Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of malignant tumor among Chinese individuals, and
typically exhibits an extremely poor prognosis (1). Surgical removal is currently the
preferred option for the treatment of HCC in the majority of cases
(2). However, only 40–50% of
patients that undergo surgery survive for ≥5 years, and the
majority ultimately succumb to HCC recurrence in the liver
(2,3). Although the high rate of tumor
recurrence may be a result of residual tumor cells, there may be an
association between the conduct of the surgery and recurrence in
the liver, as previous studies have indicated that surgical stress
itself increases the chances of tumor metastasis (4–6). Thus,
to reduce the recurrence of HCC following an hepatectomy or liver
transplantation, the cause underlying the emergence of metastasis
following surgery requires investigation.
In cases of HCC, hematogenous metastasis is the
primary cause of metastasis, during which a number of complex
interactions occur between tumor cells and the host (7,8). In the
classical process of hematogenous cancer metastasis, the critical
steps of extravasation include tumor cell adhesion onto the
vascular endothelium (docking), transition to more established cell
contacts (locking), migration through the vascular wall (foothold)
and subsequent remodeling of the target tissue (colonization)
(9,10). Various mediators, including
chemokines, adhesion molecules, kinases and matrix
metalloproteinases (MMPs) are implicated in tumor transendothelial
migration (TEM). Therefore, identifying any alterations in the
expression of these molecules following hepatic
ischemia/reperfusion (I/R) may aid in defining future targets for
tumor therapeutics.
Major blood loss during liver resection and the
requirement for perioperative blood transfusion negatively affects
perioperative morbidity, mortality and long-term outcomes (11,12).
Therefore, strategies to control intraoperative bleeding are
presently applied worldwide. However, such measures may lead to I/R
injury of the liver parenchyma, which is a major cause of hepatic
failure following surgery. I/R damage following standard clamping
is characterized by widespread liver cell death and
microcirculatory disturbances. This damage is mediated by processes
including the induction of free-radical formation, upregulation of
inflammatory cytokines and infiltration of polymorphonuclear
neutrophils (PMNs) into the hepatic parenchyma (13,14), all
of which may produce an ideal milieu for tumor cell TEM (15,16).
Approaches designed to limit I/R damage, which involve controlling
cytokine storms following I/R injury, may be capable of reducing
the incidence of metastasis following surgery.
Peroxisome proliferator-activated receptor-γ (PPARγ)
is one of the three subtypes of the nuclear receptor PPARs
(17,18). Ligands of PPARγ, such as
rosiglitazone, exert the beneficial effect of reducing serum
glucose levels in diabetic patients. However, these ligands can
also induce the negative transcriptional regulation of the nuclear
factor (NF)-κB signaling pathway, which increases the expression of
adhesion molecules and the production of chemokines, and which upon
reperfusion recruit neutrophils to the site of injury (18,19).
Therefore, we hypothesized that I/R injury accelerates the
metastasis of pre-existing tumor cells in the circulation. Thus,
the aim of the present study was to investigate the effects of the
PPARγ agonist, rosiglitazone, on I/R-associated metastasis in mice.
In addition, the influence of GW9662, a specific PPARγ antagonist,
was investigated.
Materials and methods
Reagents
Rosiglitazone and GW9662 were purchased from Cayman
Chemical Company, Inc. (Ann Arbor, MI, USA). Polyclonal rabbit
anti-mouse VCAM-1 antibody (sc8304) was acquired from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) and NF-κB and PPARγ
antibodies (#3034 and #2443, respectively) were from Cell Signaling
Technology, Inc. (Danvers, MA, USA). All other reagents were
purchased from ZSJQ Biotechnology (Beijing, China) unless otherwise
stated.
Experimental animals
All experiments were conducted in accordance with
the guidelines of the animal welfare committee of the Shandong
University Medical Center (Jenan, China). A total of 64 male BALB/c
mice, aged 6–7 weeks, were purchased from the Academy of Military
Medical Sciences of PLA (Beijing, China). All animals were housed
under standard laboratory conditions and allowed free access to
water and food. All animal experiments were conducted in accordance
with the principles and procedures outlined in the Administration
Regulations on Laboratory Animals of Beijing Municipality. The
protocols for animal experiments were approved by the Animal
Experimentation Committee of the Academy of Military Medical
Sciences of the PLA (Beijing, China).
Cell culture
H22 is a mouse HCC cell line with a high potency for
liver metastases, and was purchased from the Cell Culture Center of
the Chinese Academy of Medical Sciences (Beijing, China). The H22
cells were isolated from the ascites of BALB/c mice on day 7
following an abdominal injection of H22 cells (0.2 ml,
1×108 cells/ml). The cell culture medium consisted of
RPMI-1640 supplemented with 10% fetal bovine serum and 100 U/ml
streptomycin and penicillin solution, all of which were provided by
the Research Institute of Hepatobiliary Surgery, Chinese PLA
General Hospital (Beijing, China). Cells were incubated at 37°C in
humidified air with 5% CO2 and 95% O2. For
usage, tumor cells were suspended in phosphate-buffered saline at a
density of 1×107 cells/ml. Each mouse received an
intravenous injection of 5×105 cells suspended in 50 µl
solution.
Mouse model of tumor metastasis
following hepatic I/R
Standardized surgical procedures were performed as
described by van der Bilt et al (20) with appropriate adjustments. Briefly,
the mice were anesthetized with pentobarbital sodium (60 mg/kg,
intraperitoneally). A midline laparotomy was performed and an
atraumatic clip was used to interrupt blood supply to the left
lateral and median lobes of the liver (corresponding to ~70% of the
liver mass). After 45 min of partial hepatic ischemia and 45 min
reperfusion, H22 cells (50 µl) were injected into the portal vein
via a 29-gauge needle attached to a 1-ml syringe. To prevent
bleeding and peritoneal dissemination of the tumor cells, a sterile
cotton sponge was applied to the injection site for 1–3 min until
bleeding stopped. The abdominal wound was then closed in two
layers.
Drugs and treatments
The mice were allocated at random into four groups:
Sham, for which the vessels to the left lateral and median lobes of
the liver were dissected but not interrupted; control, administered
10% dimethyl sulfoxide (DMSO; 2 ml/kg) 1 h prior to ischemia; Ro,
administered rosiglitazone (1 mg/kg) 1 h prior to ischemia; and Ro
+ GW, administered rosiglitazone (1 mg/kg) and GW9662 (1 mg/kg) 1 h
prior to ischemia. Rosiglitazone and GW9662 were prepared in 10%
DMSO and injected intravenously 1 h prior to ischemia,
respectively. For all experiments, the drug concentrations were
calculated such that all animals received equal volumes of
DMSO.
All experimental groups are outlined in Table I. In order to establish the effect of
I/R on hepatic metastasis, mice from the sham and control groups
(n=10 per group) were sacrificed by cervical dislocation 12 days
after surgery. Metastasis of the ischemic and non-ischemic lobes
was scored as the hepatic replacement area (HRA) (20). HRA was defined as the percentage of
liver tissue replaced by tumor tissue, based on four non-sequential
hematoxylin and eosin (H&E)-stained sections. The images were
analyzed using a Leica microscope camera and Biosens Digital
Imaging System analysis system, version 1.6 (Leica Microsystems,
Beijing, China). Survival time was recorded until 12 days after the
surgery.
 | Table I.Description of experimental
groups. |
Table I.
Description of experimental
groups.
| Experiment | Rationale | Group | Procedure | n |
|---|
| 1 | Establish the
effect of I/R on hepatic metastasis | Sham | Laparotomy, liver
manipulation, intraportal injection of H22 tumor cells and closure.
