Introduction
Acquired immunodeficiency syndrome (AIDS) is among
the most complex diseases in the field of medicine. The incidence
of Pneumocystis jiroveci pneumonia (PCP) has increased
significantly and has become the most common opportunistic
infection among patients with human immunodeficiency virus (HIV)
(1). PCP is the most common cause of
AIDS-related mortality, and the mortality rate of patients with
AIDS that contract PCP in the early stages of the disease increases
to 10–20% as the necessity for mechanical ventilation is
significantly increased (2). Based
on the results of five randomized controlled trials (RCTs), the use
of corticosteroids was recommended for the treatment of patients
co-infected with HIV and PCP by an expert panel in 1990 (3). Adjunctive corticosteroid treatment
refers to the administration of corticosteroids in combination with
sulfamethoxazole-trimethoprim (SMZ-TMP) or pentamidine (3,4).
Corticosteroids may be categorized as long-acting, middle-acting or
short-acting agents. The primary therapeutic agents used to treat
PCP in patients with HIV include prednisone and prednisolone
(5). On the basis of the results of
previous clinical trials, a systematic review was conducted by
Briel et al (5) in 2006,
which demonstrated the feasibility of adjunctive corticosteroid
treatment for the treatment PCP in patients co-infected with HIV.
Adjunctive corticosteroid therapy was considered as an alternative
to SMZ-TMP alone, and effectively improved survival in moderate to
severe cases, reducing complications such as pneumothorax and
respiratory failure (6). Therefore,
adjunctive corticosteroid therapy has been recommended by the
American CDC Guidelines to treat PCP associated with HIV-1
infection (6). However,
corticosteroid therapy may increase the occurrence of opportunistic
infections, by causing deterioration of cell-mediated immunity
(7,8). Thus, the present meta-analysis aimed to
evaluate the effects of adjunctive corticosteroid treatment for PCP
in patients co-infected with HIV and to provide suggestions for
clinical practice.
Materials and methods
Search methods
In order to analyze relevant RCTs, a search of the
literature from the earliest available date to March 2014 was
performed using various websites, including PubMed (http://www.ncbi.nlm.nih.gov/pmc/), Embase
(https://www.elsevier.com/solutions/embase-biomedical-research)
and Ovid (http://gateway.ovid.com/), using the
following keywords: Corticosteroids; glucocorticoide; cortisol;
corticosterone; HIV/AIDS; P. jiroveci pneumonia; and
PCP.
Inclusion criteria
The following inclusion criteria were used in the
present meta-analysis: i) Only RCT; ii) object of study, PCP in
patients with HIV; iii) intervening measure, adjunctive
corticosteroids treatment for patients with HIV in addition to the
standardized treatment in the experimental group, and the use of
placebo based on the standardized treatment, or standardized
treatment alone administered to the control group; iv) patient
reported outcomes, the mortality rate of patients following ~1
month (28–35 days); and v) language, English.
Exclusion criteria
Studies that met the inclusion criteria were
excluded according to the following criteria: Experimental design
was not strict (i.e. lacked a control group); sample data was
unclear or missing and experimental data could not be extracted;
the information was not accurate and the data could not be used; or
the content of the literature was repetitive.
Assessment of study quality
Standard Jadad scoring was used to assess the
methodological quality of the included RCTs, based on the adequacy
of randomization, blinding and follow-up, with a maximum score of 5
points. A score of 0–2 indicated low quality, whereas a score of
3–4 indicated higher quality and a score of 5 denoted high
quality.
Data synthesis and meta-analysis
In order to calculate the relative risk (RR) with
95% confidence interval (CI), data were analyzed using Stata 11.0
software (StataCorp LP, College Station, TX, USA). The
χ2 test was used to analyze the heterogeneity of the
RCTs. P>0.05 indicated that there was no statistically
significant heterogeneity, therefore a fixed-effects model was
applied using the Mantel-Haenzel (M-H) method; whereas P<0.05
indicated statistically significant heterogeneity, therefore a
random-effects model was applied using the method proposed by
DerSimonian and Laird (9).
Evaluating bias
In the present meta-analysis, potential publication
bias was examined using a funnel plot. The symmetrical
characteristics of the funnel plot were evaluated using the test
proposed by Begg and Mazumdar (10)
where a symmetrical result demonstrated no publication bias, and
asymmetry indicated potential publication bias.
