Introduction
Coronary artery bypass grafting (CABG) has been
recognized as one of the most efficacious methods for treating
coronary heart disease (1).
Sevoflurane and propofol are commonly used anesthetics in CABG
surgery (2,3).
Propofol has a chemical structure that is similar to
that of antioxidants, and has been shown to be able to scavenge
free radicals in vivo (4).
Previous studies have shown that propofol exerts different effects
on various receptors and ion channels of the central nervous system
(5,6). Propofol is able to reduce
β-adrenoreceptor-mediated signal transduction in cardiomyocytes,
via a protein kinase C-dependent pathway. Furthermore, Sayin et
al (7) proposed that propofol is
able to attenuate myocardial lipid peroxidation during CAGB
surgery. Corcoran et al (8)
further suggested that propofol decreases free radical-mediated
lipid peroxidation and the systemic inflammatory response in
patients undergoing CAGB surgery.
Sevoflurane has been shown to serve a protective
function in the pharmacological preconditioning of cardiac events
in patients undergoing CABG (9,10).
Furthermore, sevoflurane reduces the incidence of late cardiac
events during the first year following CABG surgery, which may
occur by downregulating the expression of platelet endothelial cell
adhesion molecule-1 (9). Yao et
al (11) proposed that the
myocardial protection exerted by sevoflurane in CABG surgery is
achieved through the downregulation of troponin I. In addition,
previous meta-analyses have further confirmed the protective effect
of sevoflurane in cardiac surgery (12,13).
Numerous studies have compared the myocardial
protective effects of sevoflurane and propofol in patients
undergoing CABG surgery, and the relative advantages and
disadvantages of the two methods have been presented (14,15). The
use of sevoflurane appears to result in superior outcome compared
with propofol in patients with little or no indication of ischemic
heart disease, including patients undergoing CABG surgery (16). However, sevoflurane exhibits more
marked antioxidative properties compared with propofol in patients
undergoing off-pump CABG, as indicated by the results of a
randomized controlled study (17).
Furthermore, sevoflurane has been shown to possess stronger
myocardial protective effects compared with propofol in patients
undergoing CABG surgery (18).
However, the underlying mechanisms of this protective effect remain
unclear.
Microarray technology enables the global
determination of gene expression levels, and is thus useful for the
elucidation of underlying molecular mechanisms. Therefore,
microarray analysis may useful for determining the effects of
sevoflurane and propofol on gene expression in patients undergoing
CABG. Lucchinetti et al (19)
suggested that gene regulatory control of myocardial energy
metabolism is closely associated with postoperative cardiac
function. In the present study, a data set from the Gene Expression
Omnibus (GEO) database (GSE4386; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE4386)
was downloaded and was subjected to screening for differentially
expressed genes (DEGs). The aim of this study was to determine the
influence of propofol and sevoflurane on postoperative recovery in
patients following CABG. Gene Ontology (GO) and Kyoto Encyclopedia
of Genes and Genomes (KEGG) pathway enrichment analyses were
performed to indicate the underlying molecular mechanisms of any
effects and identify potential biomarkers. Such markers may
facilitate the appropriate selection of sevoflurane or propofol,
thereby improving the outcomes of patients undergoing CABG.
Materials and methods
Gene expression data
The microarray data set GSE4386 was downloaded from
the platform of GeneChip® Human Genome U133 Plus 2.0 Array of the
GEO database (http://www.ncbi.nlm.nih.gov/geo/) (20). The data from a total of 40 samples
was contained in the data set, including data from patients
undergoing CABG surgery combined with sevoflurane treatment (n=10),
propofol treatment (n=10) and control samples (n=20). The control
samples comprised the same patients prior to CABG surgery. Atrial
samples were collected prior to and following CABG surgery to
determine gene expression profiles. The patients were treated in
Triemli Hospital (Zurich, Switzerland). The mean ages of patients
in the propofol and sevoflurane groups were 66.9 and 65.2 years,
respectively. All patients were male. In addition, patients with
hemodynamic instability was excluded. Microarray analysis was
performed based on GSE4386, in which total RNA was prepared from
the frozen cardiac tissue using an RNeasy Mini kit (Qiagen, Hilden,
Germany).
Pretreatment of raw data and DEGs
analysis
Raw data were processed using Log2 transformation
and quantile normalization, using SPSS software, version 17.0
(SPSS, Inc., Chicago, IL, USA). The mRNA expression level was
calculated based on the annotation files of probes. Gene expression
profiles prior to and following anaesthesia with sevoflurane or
propofol were compared using the R statistical program in a Limma
software (21). The threshold of
DEGs was considered to be P<0.05 and log2 (fold-change) of
>1.
Functional enrichment analysis
The database for Annotation, Visualization and
Integration Discovery (DAVID; http://david.abcc.ncifcrf.gov/) (22) provides analytical tools for analyzing
a large list of genes, and was used to perform GO (http://geneontology.org/page/go-enrichment-analysis)
and KEGG (http://www.genome.jp/kegg/) pathway
enrichment analyses for DEGs. P<0.05 was considered to indicate
a statistically significant difference.