Sacrificed 12 days after surgery | 10 |
|
|
| Control | Intraportal
injection of H22 tumor cells after partial hepatic ischemia.
Sacrificed 12 days after surgery | 10 |
| 2 | Determine the
effect of drugs on hepatic metastasis | Ro | As in the control
group, but treated with rosiglitazone 1 h prior to ischemia | 10 |
|
|
| Ro + GW | As in the control
group, but treated with rosiglitazone + GW9662 1 h prior to
ischemia | 10 |
| 3 | Quantify the
expression of metastasis.associated proteins | Sham | Samples collected
after 45 min sham ischemia and 2, 8 and 24 h reperfusion |
6a |
|
|
| Ro | Treated with
rosiglitazone 1 h prior to ischemia. Samples collected after 45 min
ischemia and 2, 8 and 24 h reperfusion |
6a |
|
|
| Control | Samples collected
after 45 min ischemia and 2, 8 and 24 h reperfusion |
6a |
|
|
| Ro + GW | Treated with
rosiglitazone + GW9662 1 h prior to ischemia. Samples collected
after 45 min ischemia and 2, 8 and 24 h reperfusion |
6a |
The second experiment was designed to determine the
effect of the drugs on hepatic metastasis in mice. Mice from the Ro
and Ro + GW groups (n=10 per group) were sacrificed 12 days after
surgery. Liver samples were obtained and the metastasis and
survival time were scored as described above.
The third experiment was designed to quantify the
expression of various metastasis-associated proteins. Mice were
sacrificed at 2, 8 and 24 h after the initiation of reperfusion
(n=6 per group at each time-point). Liver samples were obtained for
evaluation by light microscopy or storage at −80°C until tissue
analysis.
Histochemistry and
immunohistochemistry
For light microscopy, sections of the left lobe of
the liver were fixed in 10% phosphate-buffered formalin for ≥5
days. The resulting paraffin-embedded sections (5 µm) were stained
with H&E for routine histological examination according to
standard procedures. Vascular cell adhesion molecule (VCAM)-1
protein was stained using immunohistochemical techniques
(streptavidin peroxidase). In brief, deparaffinized sections were
incubated with 3% H2O2 to block endogenous
peroxidases and with 0.5% goat normal serum to block nonspecific
binding sites. Polyclonal mouse anti-VCAM-1 antibodies (1:50) were
used as primary antibodies. Biotinylated anti-goat rabbit
immunoglobulin antibodies were used as secondary antibodies for
streptavidin-biotin complex peroxidase staining. The labeling was
visualized by immersing the slides in prepared diaminobenzidine
solution (1:20) for 3–7 min. The slides were then examined using a
light microscope (CKX31; Olympus, Shanghai Fulai Optical Technology
Co., Ltd., Shanghai, China) and the VCAM-1 positive cells were
counted in 10 high-power fields. The labeling index was expressed
as the percentage of total hepatocytes counted.
Biochemical determinations
Alanine aminotransferase (ALT) and myeloperoxidase
(MPO) levels were measured using ALT/GPT and MPO ELISA kits (ZSJQ
Biotechnology, Inc., Beijing, China), following the manufacturer's
protocol. Briefly, 100 mg liver tissue was homogenized in 2 ml
buffer A, which consisted of 3.4 mmol/l
KH2HPO4 and 16 mmol/l
Na2HPO4 at pH 7.4. After centrifugation for
20 min at 10,000 × g, the pellet was resuspended in 10 volumes of
buffer B, which consisted of 43.2 mmol/l
KH2HPO4, 6.5 mmol/l
Na2HPO4, 10 mmol/l ethylenediaminetetraacetic
acid and 0.5% hexadecyltrimethylammonium at pH 6.0. Liver samples
were subsequently sonicated for 10 sec following treatment with
3,3,5,5-tetramethylbenzidine and the optical density was recorded
at 655 nm using a UV-2000 spectrophotometer (UNICO, Dayton, NJ,
USA).
Electrophoretic mobility shift assay
(EMSA)
Nuclear extracts from the ischemic lobe of the mouse
liver tissue were prepared according to previously described
methods (17) and analyzed using an
EMSA. The 5′-biotin-labeled probes for PPARγ and NF-κB BiotinLight™
Chemiluminescent EMSA kit (Exprogen Biotechnology, Inc., Beijing,
China) were purified with QIAquick Gel Extraction kit (Qiagen,
Hilden, Germany). An EMSA was performed using a BiotinLight™
Chemiluminescent EMSA kit (Exprogen Inc., Beijing, China). A total
of 2 mg purified protein was incubated with the probe at 30°C for
20 min in a 20-ml binding reaction containing 1X binding buffer, 5
mM MgCl2, 2.5% glycerol, 0.05% NP-40, 1 mg poly(dI-dC),
and 10 fmol biotin-labeled probe. Competitor experiments with 50-
and 100-fold excesses of unlabeled probe as a specific competitor
or poly(dI-dC) as a nonspecific competitor were used to demonstrate
the specificity of protein binding. Samples were subjected to
electrophoresis at 120 V in 1% agarose gel with 0.5X
Tris-borate-EDTA for 1.5 h; the gel was then electrophoretically
transferred to a nylon membrane at 380 mA for 60 min, and
cross-linked DNA was transferred to the membrane using a UV-light
cross-linker (UVP, LLC, Upland, CA, USA). After the membrane was
cross-linked, biotin-labeled DNA was detected by chemiluminescence,
which was developed using a chemiluminescence imaging system
(Bio-Rad, Shanghai, China). The membrane was exposed to X-ray film
for 5–10 min. PPARγ and NF-κB activities were determined from the
integrated density value of the band.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Liver samples were stored at −80°C until total RNA
extraction using TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA). The expression levels of VCAM-1 and PPARγ in
the ischemic liver were quantified using RT-qPCR. The sequences for
the VCAM-1, PPARγ and β-actin specific primers are displayed in
Table II. In brief, amplification
and detection were performed using the ReverTra Ace qPCR RT kit
(FSQ-101; Toyobo, Osaka, Japan), according to the manufacturer's
instructions, and FastStart Universal SYBR Green Master (Roche,
Basel, Switzerland). The analysis was conducted using a LightCycler
qPCR apparatus (Bio-Rad, Hercules, CA, USA) with the following
reaction profile: 10 min at 95°C, 40 cycles at 95°C for 25 sec,
55°C for 25 sec, 72°C for 50 sec and 72°C for 5 min. All primers
and probes were purchased from SBS Genetech Co. Ltd. (Beijing,
China). The expression of each mRNA was normalized against β-actin
prior to the calculation of the fold change. The fold increase in
the expression of each mRNA in the ischemic liver lobe was
calculated.
 | Table II.Primers for VCAM-1, PPARγ and
β-actin. |
Table II.
Primers for VCAM-1, PPARγ and
β-actin.
| Gene (bp) | Upstream
primer | Downstream
primer |
|---|
| VCAM-1 (387) |
5′-TCGCGGTCTTGGGAGCCTCA-3′ |
5′-CCGTGACCGGCTTCCCAACC-3′ |
| PPARγ (91) |
5′-GGGCAAGAGAATCCACGAAG-3′ |
5′-GTTGTTGCTGGTCTTTCCCG-3′ |
| β-actin (93) |
5′-CAGAAGGAGATTACTGCTCTGGCT-3′ |
5′-GGAGCCACCGATCCACACA-3′ |
Gelatin zymography for MMP-2/9
activity
Zymography was used to assay MMP enzyme expression
as described by Herron et al (21) in tissue extracts following the
manufacturer's instructions. Gelatinolytic bands were scanned and
digitized for quantification of band intensity using Gel-Pro
Analyzer software, version 3.1 (Cold Spring Harbor Laboratory).