Results
Characteristics of included RCTs
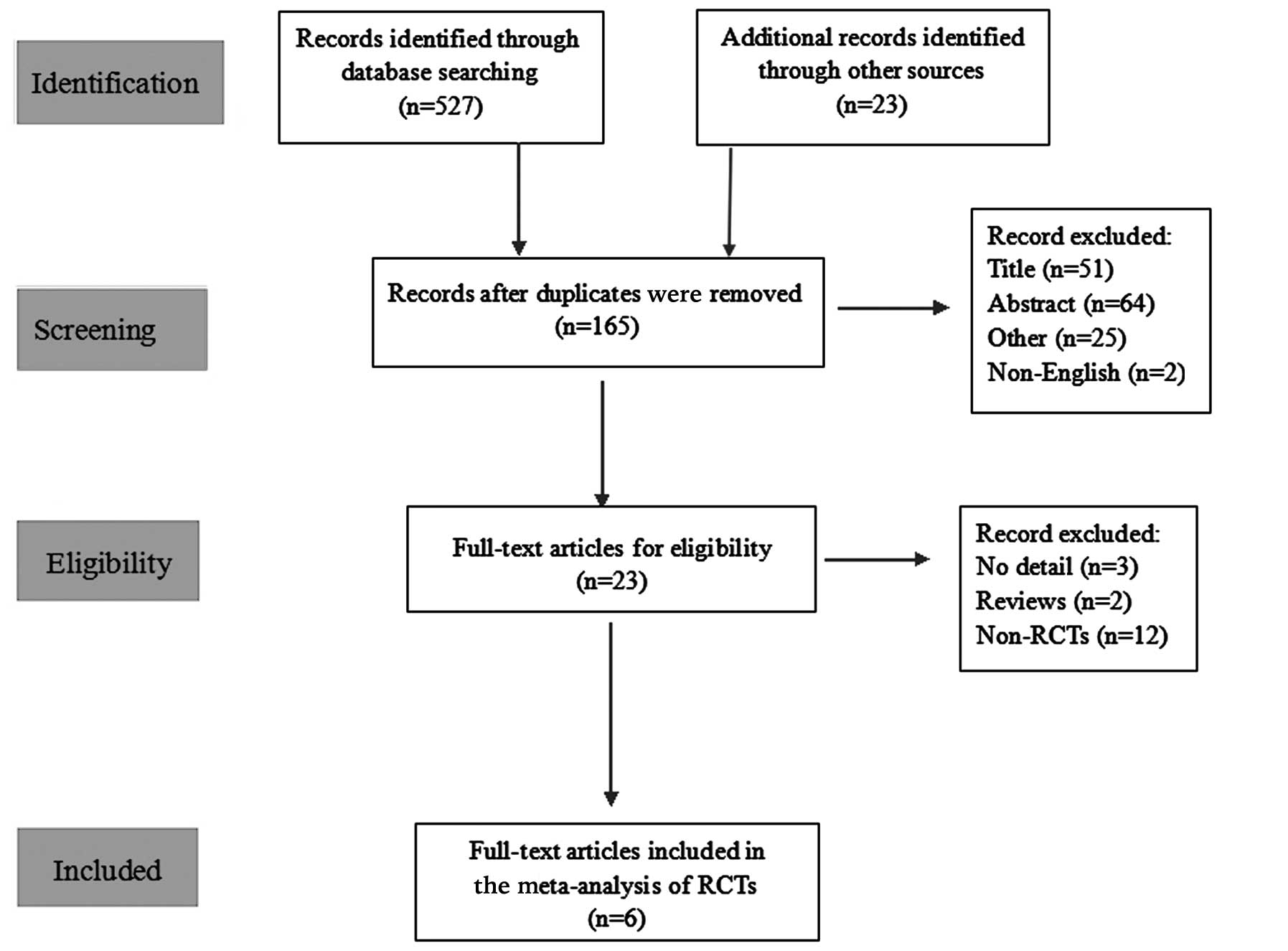
A total of 550 studies were initially included;
however, 527 were subsequently excluded due to duplicate content
(n=385), an irrelevant study topic, abstract or other dependency
(n=140) or because they were not published in English (n=2)
(Fig. 1). Following detailed
examination of the remaining 23 studies and the exclusion of
citations that lacked sufficient detail or were not RCTs, six RCTs
that met the inclusion criteria (11–16) were
finally identified and the meta-analysis was performed. Table I outlines the RCTs included in the
present meta-analysis.
 | Table I.Characteristics of the RCTs included
in the present meta-analysis. |
Table I.