Results
Sevoflurane influences more genes
compared with propofol at the transcriptional level
A total of 34,296 mRNA sequences (corresponding to
19,745 genes) were identified following raw data pretreatment.
Compared with the control samples, 242 and 560 DEGs were detected
in patients treated with propofol and sevoflurane, respectively
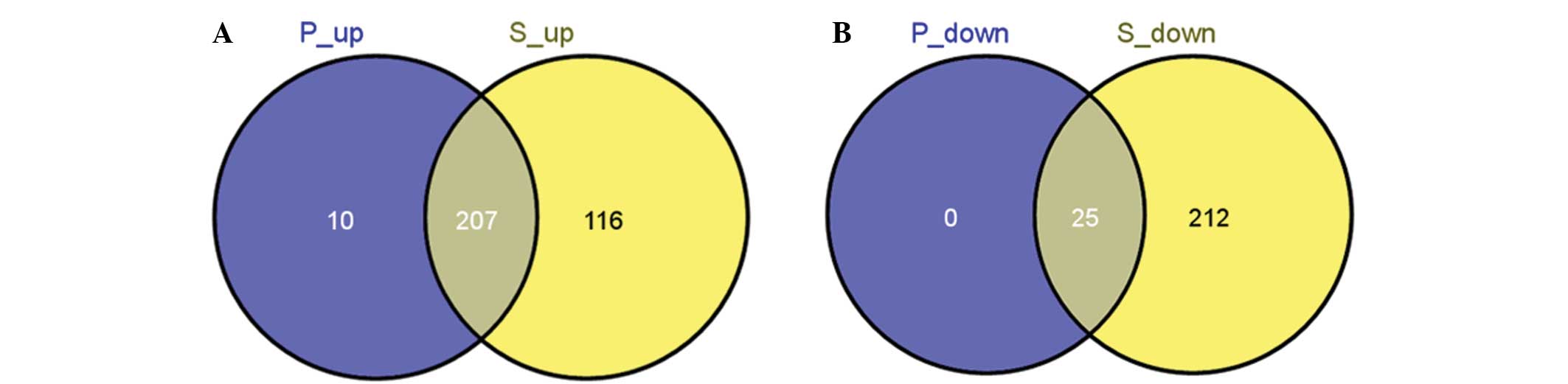
(Table I). Following the comparison
between the DEGs identified in the two treatment groups, 207
upregulated and 25 downregulated DEGs were found to overlap
(Fig. 1). By contrast, 116
upregulated and no downregulated DEGs were unique to sevoflurane,
while 10 upregulated and 212 downregulated DEGs were unique to
propofol.
 | Table I.Differentially expressed mRNA
sequences and genes in propofol and sevoflurane. |
Table I.
Differentially expressed mRNA
sequences and genes in propofol and sevoflurane.