Statistical analysis
Data are expressed as mean ± standard error of the
mean. Data were analyzed by one-way analysis of variance with a
subsequent Student-Newman-Keuls test. The Kaplan-Meier method with
log rank test was used for survival analysis. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analysis was performed using SPSS software, version
13.0 (SPSS Inc., Chicago, IL, USA).
Results
Rosiglitazone significantly inhibits
tumor metastasis following hepatic I/R
The sham and control groups were compared to
determine whether hepatic I/R affects liver metastasis following
the portal injection of H22 cells. A total of 2/10 control group
mice survived for 12 days post-surgery, and the majority of mice in
the control group (9/10) developed hepatic metastases. In the sham
group, 5/10 mice survived 12 days (Fig.
1A). Histopathological examination revealed a clear margin
between the tumor and normal liver tissue. Furthermore, necrotic
areas were observed in all liver sections, covering 5–10% of the
liver tissue in the sham group and 15–25% in the control group with
accumulated PMNs. Tumor metastasis was located predominantly in
proximity of the necrotic areas (Fig.
1B). The largest tumor metastases were observed in the Ro + GW
group (Fig. 1C). Furthermore, 2 mice
developed renal metastases (Fig. 1D)
and 1 mouse developed lung metastases in the Ro + GW group. As
presented in Fig. 1E, in the sham
group, the left lobe of the liver exhibited fewer micrometastases
compared with the left lobe of the control group, which was
ischemic, as evaluated by the percentage of HRA (P=0.0032). No
statistically significant difference was observed in tumor load
between the right lobe of the sham mice and the right lobe
(non-ischemic lobes) of mice subjected to I/R (P=0.089). Therefore,
hepatic I/R increased the development of hepatic metastasis in
portal-injected tumor cells in mice. In the Ro group, 4/10 mice
survived at the selective time-point, but none survived in the Ro +
GW group (P=0.041, Ro vs. control group; P<0.001, Ro + GW vs.
control group). A marked increase in tumor load was observed in the
control and Ro + GW groups. Significant differences were observed
in tumor load in the left ischemic lobes of the control and Ro
groups (P=0.01009). Mice in the Ro + GW group exhibited a
detectable but insignificant acceleration of tumor metastases
compared with the control group (P=0.064).
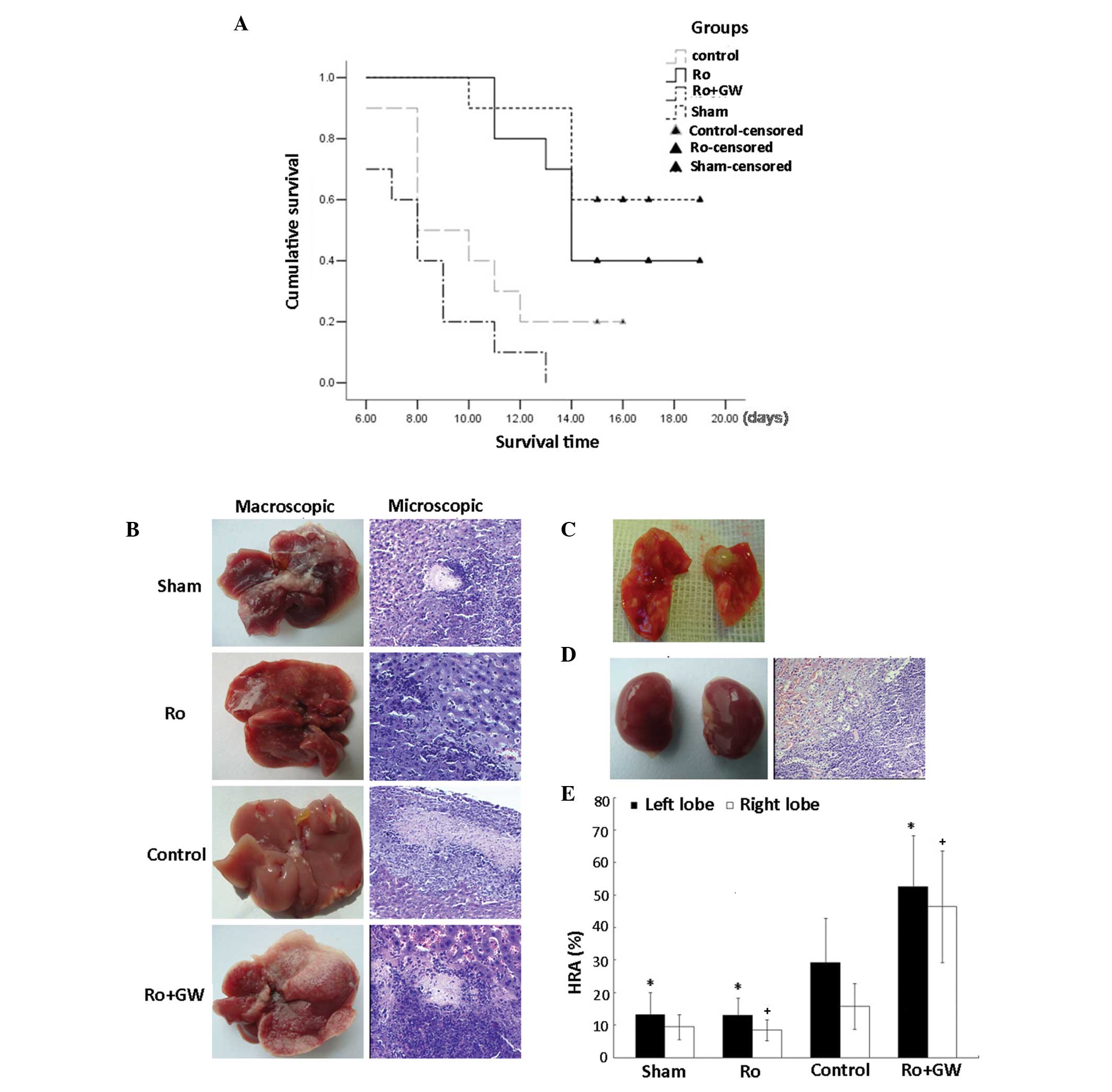 | Figure 1.Effect of ischemia/reperfusion (I/R)
on hepatic metastasis in a mouse model. (A) Median survival times
were as follows: Sham group, 16.6 days; control group, 10.3 days;
Ro group, 15.3 days; and Ro + GW group, 8.3 days. P=0.011, sham vs.
control group; P=0.041, Ro vs. control group; and P=0.138, Ro + GW
vs. control group. Tumor metastases were examined macroscopically
and using hematoxylin and eosin-stained tissue sections
(magnification, ×200). (B) Macroscopic and microscopic evaluation
in the sham, Ro, control and Ro + GW groups 12 days after the
procedure. Under macroscopic examination, metastases were
identified in all groups. (C) The greatest amount of lung
metastasis was observed in the Ro + GW group. (D) In addition,
kidney metastases were primarily observed in the Ro + GW group. (E)
Liver tumor load presented as hepatic replacement area (HRA).