Characteristics of the RCTs included
in the present meta-analysis.
|
|
|
| Mortality rate (%)
| Intervention
|
|---|
| RCT (ref.) | Year | Duration (days) | Experimental
group | Control group | Experimental
group | Control group |
|---|
| Terblanche et
al (11) | 2008 | 35 | 38.3 (18/47) | 47.2
(25/53) | SMZ-TMP +
corticosteroids | SMZ-TMP +
placebo |
| Gagnon et al
(12) | 1990 | 28 | 25 (3/12) | 81.8 (9/11) | SMZ-TMP +
corticosteroids | SMZ-TMP +
placebo |
| Bozzette et al
(13) | 1990 | 31 | 10.6
(13/123) |
21.9 (28/128) | SMZ-TMP +
corticosteroids | SMZ-TMP |
| Nielsen et al
(14) | 1992 | 34 | 6.7 (2/30) | 31.0 (9/29) | SMZ-TMP +
corticosteroids | SMZ-TMP |
| Montaner et al
(15) | 1990 | 30 | 5.6 (1/18) |
0
(0/19) | SMZ-TMP or | SMZ-TMP or |
|
|
|
|
|
| pentamidline +
corticosteroids | pentamidline +
placebo |
| Walmsley et al
(16) | 1995 | 35 | 10 (4/40) | 15.8 (6/38) | SMZ-TMP or | SMZ-TMP or |
|
|
|
|
|
| pentamidline +
corticosteroids | pentamidline +
placebo |
Quality assessment of the included
trials
The six included studies were all RCTs, four of
which were double-blinded (11,12,15,16) and
two were not (13,14). The respective control groups received
placebo (11,12,15,16) or
null (13,14) treatment (Table II). No significant differences were
determined between the characteristics from the six trials. No
patient withdrawal or dropout occurred in the six included trials.