| Parameter | P_up | P_down | S_up | S_down |
|---|
| mRNAs (n) | 353 | 41 | 558 | 408 |
| Genes (n) | 217 | 25 | 323 | 237 |
GO term and KEGG pathway enrichment
analyses
Significantly enriched KEGG pathways and GO terms,
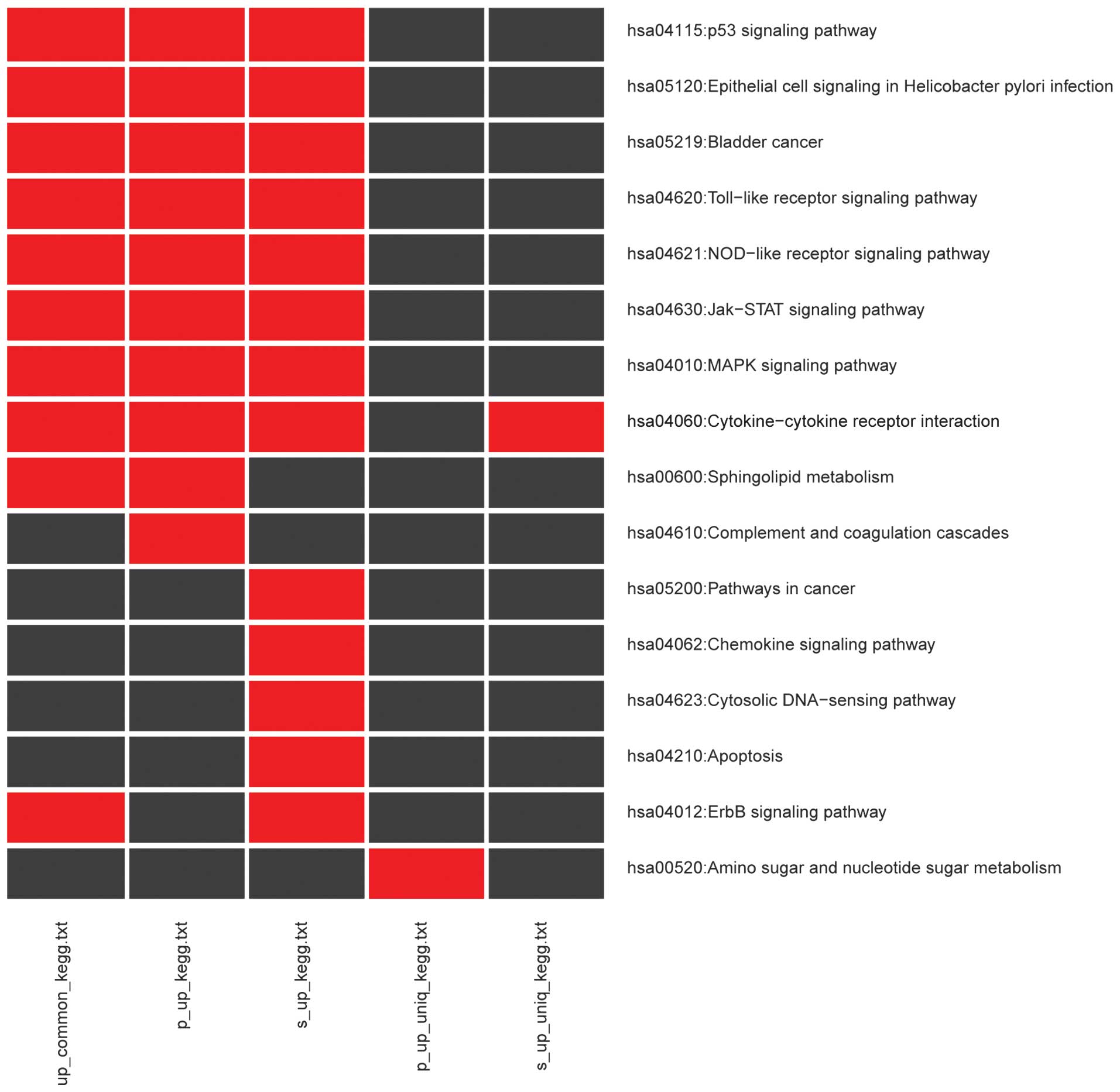
and their enriched DEGs, are presented in Table II. A total of 10 KEGG pathways were
enriched by 207 upregulated DEGs, while these DEGs enriched into
243 GO terms. As shown in Fig. 2,
the majority of the pathways of upregulated overlapping DEGs were
associated with immune responses, such as Toll-like receptor (TLR),
NOD-like receptor (NLR), Jak-STAT and MAPK signaling pathways, in
addition to cytokine-cytokine receptor interaction. The KEGG
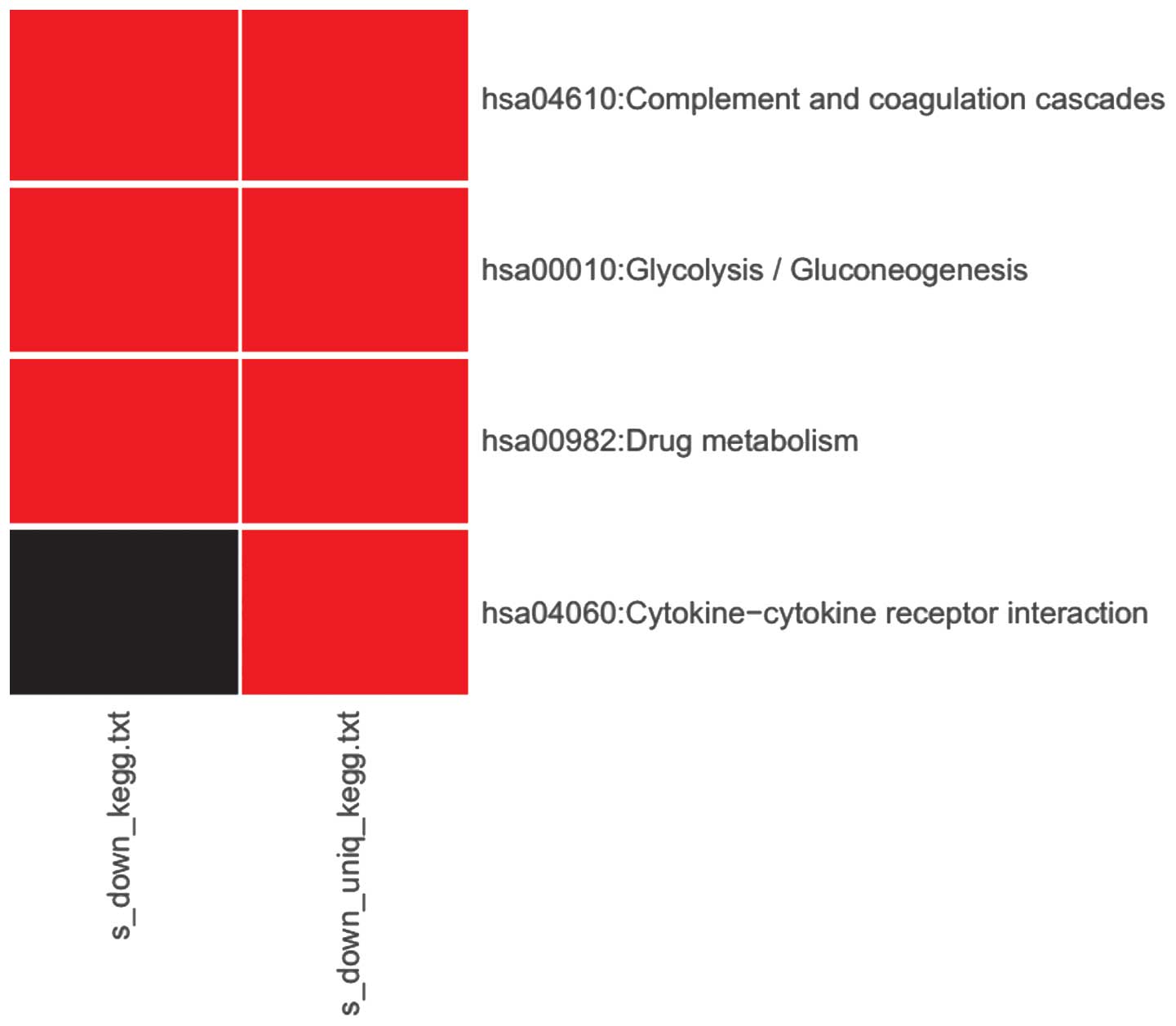
enrichment results for downregulated DEGs associated with
sevoflurane are presented in Fig. 3.