*P<0.05 vs. control group left lobe; +P<0.05, vs.
control group right lobe. Ro, rosiglitazone; Ro + GW, rosiglitazone
and GW9662. |
Protective effect of PPARγ activation
on liver function
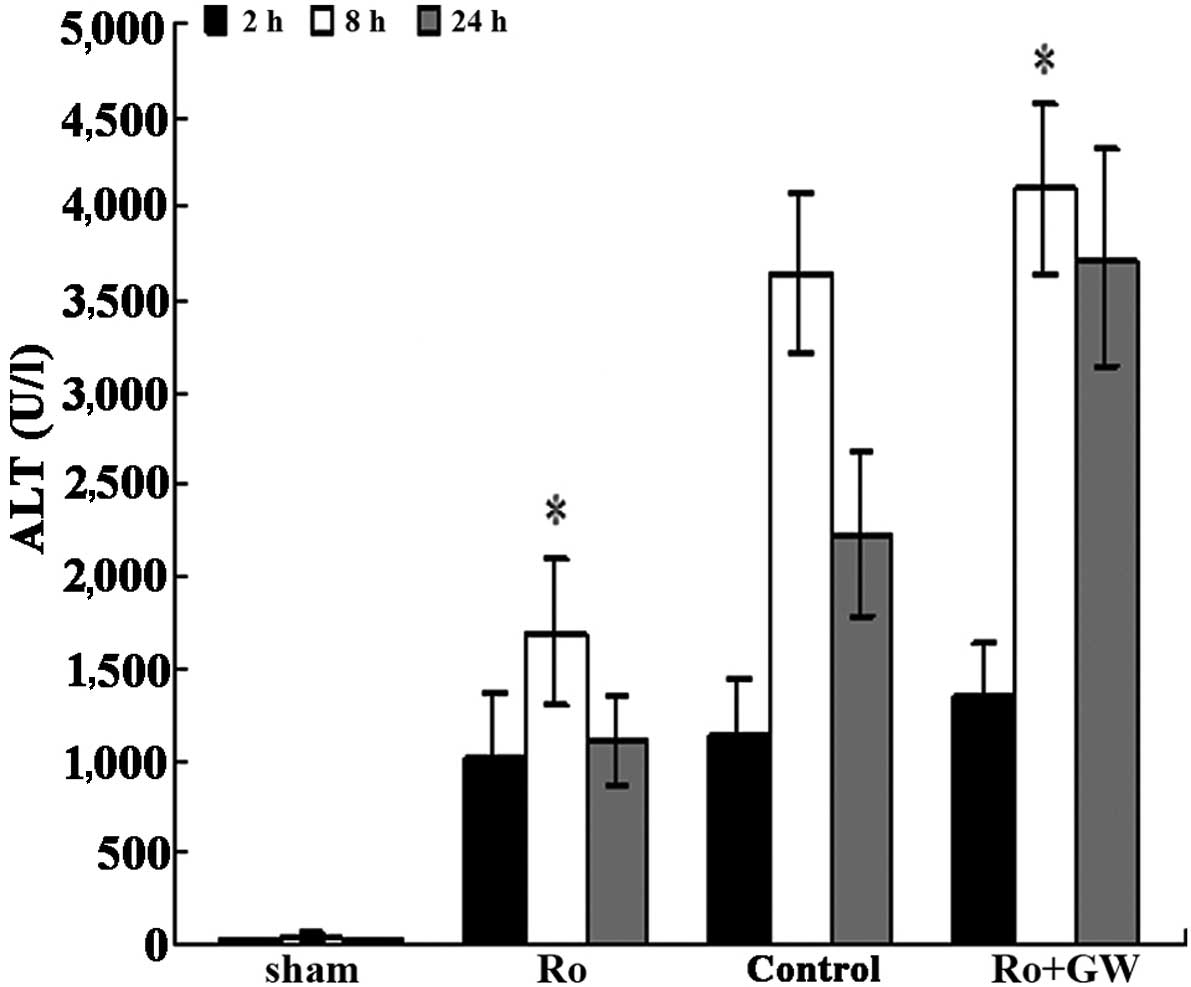
The degree of damage to liver function was
determined by measuring ALT expression levels. Mice that were
subjected to 70% hepatic ischemia followed by 8 h of reperfusion
exhibited a significant increase in ALT expression levels compared
with those in the sham group; the increase observed at 8 h was
particularly marked (3,649.1±440.1 vs. 45.5±18.3 U/l,
respectively). Rosiglitazone appeared to exert an insignificant
effect on I/R liver injury at 2 h reperfusion compared with that in
the control group (ALT, 1,017.3±365.9 vs. 1,134.2±320.5 U/l,
respectively; P=0.191). However, PPARγ activation caused a
significant reduction in ALT expression levels after 8 h
reperfusion in the Ro group compared with the control group (ALT,
1,691.9±398.6 vs. 3,649.1±440.1 U/l, respectively; P<0.0001). In
the mice of the Ro + GW group, the protective action of
rosiglitazone on ALT expression levels was significantly diminished
by GW9662 at the 8 and 24 h time points (P<0.001, Ro + GW group
vs. the Ro group; Fig. 2).
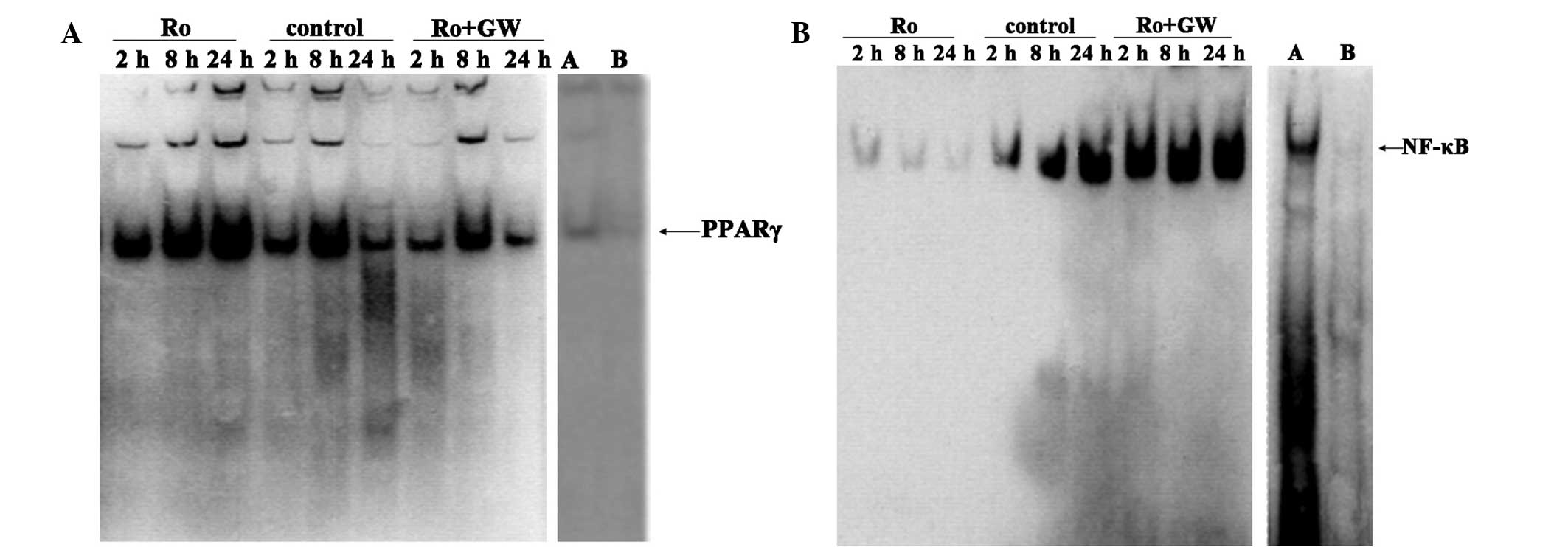
PPARγ agonist inhibits local immune
activation
To clarify the potential molecular mechanisms
underlying the protective effect of the PPARγ agonist on liver
I/R-associated metastasis, the local expression levels of VCAM-1
and MPO were evaluated in the liver at 2, 8 and 24 h after
reperfusion (Fig. 3A–C and E). The
data indicate that after 8 h of reperfusion, there was a ≥4-fold
increase in hepatic VCAM-1 mRNA levels in the control group
compared with the sham group (P<0.001). Furthermore, PPARγ
agonist treatment significantly downregulated local VCAM-1 mRNA
expression levels compared with those in the control group (P=0.002
at 2 h; P=0.0037 at 8 h; P=0.035 at 24 h). Immunohistochemistry was
used to determine the expression of VCAM-1 at the protein level and
the results were similar to those for VCAM-1 mRNA (Fig. 3B and E). To determine whether
rosiglitazone pretreatment was accompanied by reduced PMN
sequestration, the MPO levels in the liver were determined. Mice
that were treated with rosiglitazone prior to I/R injury exhibited
reduced MPO levels, indicating reduced neutrophil accumulation,
compared with those in the control group (P=0.104 at 2 h; P=0.056
at 8 h; P=0.037 at 24 h). The effects of rosiglitazone on MPO
levels were inhibited in the Ro + GW group mice at all time points
(Fig. 3C), as were the effects on
PPARγ (Fig. 3D).