Jadad scores were as follows: 3 (n=3), 4 (n=2) and 5 (n=1).
 | Table II.Quality assessment of the included
trials. |
Table II.
Quality assessment of the included
trials.
| RCT (ref.) | Randomization | Blinding | Jadad |
|---|
| Terblanche et
al (11) |
Adequate | Double-blind | 5 |
| Gagnon et al
(12) |
Unclear | Unclear | 3 |
| Bozzette et al
(13) |
Adequate | Non-blind | 3 |
| Nielsen et al
(14) |
Adequate | Non-blind | 3 |
| Montaner et al
(15) |
Adequate | Unclear | 4 |
| Walmsley et al
(16) |
Adequate | Unclear | 4 |
Meta-analysis
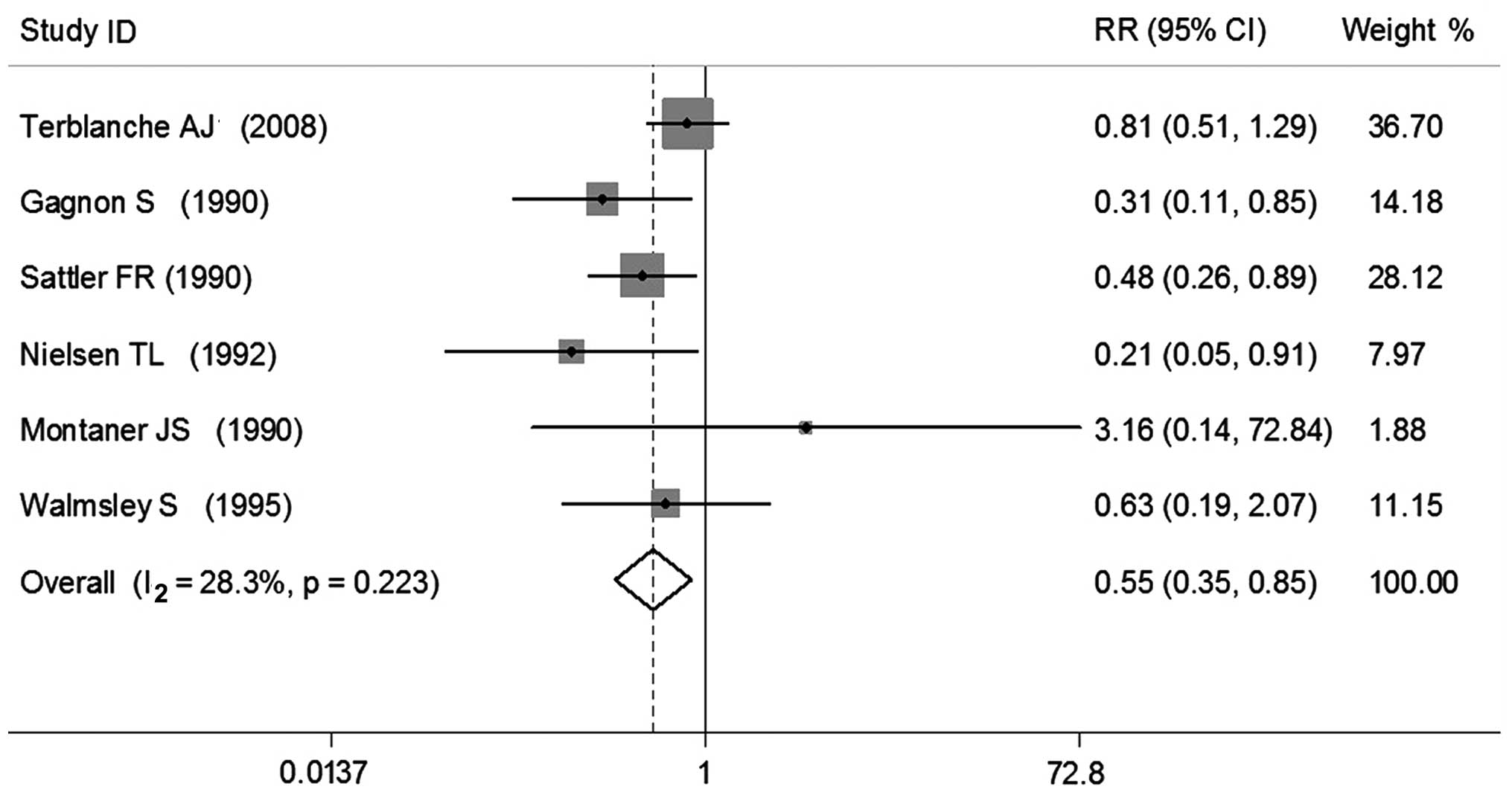
Six RCTs were enrolled in the present meta-analysis,
which included a total of 548 patients with HIV suffering from PCP.
The duration of observation was ~1 month (28–37 days), defined in
terms of the patients' mortality rate. A total of were enrolled in
The experimental groups, which contained 270 patients, demonstrated
a mortality rate of 15.2% (n=41), as compared with 27.7% (n=77) in
the control groups, (n=278). A heterogeneity test demonstrated that
χ2=6.97 (P=0.223; I2=28.3%), therefore no
significant heterogeneity was detected and the M-H method was
employed. Subsequent meta-analysis demonstrated that the
experimental group had a significantly decreased risk of mortality
(0.55 times; P<0.05), as compared with the control group (RR,
0.55; 95% CI, 0.35–0.85) (Fig.
2).