A total of three pathways were associated, including complement and
coagulation cascades, glycolysis and drug metabolism. The pathway
associated with cytokine-cytokine receptor interaction was found to
be uniquely downregulated by sevoflurane.
 | Table II.Numbers of significantly enriched KEGG
pathways and GO terms. |
Table II.
Numbers of significantly enriched KEGG
pathways and GO terms.
| Parameter | Up_common | P_up | S_up | P_up_uniq | S_up_uniq | down_common | P_down | S_down | P_done_uniq | S_done_uniq |
|---|
| Gene (n) | 207 | 217 | 323 | 10 | 116 | 25 | 25 | 237 | 0 | 212 |
| KEGG pathway
(n) | 10 | 10 | 13 | 1 | 1 | 0 | 0 | 3 | 0 | 4 |
| GO biological
pathway (n) | 243 | 250 | 321 | 4 | 95 | 0 | 0 | 49 | 0 | 50 |
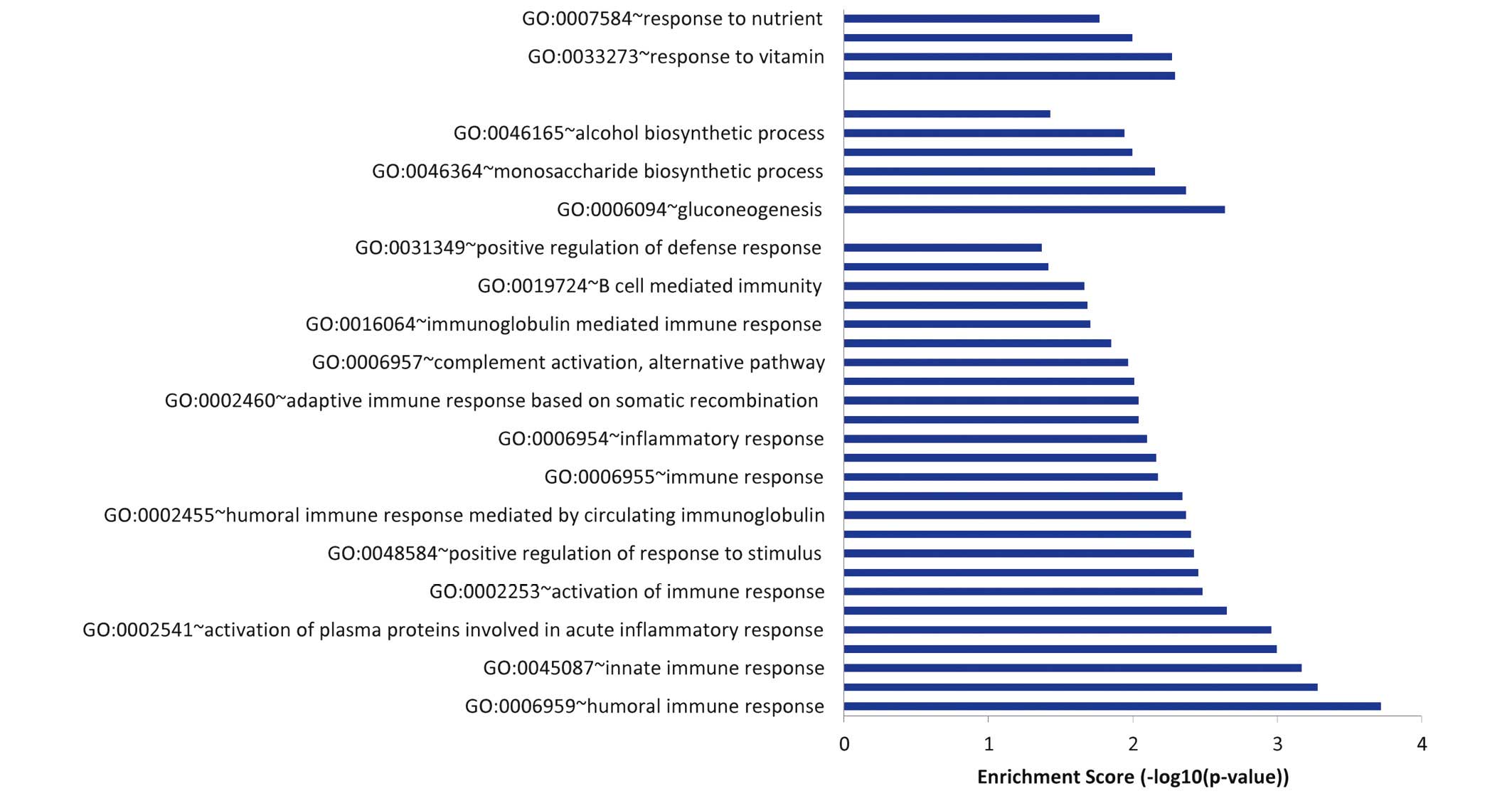
As shown in Fig. 4,