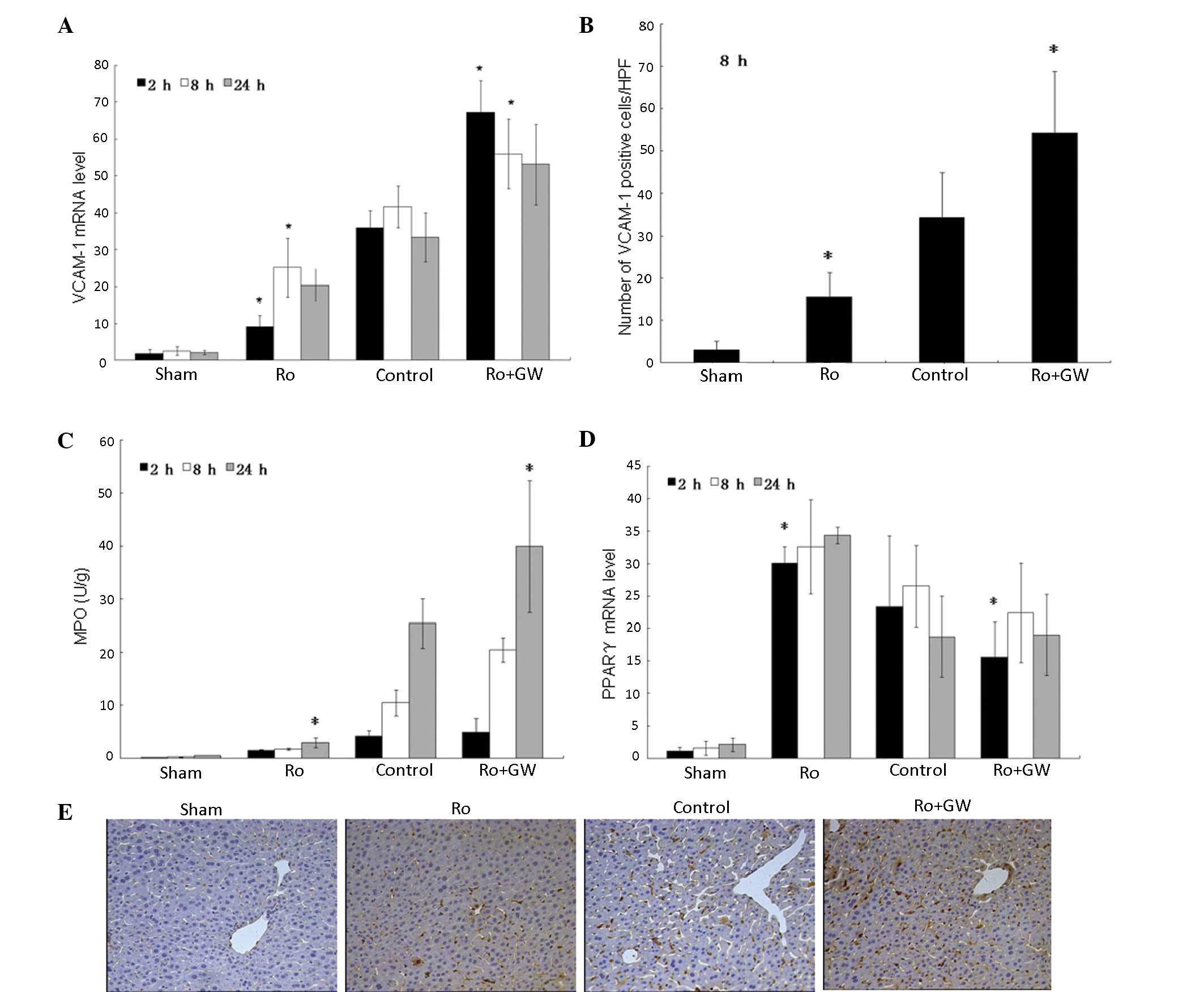 | Figure 3.Expression of VCAM-1, MPO and PPARγ
following I/R of the liver in the four groups. (A, B and E) VCAM-1,
(C) MPO and (D) PPARγ levels were detected in the liver homogenates
of the four groups. VCAM-1 and MPO were detected after 2 h of
reperfusion, and the proteins and mRNA were highly expressed after
8 and 24 h of reperfusion. (A) After 8 h of reperfusion, there was
a ≥4-fold increase in hepatic VCAM-1 mRNA levels in the control
group compared with the sham group (P<0.001) and PPARγ agonist
treatment significantly downregulated local VCAM-1 expression
compared with that in the control group (P=0.002 at 2 h; P=0.0037
at 8 h; P=0.035 at 24 h). (B and E) The expression of VCAM-1 in the
liver at 8 h after reperfusion showed similar results to the VCAM-1
mRNA levels. Positive cells are stained brown (magnification,
×100). To determine whether rosiglitazone pretreatment was
accompanied by decreased PMN sequestration, liver MPO levels were
measured and showed that mice that were treated with rosiglitazone
prior to I/R injury had reduced neutrophil accumulation compared
with the control group (P=0.104 at 2 h; P=0.056 at 8 h; P=0.037 at
24 h), and the effects were abolished by GW9662 at all time-points.