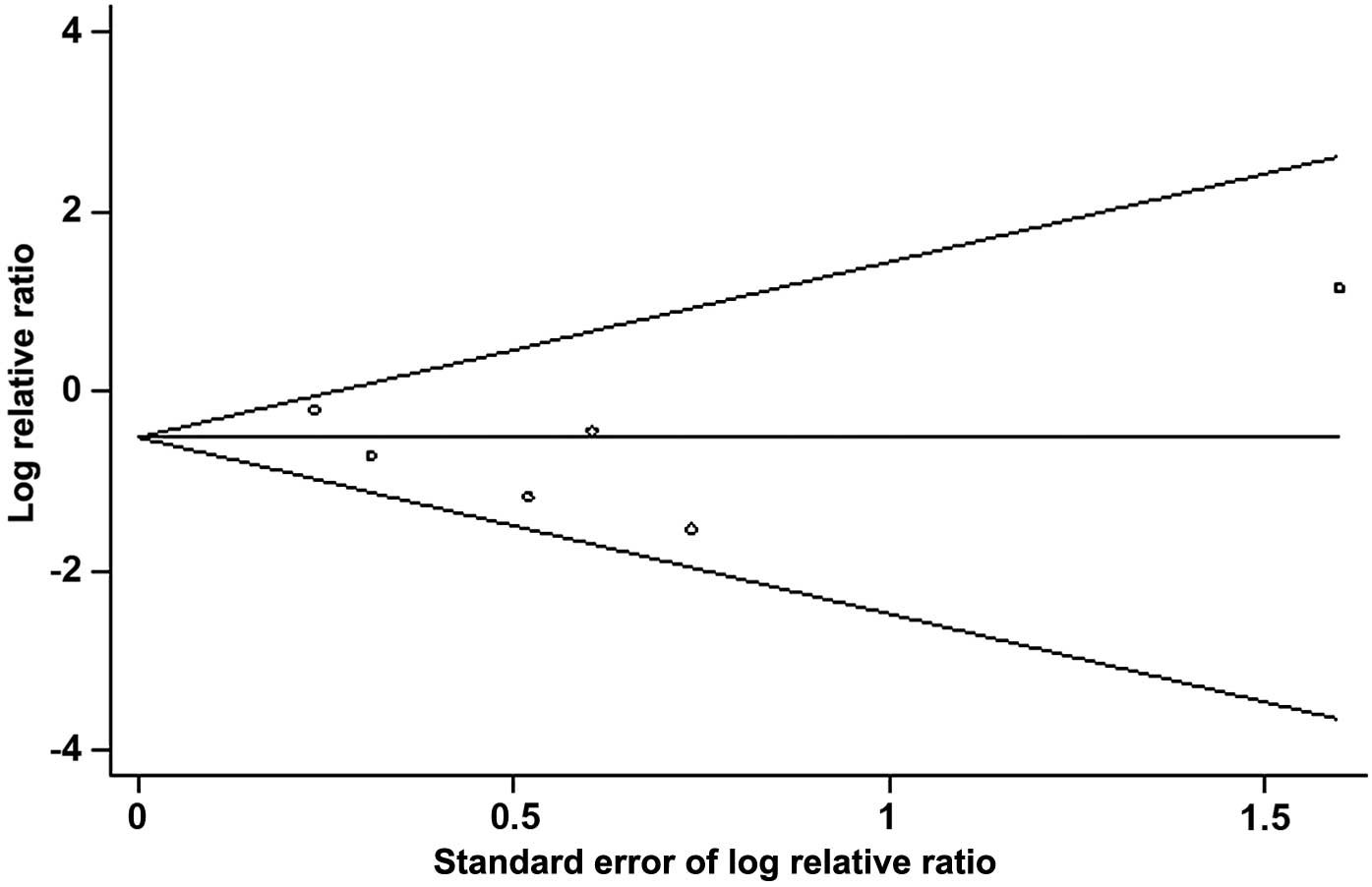
Publication bias
Funnel plot analysis of publication bias in the six
RCTs demonstrated that all six trials were present in the funnel
plot and the majority were in the middle or top sections (Fig. 3). Funnel chart linear regression
showed the bias coefficient was t=0.38 [Pr >|t|=0.707
(continuity corrected)], therefore the result was not statistically
significant. The 95% CI was −3.52 to 2.34. The funnel plot was
considered symmetrical, indicating no publication bias.