the GO terms which were found to be significantly enriched in the
212 downregulated genes that were unique to sevoflurane could be
divided into three groups: Immune response, glucose metabolism and
response to vitamin and nutrient.
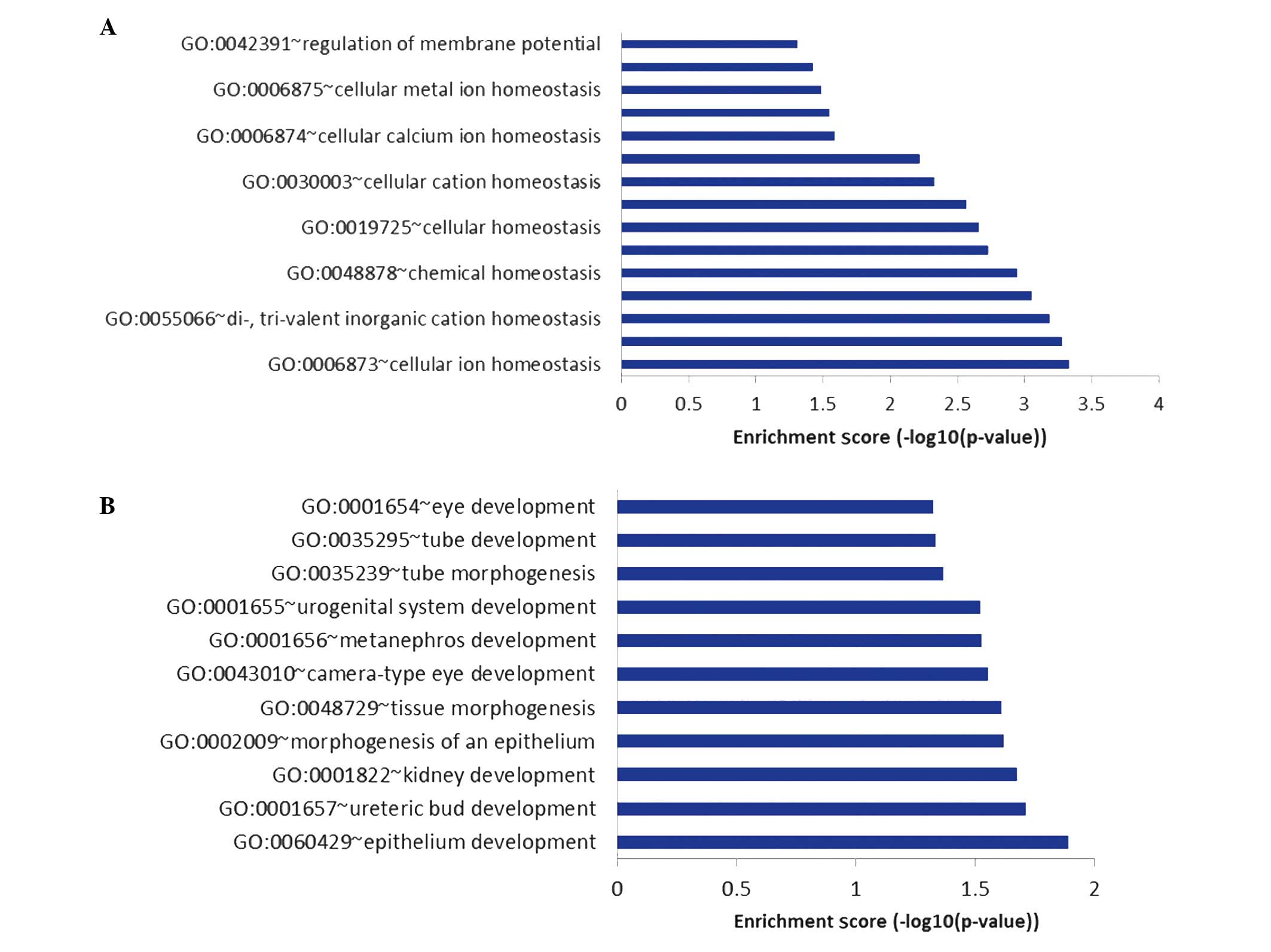
The GO biological pathways, which were found to be
significantly over-represented in the 116 upregulated genes that
were unique to sevoflurane could be divided into seven groups,
including pathways associated with the immune response, apoptosis,
cellular ion homeostasis and epithelial cell development. Among
these pathways, those associated with cellular ion homeostasis
(Fig. 5A) and epithelial cell
development (Fig. 5B) were not
detected among the pathways enriched by DEGs commonly upregulated
by propofol and sevoflurane.
