
USA. 81:4510–4514. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lindhout E, Lakeman A, Mevissen ML and de
Groot C: Functionally active Epstein-Barr virus-transformed
follicular dendritic cell-like cell lines. J Exp Med.
179:1173–1184. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kapucuoglu N, Percinel S, Ventura T, Lang
R, Al-Daraji W and Eusebi V: Dendritic cell sarcomas/tumours of the
breast, Report of two cases. Virchows Arch. 454:333–339. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uluoğlu O, Akyürek N, Uner A, Coşkun U,
Ozdemir A and Gökçora N: Interdigitating dendritic cell tumor with
breast and cervical lymph-node involvement A case report and review
of the literature. Virchows Arch. 446:546–554. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fraser CR, Wang W, Gomez M, Zhang T,
Mathew S, Furman RR, Knowles DM, Orazi A and Tam W: Transformation
of chronic lymphocytic leukemia/small lymphocytic lymphoma to
interdigitating dendritic cell sarcoma, Evidence for
transdifferentiation of the lymphoma clone. Am J Clin Pathol.
132:928–939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Franke WW and Moll R: Cytoskeletal
components of lymphoid organs. Histopathology. Differentiation.
36:145–163. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sundersingh S, Majhi U, Krishnamurthy A
and Velusami SD: Cytokeratin-positive interstitial reticulum cell
sarcoma, Extranodal presentations mimicking carcinoma. Indian J
Pathol Microbiol. 56:172–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pruneri G, Masullo M, Renne G, Taccagni G,
Manzotti M, Luini A and Viale G: Follicular dendritic cell sarcoma
of the breast. Virchows Arch. 441:194–199. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gaertner EM, Tsokos M, Derringer GA,
Neuhauser TS, Arciero C and Andriko JA: Interdigitating dendritic
cell sarcoma, A report of four cases and review of the literature.
Am J Clin Pathol. 115:589–597. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Toesca A, Spitaleri G, De Pas T, Botteri
E, Gentilini O, Bottiglieri L, Rotmentsz N, Sangalli C, Marrazzo E,
Cassano E, et al: Sarcoma of the breast: Outcome and reconstructive
options. Clin Breast Cancer. 12:438–444. 2012. View Article : Google Scholar : PubMed/NCBI
|