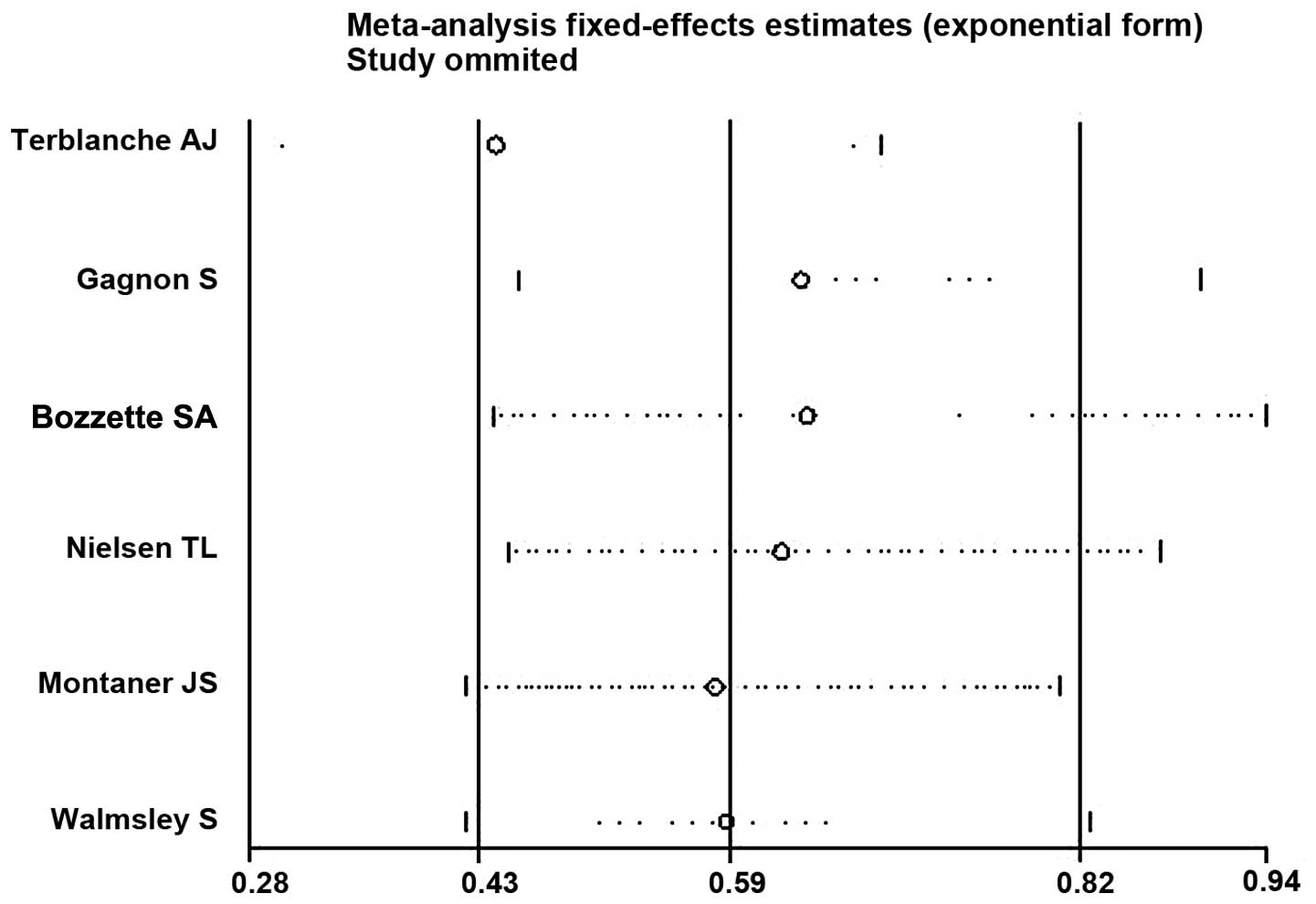
Sensitivity analysis
Sensitivity analysis of the included RCTs was
performed by altering the inclusion criteria, excluding low quality
RCTs and employing different statistical methods for analysis,
using Stata 11.0 software. The results demonstrated that there were
minor differences between the studies and the exclusion of any one
study had minimal effects on the results (Fig. 4). When the random-effects model was
used, the RR was 0.55 (95%CI, 0.35–0.85), approximated to the
fixed-effects model, indicating a small but statistically
insignificant heterogeneity among the included studies.
Discussion
The effective treatment of AIDS and its
complications remains a complex worldwide problem. The incidence of
PCP has increased significantly and it has become the most common
opportunistic infection in patients with HIV (17,18).
Adjunctive corticosteroids were initially suggested as a treatment
for PCP in patients with HIV in 1990, and current treatment
guidelines remain in agreement with this consensus statement
(19).
Six RCTs with 548 cases of PCP in patients with HIV
were enrolled in the present meta-analysis. The smallest sample
size was 23 patients and the largest was 253 patients. The
intervening measure was the use of adjunctive corticosteroids for
the treatment of PCP in patients with HIV, with SMZ-TMP or
pentamidline used as the standard treatment. Various types of
control were used: Bozzette et al (13) and Nielsen et al (14) used blank controls, whereas the
remaining RCTs (11,12,15,16) used
placebo treatments. The results of one study differed from the
other five RCTs. In the study conducted by Montaner et al
(15) the RR was 3.16, suggesting
that patients in the experimental group had a risk of mortality
that was 3.16 times higher compared with the control group.