Discussion
Sevoflurane and propofol are the two most commonly
used anesthetics in CABG surgery (23). In the present study, a CABG patient
data set (GSE4386) was downloaded from the GEO database to
determine the molecular mechanisms underlying the effects of the
two anesthetics. A total of 242 and 560 DEGs were identified in
patients treated with propofol and sevoflurane, respectively. The
two groups were found to have 207 downregulated DEGs in common, 116
that were unique to sevoflurane and 10 that were unique to
propofol. In total, 25 downregulated genes were shared and 212 were
unique to sevoflurane, while none was unique to propofol. The
upregulated DEGs unique to sevoflurane were associated with
cellular ion homeostasis and epithelial cell development, while the
unique downregulated genes were associated with glucose metabolism,
immune response and response to vitamin.
In the present study, pathways associated with
cellular ion homeostasis, such as the regulation of membrane
potential, cellular metal ion homeostasis and cellular calcium ion
homeostasis, were enriched by upregulated DEGs unique to
sevoflurane. Sevoflurane anesthesia has previously been shown to
alter the electrophysiological activity of neurons by reducing
hypoxic depolarization and enhancing the hypoxic hyperpolarization,
thus protecting neurons against ischemia (24). Furthermore, sevoflurane has been
found to increase coronary collateral blood flow through the
activation of calcium-activated potassium channels (25). In the present study, the upregulated
DEGs that were unique to sevoflurane were additionally associated
with epithelial cell development, such as tissue morphogenesis, and
epithelium and blood vessel development. Previously, sevoflurane
treatment was shown to increase the synthesis of heat shock protein
(HSP)-70 without affecting HSP-32 and HSP-27 synthesis (26). In the process of ischemia and
reperfusion, HSP-70 was able to protect the heart through the
expression of CD69 and by inducing a reduction in intracellular
calcium (27). Sevoflurane may
therefore serve a crucial function in mediating cardioprotection by
influencing the pathways associated with cellular ion homeostasis
and epithelial cell development.
In addition, unique downregulated DEGs associated
with sevoflurane anesthesia were enriched in the pathways
associated with glucose metabolism, immune response and response to
vitamin in the present study. Saho et al (28) previously found that sevoflurane
anesthesia may reversibly inhibit basal and glucose-stimulated
insulin secretion, and further induce insulin resistance. Insulin
resistance has been identified as an independent risk factor for
coronary heart disease (29).
Therefore, understanding the glycometabolic state of patients prior
to CABG surgery is crucial in order to reduce the postoperative
complications.
In the present study, these upregulated DEGs were
enriched in pathways associated with the immune response, including
innate, immunoglobulin-mediated and humoral immune responses;
however, these upregulated pathways were additionally associated
with the physical injury caused by CABG surgery, and thus were
shared between sevoflurane and propofol. During the process of the
immune response, TLRs have been confirmed as the signaling receptor
for HSPs, mediating the synthesis of inflammatory cytokines
(30). In addition, NLRs, TLRs and
RIG-1-like receptors have been found to be involved in immune
responses (31–36), and response to vitamin was shown to
be a key pathway enriched by downregulated DEGs associated with
sevoflurane anesthesia by the present results. Samadikhah et
al (37) found that vitamin C
combined with oral atorvastatin was significantly effective at
preventing post-CABG atrial fibrillation. Furthermore, vitamin D,
which is activated by a low-calcemic agonist, has been shown to
modulate the humoral immune response, further affecting the
efficacy of the surgical outcome of CABG (38). Collectively, these results suggest
that sevoflurane exhibits a more marked effect on biological
pathways compared with propofol, but exhibits a number of
shortcomings. In order to achieve a more beneficial use of
sevoflurane anesthesia in patients undergoing CABG, certain
complementary therapies, such as the regulation of glucose balance
and the use of vitamin supplements, should be considered.
In conclusion, the pathways enriched by DEGs,
particularly those that were unique to sevoflurane and propofol,
may affect surgical outcomes in patients undergoing CABG. In the
present study, sevoflurane exhibited a more marked impact on the
biological pathways investigated compared with propofol; however,
the identified DEGs and pathways in this study were not
investigated in animal models. Further study of this subject in
animal models may be required in the future.