(D) PPARγ levels in the four groups. VCAM-1, vascular cell adhesion
molecule 1; Ro, rosiglitazone; HPF; high power field; GW, GW9662;
MPO, myeloperoxidase; PPARγ, peroxisome proliferator-activated
receptor-γ; I/R, ischemia/reperfusion. *P<0.05 vs. control group
at the same time-point. |
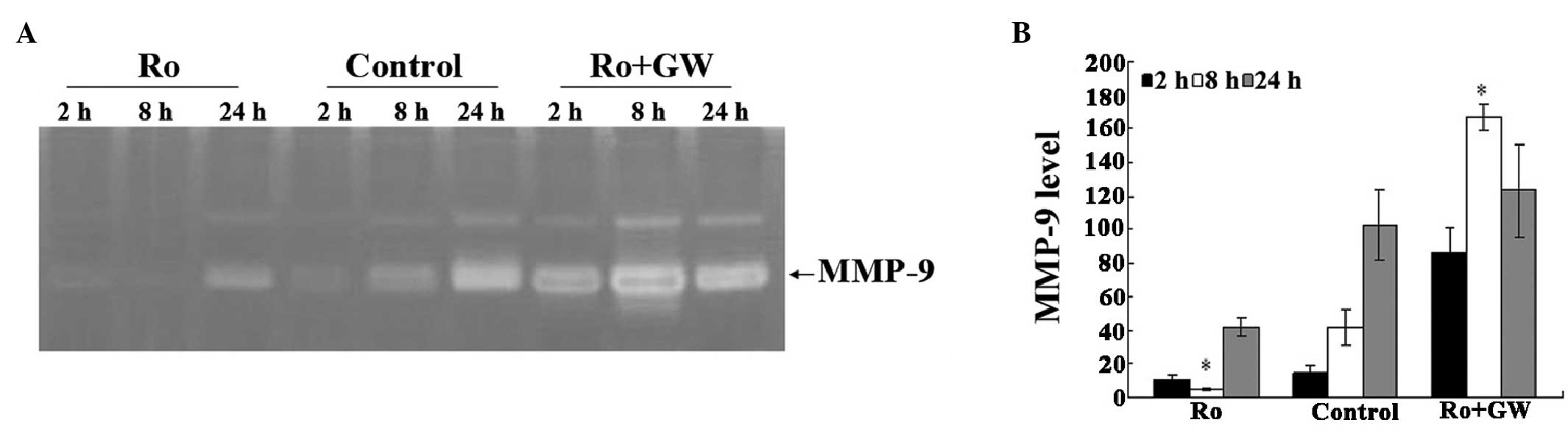
I/R-induced expression of MMP-9 is
inhibited by rosiglitazone in the liver of the mice
Whether activation by a PPARγ agonist inhibited
matrix degradation at the protein level was investigated. Samples
were assessed via gelatin zymography. As hypothesized, MMP activity
was detected in the hepatic homogenates after 2 h reperfusion, and
MMP was highly expressed at 8 and 24 h after reperfusion. I/R
significantly increased the activity of MMP in the liver as the
reperfusion time increased. An intravenous injection of
rosiglitazone notably reduced MMP activity. Almost undetectable
bands were observed in the liver homogenates of the
rosiglitazone-treated mice after 2 h perfusion compared with the
other groups. By contrast, a prominent band was observed in the
control and Ro + GW groups compared with the Ro group at the same
time-point (Fig. 4). Furthermore,
the molecular markers indicated that the band observed corresponded
to MMP-9. Conversely, MMP-2 activity was almost undetectable in all
groups at 2, 8 and 24 h after reperfusion (data not shown). Thus,
the results indicate that MMP-9 is the major MMP involved in
gelatinolysis.
Effects of rosiglitazone on NF-κB
signaling
To identify the intracellular signaling pathways
potentially involved in the protective effect exerted by
rosiglitazone pretreatment, EMSA analysis was used to measure PPARγ
and NF-κB p65 activation. Liver I/R activated NF-κB p65 in a
time-dependent manner. NF-κB p65 was maximally activated after 8 h
reperfusion and the activation persisted until 24 h reperfusion.
Rosiglitazone inhibited the I/R-induced activation of NF-κB p65
after 8 and 24 h reperfusion. The preservation of NF-κB p65
activity afforded by rosiglitazone was attenuated by GW9662
pretreatment at all reperfusion time points (Fig. 5).
Discussion
Tumor metastasis is influenced by a wide range of
factors, including cellular adhesion molecules, extracellular
matrix proteins, proteases and chemokines (7). In the current study, short-term
treatment of mice with rosiglitazone, a potent PPARγ agonist,
conferred protection against hepatic I/R-induced tumor metastasis
via a number of mechanisms.
It is widely accepted that tumor metastases occur
more frequently following surgical stress (4–6,16); however, the molecular and cellular
mechanisms underlying this phenomenon remain largely unknown.
Previous studies have demonstrated that hepatic I/R-induced injury
during surgery may activate a number of proinflammatory cytokines,
including E-selectin (5), vascular
endothelial growth factor (22) and
MMPs (16), which promote tumor
invasion and metastasis. Adhesion of tumor cells onto the vascular
endothelium is a prerequisite for tumor cell extravasation.
Inhibition of the cytokines involved in this mechanism may
represent a potential approach to limiting metastasis following
hepatic I/R. Therefore, promoting tumor metastasis through hepatic
I/R may be a multifactorial process. Reducing the cytokines
involved may serve additional key functions in the reduction of
tumor metastasis following I/R.
First, hepatic I/R was confirmed to promote the
metastases of liver tumor cells. Intraportal injection of H22 tumor
cells following I/R resulted in the formation of a number of
metastatic foci on the surface of the liver. The tumor load (scored
using HRA) in the left lobes of the control group was significantly
increased compared with that of the sham group at 12 days after
surgery. Furthermore, it was observed that metastases were
preferentially located in the margin of the visceral surface of the
ischemic lobes. Potential explanations for this observation
include: i) The margin of the visceral surface of the ischemic lobe
is more susceptible to I/R injury compared with other sites of the
liver; ii) metastases of H22 tumor cells are more easily captured
within the microvasculature of the margin of the liver in mice.
In addition, no statistical difference in tumor load
was observed between the right lobe in the sham-operated mice and
the right (non-ischemic) lobes of the mice subjected to I/R
(P=0.089). This result contrasts with a study by Tamagawa et
al (22), which indicated that
cytokines produced locally in response to hepatic ischemia may be
released into the circulatory system, reach the non-ischemic lobe
and bind to receptors on the cancer cells to promote metastases.
However, the authors induced partial hepatic ischemia 3 days after
the tumor cell inoculation, which is inconsistent with the protocol
of the present study. The present study design may better gauge the
effect of I/R on tumor TEM. The present results indicate that
hepatic I/R exerts a local inflammatory effect on the invasion of
tumor cells into circulation and does not involve systemic
cytokines in the blood.
Previous studies have indicated that PPARγ
activation confers hepatoprotective effects against hepatic I/R
(17,23). The results of the present study
verified that the PPARγ-selective agonist rosiglitazone
significantly reduced hepatic injury suffered following I/R,
potentially via ALT and neutrophil sequestration, compared with
that in the control and Ro + GW groups. Furthermore, PPARγ
activation was more evident in the Ro group than in the other
groups. Next, the effects of PPARγ on hepatic I/R-associated
metastasis were investigated. PPARγ treatment significantly
inhibited the increase in tumor load in the mice subjected to
hepatic I/R compared with that in the control group (P<0.05). By
contrast, GW9662 treatment increased the tumor load induced by I/R.
Then, to investigate the pathophysiological role of PPARγ in
I/R-associated metastasis, the expression of a number of
inflammatory molecules associated with liver metastases in mice
were detected after 2, 8 and 24 h reperfusion. These results were
analyzed in an attempt to determine the correlation between
inflammatory mediators and post-operative metastases. The results
indicate that VCAM-1 protein expression, similar to MPO and MMP-9
expression, was virtually undetectable in the sham group, but
significantly increased and peaked after 8 h of reperfusion in the
other 3 groups at all time points. The levels were particularly
elevated during the initial 24 h after I/R, which is a crucial
period for liver cancer metastases. Therefore, elevated
proinflammatory cytokines may be involved in early intrahepatic
metastases.
The present results are consistent with those of
previous studies, which indicate that hepatic I/R induces the
expression of E-selectin and promotes liver metastases of colon
cancer in rats (5,24,25). The
following phenomena may explain how tumor recurrence is enhanced by
I/R-induced inflammatory cytokines. First, inflammatory cytokines
may promote cell adhesion. Tumor cells with higher metastatic
potentials exhibit a significantly higher adhesion capability for
microvascular endothelial cells 24 h after hepatic I/R compared
with that in the absence of I/R (26,27).