Notably, the remaining 5 RCTs all indicated that the use of
adjunctive corticosteroids for the treatment of PCP in patients
with HIV may reduce patient mortality. The results of the present
meta-analysis demonstrated that the risk of mortality in the
experimental group was 0.55 times lower compared with the control
group, indicating that the use of adjunctive corticosteroids for
the treatment of PCP in patients with HIV may reduce mortality in
the early phase of the disease.
As bias may have an effect on the credibility of the
present meta-analysis, potential biases were analyzed. Publication
bias is the most common bias to consider when conducting
meta-analyses as positive results are more frequently published. In
the present meta-analysis, potential publication bias was examined
using a funnel plot. The funnel plot analysis of the 6 RCTs
indicated that all the trials were represented in the plot and the
majority were in the middle or top sections. Furthermore, the
results of the linear regression indicated that the funnel plot was
symmetrical, therefore no publications bias was detected in the
present meta-analysis. Two of the studies (15,16)
enrolled in the present meta-analysis used SMZ-TMP or pentamidline
as standard treatment, whereas the others (11–14) used
SMZ-TMP. The results of the heterogeneity test demonstrated that
there was little heterogeneity between them (P=0.223;
I2=28.3%). Furthermore, the results of the sensitivity
analyses demonstrated that this did not have any influence on the
results of the meta-analysis even following the exclusion of any
one study. These results demonstrated that the difference between
using SMZ-TMP/pentamidline and SMZ-TMP monotherapy was within the
acceptable limits, therefore the results of the six RCTs could be
combined for analysis. The results did not change when a
random-effects model was used, demonstrating that there was minimal
publication bias, and that any potential bias would have no
substantial influence on the results of the present
meta-analysis.
The results of the present meta-analysis indicated
that the use of adjunctive corticosteroids for the treatment of PCP
in patients with HIV may reduce mortality in the early stages of
the disease. Therefore, adjunctive corticosteroids may be
considered for the treatment of PCP in patients with HIV. However,
there were a number of limitations to the present meta-analysis: An
insufficient number of RCTs were analyzed; the sample sizes of
certain studies were too low; and there were confounding factors,
such as the origin of literature and the quantity of information,
as not all six of the RCTs were double-blinded studies. Therefore,
the conclusions of the present meta-analysis have certain
limitations. Future studies will require RCTs with larger sample
sizes in order to ensure the analyses of the effects of adjunctive
corticosteroids on PCP in patients with HIV are more comprehensive,
leading to a more reliable conclusion.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81360259), the
Extracurricular Scientific Research Project of Guangxi Medical
University (grant no. WLXSZX1411; Guangxi, China) and the Guangxi
University Science and Technology Research Project (grant no.
KY2015ZD026).
References
|
1
|
Kaplan JE, Hanson D, Dworkin MS, Frederick
T, Bertolli J, Lindegren ML, Holmberg S and Jones JL: Epidemiology
of human immunodeficiency virus-associated opportunistic infections
in the United States in the era of highly active antiretroviral
therapy. Clin Infect Dis. 30((Suppl 1)): S5–S14. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Curtis JR, Yarnold PR, Schwartz DN,
Weinstein RA and Bennett CL: Improvements in outcomes of acute
respiratory failure for patients with human immunodeficiency
virus-related Pneumocystis carinii pneumonia. Am J Respir
Crit Care Med. 162:393–398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
The National: Institutes of H
ealth-University of California Expert Panel for Corticosteroids as
Adjunctive Therapy for Pneumocystis Pneumonia: Consensus statement
on the use of corticosteroids as adjunctive therapy for
pneumocystis pneumonia in the acquired immunodeficiency syndrome. N
Engl J Med. 323:1500–1504. 1990.PubMed/NCBI
|
|
4
|
Klein NC, Duncanson FP, Lenox TH,
Forszpaniak C, Sherer CB, Quentzel H, Nunez M, Suarez M, Kawwaff O,
Pitta-Alvarez A, et al: Trimethoprim-sulfamethoxazole versus
pentamidine for Pneumocystis carinii pneumonia in AIDS
patients: Results of a large prospective randomized treatment
trial. AIDS. 6:301–305. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Briel M, Bucher HC, Boscacci R and Furrer
H: Adjunctive corticosteroids for Pneumocystis jiroveci
pneumonia in patients with HIV-infection. Cochrane Database Syst
Rev. 3:CD0061502006.PubMed/NCBI
|
|
6
|
Panel on Opportunistic Infections in
HIV-Infected Adults and Adolescents, Guidelines for Prevention and
Treatment of Opportunistic Infections in HIV-Infected Adults and
Adolescents: Recommendations from the Centers for Disease Control
and Prevention the National Institutes of Health, and the HIV
Medicine Association of the Infectious Diseases Society of America.
http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdfAccessed.