Acknowledgements
This study was supported by the Shanghai Natural
Science Foundation Project (grant no. 14ZR1406900). The authors
thank Dr Hao Wang of the Departments of Anesthesia and
Anesthesiology (Shanghai Medical College, Fudan University,
Shanghai, China) for assistance with data analysis and valuable
discussion.
References
|
1
|
Møller C, Penninga L, Wetterslev J,
Steinbrüchel D and Gluud C: Off-pump versus on-pump coronary artery
by-pass grafting for ischaemic heart disease. Cochrane Database
Syst Rev. 3:CD0072242012.PubMed/NCBI
|
|
2
|
Wang J, Zheng H, Chen CL, Lu W and Zhang
YQ: Sevoflurane at 1 MAC provides optimal myocardial protection
during off-pump CABG. Scand Cardiovasc J. 47:175–184. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hellström J, Öwall A, Bergström J and
Sackey P: Cardiac outcome after sevoflurane versus propofol
sedation following coronary bypass surgery, A pilot study. Acta
Anaesthesiol Scand. 55:460–467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gülçin I, Alici HA and Cesur M:
Determination of in vitro antioxidant and radical scavenging
activities of propofol. Chem Pharm Bull (Tokyo). 53:281–285. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marik PE: Propofol: Therapeutic
indications and side-effects. Curr Pharm Des. 10:3639–3649. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vakkuri A, Yli-Hankala A, Talja P, Mustola
S, Tolvanen-Laakso H, Sampson T and Viertiö-Oja H: Time-frequency
balanced spectral entropy as a measure of anesthetic drug effect in
central nervous system during sevoflurane, propofol and thiopental
anesthesia. Acta Anaesthesiol Scand. 48:145–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sayin MM, Özatamer O, Taşöz R, Kilinc K
and Ünal N: Propofol attenuates myocardial lipid peroxidation
during coronary artery bypass grafting surgery. Br J Anaesth.
89:242–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Corcoran TB, Engel A, Sakamoto H, O'Shea
A, O'Callaghan-Enright S and Shorten G: The effects of propofol on
neutrophil function, lipid peroxidation and inflammatory response
during elective coronary artery bypass grafting in patients with
impaired ventricular function. Br J Anaesth. 97:825–831. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garcia C, Julier K, Bestmann L, Zollinger
A, von Segesser LK, Pasch T, Spahn DR and Zaugg M: Preconditioning
with sevoflurane decreases PECAM-1 expression and improves one-year
cardiovascular outcome in coronary artery bypass graft surgery. Br
J Anaesth. 94:159–165. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lucchinetti E, da Silva R, Pasch T, Schaub
MC and Zaugg M: Anaesthetic preconditioning but not
postconditioning prevents early activation of the deleterious
cardiac remodelling programme, Evidence of opposing genomic
responses in cardioprotection by pre-and postconditioning. Br J
Anaesth. 95:140–152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao YT and Li LH: Sevoflurane versus
propofol for myocardial protection in patients undergoing coronary
artery bypass grafting surgery, A meta-analysis of randomized
controlled trials. Chin Med Sci J. 24:133–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Landoni G, Biondi-Zoccai GG, Zangrillo A,
Bignami E, D'Avolio S, Marchetti C, Calabrò MG, Fochi O, Guarracino
F, Tritapepe L, et al: Desflurane and sevoflurane in cardiac
surgery: A meta-analysis of randomized clinical trials. J
Cardiothorac Vasc Anesth. 21:502–511. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu CH and Beattie WS: The effects of
volatile anesthetics on cardiac ischemic complications and
mortality in CABG, A meta-analysis. Can J Anaesth. 53:906–918.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yao YT and Li LH: Sevoflurane versus
propofol for myocardial protection in patients undergoing coronary
artery bypass grafting surgery, A meta-analysis of randomized
controlled trials. Chin Med Sci J. 24:133–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jakobsen CJ, Berg H, Hindsholm KB, Faddy N
and Sloth E: The influence of propofol versus sevoflurane
anesthesia on outcome in 10,535 cardiac surgical procedures. J
Cardiothorac Vasc Anesth. 21:664–671. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jakobsen CJ, Berg H, Hindsholm KB, Faddy N
and Sloth E: The influence of propofol versus sevoflurane
anesthesia on outcome in 10,535 cardiac surgical procedures. J
Cardiothorac Vasc Anesth. 21:664–671. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ballester M, Llorens J,
Garcia-de-la-Asuncion J, Perez-Griera J, Tebar E, Martinez-Leon J,
Belda J and Juez M: Myocardial oxidative stress protection by
sevoflurane vs. propofol, A randomised controlled study in patients
undergoing off-pump coronary artery bypass graft surgery. Eur J
Anaesthesiol. 28:874–881. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao YT and Li LH: Sevoflurane versus
propofol for myocardial protection in patients undergoing coronary
artery bypass grafting surgery, A meta-analysis of randomized
controlled trials. Chin Med Sci J. 24:133–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lucchinetti E, Hofer C, Bestmann L,
Hersberger M, Feng J, Zhu M, Furrer L, Schaub MC, Tavakoli R,
Genoni M, et al: Gene regulatory control of myocardial energy
metabolism predicts postoperative cardiac function in patients
undergoing off-pump coronary artery bypass graft surgery:
Inhalational versus intravenous anesthetics. Anesthesiology.