Second, inflammatory cytokines promote angiogenesis (28,29).
Third, pro-inflammatory cytokines indirectly stimulate cell
proliferation (30,31) and inhibit cell apoptosis (32).
Extracellular matrix and basement membranes function
as physical barriers to tumor cell metastasis from their primary
site to target organs (33). The
ability of cancer cells to metastasize depends on their ability to
degrade type-IV collagen. MMPs are the primary proteolytic enzymes
involved in the invasion of tumor cells (33,34). In
the present model of H22 cell metastatic tumors, significant
gelatinase activity was detected in the metastatic tumor-bearing
mouse liver. Furthermore, MMP-9 expression was evident in the
homogenates of the tumor-bearing liver tissue. Gelatin zymographic
analysis of the liver homogenates clearly demonstrated that MMP-9
is a major contributor to the gelatinolysis in the tumor-bearing
mouse liver following the intraportal inoculation of H22 tumor
cells. MMP-9 activity was markedly suppressed following the
intravenous injection of rosiglitazone at all time points.
Although PMNs may be cytotoxic to tumor cells, they
have been demonstrated to promote tumor adhesion, transendothelial
migration and facilitate the activation of angiogenesis under
certain circumstances (35,36). MPO is an enzyme restricted primarily
to PMNs and may reflect the number of PMNs in the tissue.
Rosiglitazone reduced MPO activity in the liver compared with that
in the control and Ro + GW groups. This indicates that the PPARγ
agonist reduces PMN infiltration into the liver parenchyma. These
data suggest that the protective ability of rosiglitazone against
hepatic I/R-associated metastases was partially a result of the
reduction in neutrophil sequestration. There are two possible
mechanisms by which PMN may assist tumor cell migration across the
endothelial barrier. The first possibility is that I/R produces
reactive oxygen species from PMNs via the action of cytokines
(33,37), which are able to damage endothelial
cells and produce circulatory disturbances. Tumor cells present in
the bloodstream under these conditions may be rapidly invaded. The
other possibility is that an interaction may occur between PMNs and
tumor cells wherein PMNs, via an adhesion receptor-dependent
mechanism, may bind to tumor cells and facilitate their migration
through the vascular endothelium (36). However, it was not possible to
determine which cells in the liver contributed to the cytokine
activity discussed in the present experimental model. Certain tumor
cells, endothelial cells, macrophages and hepatocytes produce large
amounts of adhesion molecules, chemotactic molecules, inducible
nitric oxide synthase and MMPs (38,39).
Additional data are required to identify the source of these
inflammatory factors in vivo. Collectively, the beneficial
effect of PPARγ agonists on hepatic I/R-associated metastases may
be, at least in part, dependent on the restraints of the local
inflammatory response in the liver.
Aberrantly produced PPARγ may bind to its receptors
and result in the altered activation of particular signaling
pathways, including the NF-κB pathway (17–19). The
NF-κB signaling pathway has been demonstrated to be actively
involved in HCC development by controlling angiogenesis (38), cell motility and cell proliferation
(40,41). Furthermore, the NF-κB pathway is a
key factor in inflammation (39,42).
NF-κB regulates the expression of VCAM-1, MPO and MMP-9, which are
associated with tumor metastases and inflammation (38,39,42).
Thus, it may be hypothesized that the activation of NF-κB by
rosiglitazone, a marker of inflammatory responses frequently
detected in tumors, constitutes a mechanistic link between I/R and
cancer. Thus, NF-κB activation in hepatic I/R is essential for
promoting tumor metastases.
A number of studies have indicated that PPARγ
ligands are potential chemopreventive agents for liver
carcinogenesis (32,40). The mechanisms underlying their
actions appear to involve the inhibition of cell proliferation and
the induction of apoptosis. However, this anticarcinogenic effects
requires an extended treatment period and a flushing dose (>40
mg/kg). In the present study, rosiglitazone (1 mg/kg) was
administered 1 h prior to hepatic I/R and the intravenous injection
of the H22 cells. On the basis of these results, the inhibition of
tumor metastasis in the rosiglitazone-treated mice was highly
unlikely to be due to the direct cytotoxic effects of injected
tumor cells. Further studies are required to eliminate the
possibility of the direct cytotoxic effects of rosiglitazone on H22
cells.
The short-term administration of rosiglitazone can
limit I/R-induced hepatic injury. Thus, this drug may be used in
certain I/R processes, particularly in emergency procedures such as
liver surgery and transplantation, as there is limited time in
which to pretreat patients with PPARγ agonists.
In summary, hepatic I/R results in microcirculatory
disturbances and excessive inflammation, which induce PMNs, VCAM-1
and MMP-9, all of which may serve key functions in the accelerated
metastases of HCCs following I/R. PPARγ activation appears to offer
a promising strategy in metastases therapy by reducing the strong
stimulus of I/R, which promotes hematogenous micrometastases in the
liver. Therefore, the PPARγ agonist rosiglitazone may be an
efficient agent for preventing hepatic I/R-associated
metastases.
Acknowledgements
The present study was funded by a grant from the
China Postdoctoral Science Foundation (no. 2009045513).
References
|
1
|
Fu SY, Lau WY, Li AJ, Yang Y, Pan ZY, Sun
YM, Lai EC, Zhou WP and Wu MC: Liver resection under total vascular
exclusion with or without preceding Pringle manoeuvre. Br J Surg.
97:50–55. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Makuuchi M, Takayama T, Kubota K, Kimura
W, Midorikawa Y, Miyagawa S and Kawasaki S: Hepatic resection for
hepatocellular carcinoma - Japanese experience.
Hepatogastroenterology. 45(Suppl 3): 1267–1274. 1998.PubMed/NCBI
|
|
3
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
Chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsuchiya Y, Sawada S, Yoshioka I, Ohashi
Y, Matsuo M, Harimaya Y, Tsukada K and Saiki I: Increased surgical
stress promotes tumor metastasis. Surgery. 133:547–555. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uotani H, Yamashita I, Nagata T, Kishimoto
H, Kashii Y and Tsukada K: Induction of E-selectin after partial
hepatectomy promotes metastases to liver in mice. J Surg Res.
96:197–203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kooby DA, Stockman J, Ben-Porat L, Gonen
M, Jarnagin WR, Dematteo RP, Tuorto S, Wuest D, Blumgart LH and
Fong Y: Influence of transfusions on perioperative and long-term
outcome in patients following hepatic resection for colorectal
metastases. Ann Surg. 237:860–869; discussion 869–870. 2003.
View Article : Google Scholar
|
|
7
|
Jarrar D, Chaudry IH and Wang P: Organ
dysfunction following hemorrhage and sepsis, Mechanisms and
therapeutic approaches (Review). Int J Mol Med. 4:575–583.
1999.PubMed/NCBI
|
|
8
|
Selzner M and Clavien PA: Failure of
regeneration of the steatotic rat liver: Disruption at two
different levels in the regeneration pathway. Hepatology. 31:35–42.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jaeschke H, Bautista AP, Spolarics Z and
Spitzer JJ: Superoxide generation by Kupffer cells and priming of
neutrophils during reperfusion after hepatic ischemia. Free Radic
Res Commun. 15:277–284. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Higashiyama A, Watanabe H, Okumura K and
Yagita H: Involvement of tumor necrosis factor alpha and very late
activation antigen 4/vascular cell adhesion molecule 1 interaction
in surgical-stress-enhanced experimental metastasis. Cancer Immunol
Immunother. 42:231–236. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nicoud IB, Jones CM, Pierce JM, Earl TM,
Matrisian LM, Chari RS and Gorden DL: Warm hepatic
ischemia-reperfusion promotes growth of colorectal carcinoma
micrometastases in mouse liver via matrix metalloproteinase-9
induction. Cancer Res. 67:2720–2728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miles FL and Pruitt FL: vanG olen KL and
Cooper CR: Stepping out of the flow: Capillary extravasation in
cancer metastasis. Clin Exp Metastasis. 25:305–324. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Da Costa ML, Redmond HP, Finnegan N, Flynn
M and Bouchier-Hayes D: Laparotomy and laparoscopy differentially
accelerate experimental flank tumour growth. Br J Surg.