November 20–2015
|
|
7
|
Wolfe F, Caplan L and Michaud K: Treatment
for rheumatoid arthritis and the risk of hospitalization for
pneumonia, Associations with prednisone, disease-modifying
antirheumatic drugs, and anti-tumor necrosis factor therapy.
Arthritis Rheum. 54:628–634. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ko JH, Peck KR, Lee WJ, Lee JY, Cho SY, Ha
YE, Kang CI, Chung DR, Kim YH, Lee NY, et al: Clinical presentation
and risk factors for cytomegalovirus colitis in immunocompetent
adult patients. Clin Infect Dis. 60:e20–e26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–187. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Terblanche AJ, Green RJ, Rheeder P and
Wittenberg DF: Adjunctive corticosteroid treatment of clinical
Pneumocystis jiroveci pneumonia in infants less than 18
months of age - a randomised controlled trial. S Afr Med J.
98:287–290. 2008.PubMed/NCBI
|
|
12
|
Gagnon S, Boota AM, Fischl MA, Baier H,
Kirksey OW and La Voie L: Corticosteroids as adjunctive therapy for
severe Pneumocystis carinii pneumonia in the acquired
immunodeficiency syndrome. A double-blind, placebo-controlled
trial. N Engl J Med. 323:1444–1450. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bozzette SA, Sattler FR, Chiu J, Wu AW,
Gluckstein D, Kemper C, Bartok A, Niosi J, Abramson I, Coffman J,
et al: California collaborative treatment group: A controlled trial
of early adjunctive treatment with corticosteroids for
Pneumocystis carinii pneumonia in the acquired
immunodeficiency syndrome. N Engl J Med. 323:1451–1457. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nielsen TL, Schattenkerk Eeftinck JK,
Jensen BN, Lundgren JD, Gerstoft J, van Steenwijk RP, Bentsen K,
Frissen PH, Gaub J, Orholm M, et al: Adjunctive corticosteroid
therapy for Pneumocystis carinii pneumonia in AIDS: A
randomized European multicenter open label study. J Acquir Immune
Defic Syndr. 5:726–731. 1992.PubMed/NCBI
|
|
15
|
Montaner JS, Lawson LM, Levitt N, Belzberg
A, Schechter MT and Ruedy J: Corticosteroids prevent early
deterioration in patients with moderately severe Pneumocystis
carinii pneumonia and the acquired immunodeficiency syndrome
(AIDS). Ann Intern Med. 113:14–20. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Walmsley S, Levinton C, Brunton J,
Muradali D, Rappaport D, Bast M, Spence D and Salit I: A
multicenter randomized double-blind placebo-controlled trial of
adjunctive corticosteroids in the treatment of Pneumocystis
carinii pneumonia complicating the acquired immune deficiency
syndrome. J Acquir Immune Defic Syndr Hum Retrovirol. 8:348–357.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thomas CF: Jr andL imper AH: Pneumocystis
pneumonia. N Engl J Med. 350:2487–2498. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ewald H, Raatz H, Boscacci R, Furrer H,
Bucher HC and Briel M: Adjunctive corticosteroids for
Pneumocystis jiroveci pneumonia in patients with HIV
infection. Cochrane Database Syst Rev. 4:D61502015.
|
|
19
|
Benson CA, Kaplan JE, Masur H, Pau A and
Holmes KK: CDC; National Institutes of Health; Infectious Diseases
Society of America: Treating opportunistic infections among
HIV-infected adults and adolescents. Recommendations from CDC, the
National Institutes of Health and the HIV Medicine
Association/Infectious Diseases Society of America. MMWR Recomm
Rep. 53(RR-15): 1–112. 2004.PubMed/NCBI
|