106:444–457. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barrett T, Suzek TO, Troup DB, Wilhite SE,
Ngau WC, Ledoux P, Rudnev D, Lash AE, Fujibuchi W and Edgar R: NCBI
GEO, Mining millions of expression profiles-database and tools.
Nucleic Acids Res. 33:(Database Issue). D562–D566. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smyth GK: Linear models and empirical
bayes methods for assessing differential expression in microarray
experiments. Stat Appl Genet Mol Biol. 3:Article32004.PubMed/NCBI
|
|
22
|
Dennis G Jr, Sherman BT, Hosack DA, Yang
J, Gao W, Lane HC and Lempicki RA: DAVID: Database for annotation,
visualization and integrated discovery. Genome biol. 4:P32003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soro M, Gallego L, Silva V, Ballester MT,
Lloréns J, Alvariño A, García-Perez ML, Pastor E, Aguilar G, Martí
FJ, et al: Cardioprotective effect of sevoflurane and propofol
during anaesthesia and the postoperative period in coronary bypass
graft surgery: a double-blind randomised study. Eur J Anaesthesiol.
29:561–569. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang J, Lei B, Popp S, Meng F, Cottrell J
and Kass I: Sevoflurane immediate preconditioning alters hypoxic
membrane potential changes in rat hippocampal slices and improves
recovery of CA1 pyramidal cells after hypoxia and global cerebral
ischemia. Neuroscience. 145:1097–1107. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kehl F, Krolikowski JG, Tessmer JP, Pagel
PS, Warltier DC and Kersten JR: Increases in coronary collateral
blood flow produced by sevoflurane are mediated by
calcium-activated potassium (BKCa) channels in vivo.
Anesthesiology. 97:725–731. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee HT, Kim M, Jan M and Emala CW:
Anti-inflammatory and antinecrotic effects of the volatile
anesthetic sevoflurane in kidney proximal tubule cells. Am J
Physiol Renal Physiol. 291:F67–F78. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sharma M, Ganguly NK, Chaturvedi G,
Thingnam SK, Majumdar S and Suri RK: A possible role of HSP70 in
mediating cardioprotection in patients undergoing CABG. Mol Cell
Biochem. 247:31–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saho S, Kadota Y, Sameshima T, Miyao J,
Tsurumaru T and Yoshimura N: The effects of sevoflurane anesthesia
on insulin secretion and glucose metabolism in pigs. Anesth Analg.
84:1359–1365. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ai M, Otokozawa S, Asztalos BF, White CC,
Cupples LA, Nakajima K, Lamon-Fava S, Wilson PW, Matsuzawa Y and
Schaefer EJ: Adiponectin. An independent risk factor for coronary
heart disease in men in the Framingham offspring study.
Atherosclerosis. 217:543–548. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dybdahl B, Wahba A, Lien E, Flo TH, Waage
A, Qureshi N, Sellevold OF, Espevik T and Sundan A: Inflammatory
response after open heart surgery, Release of heat-shock protein 70
and signaling through toll-like receptor-4. Circulation.
105:685–690. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Akira S and Takeda K: Toll-like receptor
signalling. Nat Rev Immunol. 4:499–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanneganti TD, Lamkanfi M and Núñez G:
Intracellular NOD-like receptors in host defense and disease.
Immunity. 27:549–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kaminska B: MAPK signalling pathways as
molecular targets for anti-inflammatory therapy-from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murray PJ: The JAK-STAT signaling pathway:
Input and output integration. J Immunol. 178:2623–2629. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Appledorn DM, Patial S, McBride A,
Godbehere S, Van Rooijen N, Parameswaran N and Amalfitano A:
Adenovirus vector-induced innate inflammatory mediators, MAPK
signaling, as well as adaptive immune responses are dependent upon
both TLR2 and TLR9 in vivo. J Immunol. 181:2134–2144. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang G, Shi LZ and Chi H: Regulation of
JNK and p38 MAPK in the immune system, Signal integration,
propagation and termination. Cytokine. 48:161–169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Samadikhah J, Golzari SE, Sabermarouf B,
Karimzadeh I and Tizro P: MohammadK hanli H and Ghabili K: Efficacy
of combination therapy of statin and vitamin C in comparison with
statin in the prevention of post-CABG atrial fibrillation. Adv
Pharm Bull. 4:97–100. 2014.PubMed/NCBI
|
|
38
|
Baeke F, Takiishi T, Korf H, Gysemans C
and Mathieu C: Vitamin D, Modulator of the immune system. Curr Opin
Pharmacol. 10:482–496. 2010. View Article : Google Scholar : PubMed/NCBI
|