85:1439–1442. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Konstantopoulos K and Thomas SN: Cancer
cells in transit: The vascular interactions of tumor cells. Annu
Rev Biomed Eng. 11:177–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abdelrahman M, Sivarajah A and Thiemermann
C: Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion
injury, inflammation and shock. Cardiovasc Res. 65:772–781. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cuzzocrea S: Peroxisome
proliferator-activated receptors gamma ligands and ischemia and
reperfusion injury. Vascul Pharmacol. 41:187–195. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuboki S, Shin T, Huber N, Eismann T,
Galloway E, Schuster R, Blanchard J, Zingarelli B and Lentsch AB:
Peroxisome proliferator-activated receptor-gamma protects against
hepatic ischemia/reperfusion injury in mice. Hepatology.
47:215–224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kohn EC: Invasion and metastasis: B iology
and clinical potential. Pharmacol Ther. 52:235–244. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tantivejkul K, Kalikin LM and Pienta KJ:
Dynamic process of prostate cancer metastasis to bone. J Cell
Biochem. 91:706–717. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van der Bilt JD, Kranenburg O, Nijkamp MW,
Smakman N, Veenendaal LM, Te Velde EA, Voest EE, van Diest PJ and
Borel Rinkes IH: Ischemia/reperfusion accelerates the outgrowth of
hepatic micrometastases in a highly standardized murine model.
Hepatology. 42:165–175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herron GS, Banda MJ, Clark EJ, Gavrilovic
J and Werb Z: Secretion of metalloproteinases by stimulated
capillary endothelial cells. Histopathology. J Biol Chem.
261:2814–2818. 1986.PubMed/NCBI
|
|
22
|
Tamagawa K, Horiuchi T, Uchinami M, Doi K,
Yoshida M, Nakamura T, Sasaki H, Taniguchi M and Tanaka K: Hepatic
ischemia-reperfusion increases vascular endothelial growth factor
and cancer growth in rats. J Surg Res. 148:158–163. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akahori1 T, Sho M, Hamada K, Suzaki Y,
Kuzumoto Y, Nomi T, Nakamura S, Enomoto K, Kanehiro H and Nakajima
Y: Importance of peroxisome proliferator-activated receptor γ in
hepatic ischemia/reperfusion injury in mice. J Hepatol. 47:784–792.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu J, Qiao L, Zimmermann L, Ebert MP,
Zhang H, Lin W, Röcken C, Malfertheiner P and Farrell GC:
Troglitazone inhibits tumor growth in hepatocellular carcinoma in
vitro and in vivo. Hepatology. 43:134–143. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang S and Dong C: Integrin VLA-4
enhances sialyl-Lewisx/a-negative melanoma adhesion to and
extravasation through the endothelium under low flow conditions. Am
J Physiol Cell Physiol. 295:C701–C707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Conrad R, Remberger M, Cederlund K,
Hentschke P, Sundberg B, Ringdén O and Barkholt L: Inflammatory
cytokines predominate in cases of tumor regression after
hematopoietic stem cell transplantation for solid cancer. Biol
Blood Marrow Transplant. 12:346–354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hirai T, Matsumoto H, Yamashita K, Urakami
A, Iki K, Yamamura M and Tsunoda T: Surgical oncotaxis - excessive
surgical stress and postoperative complications contribute to
enhancing tumor metastasis, resulting in a poor prognosis for
cancer patients. Ann Thorac Cardiovasc Surg. 11:4–6.
2005.PubMed/NCBI
|
|
28
|
McGary EC, Lev DC and Bar-Eli M: Cellular
adhesion pathways and metastatic potential of human melanoma.
Cancer Biol Ther. 1:459–465. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mendoza L, Carrascal T, De Luca M, Fuentes
AM, Salado C, Blanco J and Vidal-Vanaclocha F: Hydrogen peroxide
mediates vascular cell adhesion molecule-1 expression from
interleukin-18-activated hepatic sinusoidal endothelium
Implications for circulating cancer cell arrest in the murine
liver. Hepatology. 34:298–310. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mendoza L, Valcárcel M, Carrascal T,
Egilegor E, Salado C, Sim BK and Vidal-Vanaclocha F: Inhibition of
cytokine-induced microvascular arrest of tumor cells by recombinant
endostatin prevents experimental hepatic melanoma metastasis.
Cancer Res. 64:304–310. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grommes C, Landreth GE, Sastre M, Beck M,
Feinstein DL, Jacobs AH, Schlegel U and Heneka MT: Inhibition of in
vivo glioma growth and invasion by peroxisome
proliferator-activated receptor gamma agonist treatment. Mol
Pharmacol. 70:1524–1533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nishikawa M, Tamada A, Hyoudou K, Umeyama
Y, Takahashi Y, Kobayashi Y, Kumai H, Ishida E, Staud F, Yabe Y, et
al: Inhibition of experimental hepatic metastasis by targeted
delivery of catalase in mice. Clin Exp Metastasis. 21:213–221.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Janani P, Sivakumari K, Geetha A, Yuvaraj
S and Parthasarathy C: Bacoside A downregulates matrix
metalloproteinases 2 and 9 in DEN-induced hepatocellular carcinoma.
Cell Biochem Funct. 28:164–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshida M, Horiuchi T, Uchinami M, Tabo T,
Kimura N, Yokomachi J, Doi K, Nakamura T, Tamagawa K and Tanaka K:
Intermittent hepatic ischemia-reperfusion minimizes liver
metastasis in rats. J Surg Res. 111:255–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Doi K, Horiuchi T, Uchinami M, Tabo T,
Kimura N, Yokomachi J, Yoshida M and Tanaka K: Hepatic
ischemia-reperfusion promotes liver metastasis of colon cancer. J
Surg Res. 105:243–247. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nozawa H, Chiu C and Hanahan D:
Infiltrating neutrophils mediate the initial angiogenic switch in a
mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA.
103:12493–12498. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Orr FW and Warner DJ: Effects of systemic
complement activation and neutrophil-mediated pulmonary injury on
the retention and metastasis of circulating cancer cells in mouse
lungs. Lab Invest. 62:331–338. 1990.PubMed/NCBI
|
|
38
|
Wu QD, Wang JH, Condron C, Bouchier-Hayes
D and Redmond HP: Human neutrophils facilitate tumor cell
transendothelial migration. Am J Physiol Cell Physiol.
280:C814–C822. 2001.PubMed/NCBI
|
|
39
|
Rogers AB and Fox JG: Inflammation and
Cancer. Histopathology. Am J Physiol Gastrointest Liver Physiol.
286:G361–G366. 2004.PubMed/NCBI
|
|
40
|
Elsharkawy AM and Mann DA: Nuclear
factor-kappaB and the hepatic inflammation-fibrosis-cancer axis.
Hepatology. 46:590–597. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vainer GW, Pikarsky E and Ben-Neriah Y:
Contradictory functions of NF-kappaB in liver physiology and
cancer. Cancer Lett. 267:182–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Baud V and Karin M: Is NF-kappaB a good
target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov.
8:33–40. 2009. View Article : Google Scholar : PubMed/NCBI
|