Introduction
A successful and classical animal model of
heterotopic intestinal transplantation (HIT) was initially
described by Monchick and Russell (1) in 1971, and has since been improved. At
present, a rat model of HIT is widely used, which has particular
value for use in immunological studies. The majority of technical
difficulties associated with this model have been surmounted;
however, the present study hypothesized that the rat model of HIT
may be improved by the application of fast-track surgery (FTS)
concepts and exclusive enteral nutrition (EEN) gavage.
FTS comprises a combination of perioperative
interventions, which aim to reduce postoperative stress, the
incidence of postoperative complications, and the length of
hospital stays (2). In particular,
FTS consists of revised traditional surgical practices, improved
anesthesia and minimized surgical stress, which have proved
effective in major human surgical procedures since the introduction
of FTS in the early 1990s (3,4). EEN is
a nutritional therapy used for inducing remission in patients with
Crohn's disease, and involves a period of 6–8 weeks, during which
the patient subsists on an exclusively liquid diet, consisting of
either elemental or polymeric formulae (5,6). During
this period, the patients are not allowed to consume other dietary
items, with the exception of water and various beverages (5,6). To the
best of our knowledge, the application of EEN gavage to a rat model
of HIT has yet to be reported. The present study hypothesized that
EEN gavage may help to stabilize the structure of the mucosal
barrier, re-establish the gut bacterial environment and ameliorate
the survival rate in a rat model of HIT.
Materials and methods
Rats and experimental design
A total of 192 male Sprague-Dawley rats (weight,
250–330 g; age, 8–9 weeks) from the Department of Comparative
Medicine, Jinling Hospital (Nanjing, China), underwent HIT. The
rats were housed in rodent facilities, and were maintained on a 12
h light/dark cycle, with ad libitum access to commercially
available chow and tap water. The temperature was maintained at
~20°C. Surgical procedures were conducted by two junior
microsurgeons. Rat care protocols and experiments were conducted in
accordance with the Principles of Laboratory Animal Care and the
Guide for the Care and Use of Laboratory Animals (National
Institutes of Health, Bethesda, MA, USA), and were designed
according to the Rules for the Medical Laboratory Animal, as
published by the Ministry of Health of the People's Republic of
China. The present study was approved by the Ethics Committee of
Jinling Hospital, and Institutional Review Board approval was
obtained.
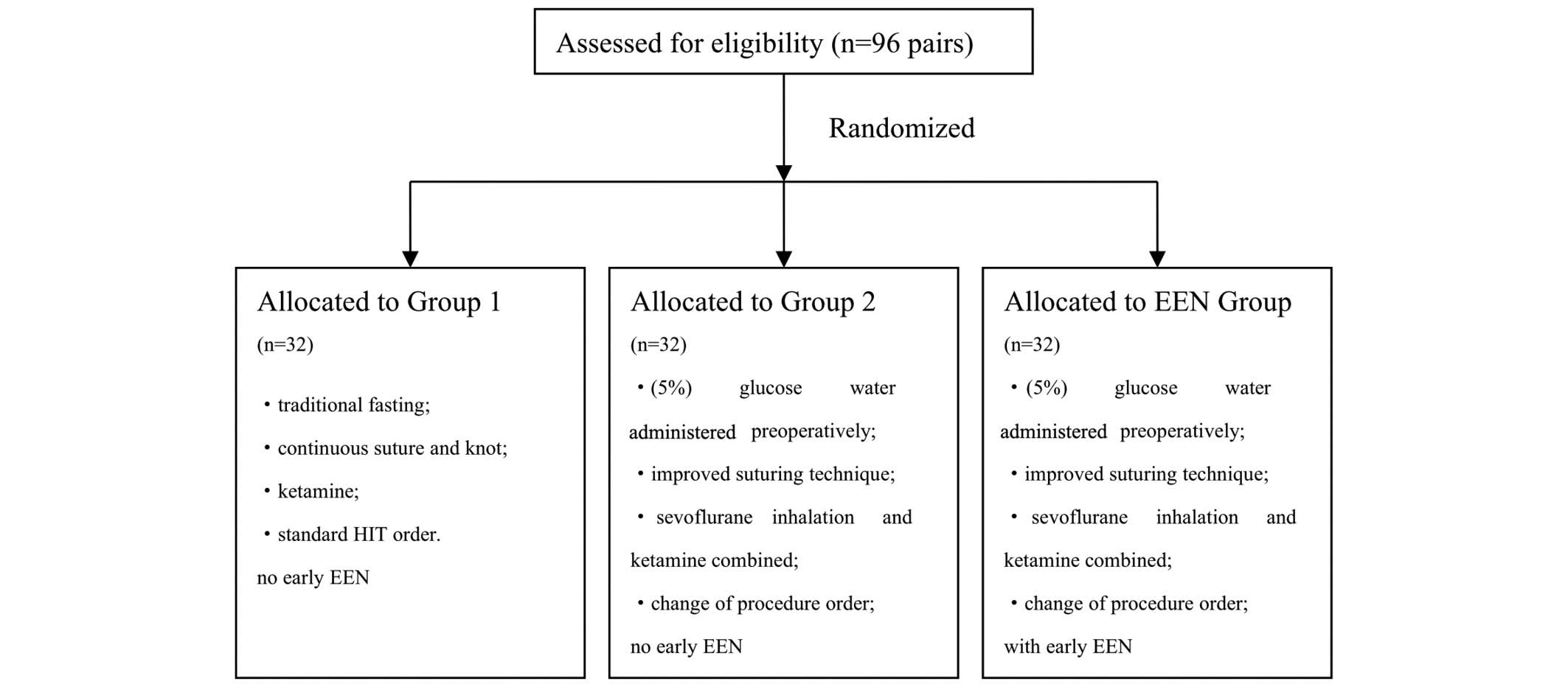
The rats were randomly divided into 96 pairs,
including a donor and recipient rat, and were distributed into
three groups (n=32/group): i) The conventional group (group 1); ii)
the FTS group (group 2); and iii) the FTS with EEN group (EEN
group; Fig. 1). For group 1 rats,
the protocols were consistent with the standard and perioperative
care procedures outlined in a previous study (5). For group 2 rats, various modifications
were made according to FTS concepts, including preoperative
treatment with glucose water (5%), the use of sevoflurane
inhalation anesthesia, and alterations to the surgical procedure
order and suturing technique. In addition, for the EEN group, an
early EEN gavage was conducted. The rats were not treated with any
immunosuppressive therapies postoperatively.
Interventions
Anesthesia
The rats in group 1 were anesthetized with an
intraperitoneal injection of ketamine (100 mg/kg; Jiangsu Hengrui
Medicine Co. Ltd., Lianyungang, China) dissolved in saline. In
addition, 50 mg/kg ketamine was administered perioperatively when
required. The rats in group 2 and the EEN group were anaesthetized
with sevoflurane via a self-made plastic bottle containing gauze
saturated in sevoflurane (Baxter International, Inc., Deerfield,
IL, USA). Anesthesia was initiated and induced by placing the
bottle over the head of the rats. Following the initial
anesthetization, 60 mg/kg ketamine was administered in order to
achieve an adequate depth of anesthetization. Anesthesia was
maintained throughout the surgery by administering sevoflurane
every 20 min. The gauze was renewed in order to maintain the
desired concentration of sevoflurane in the bottle.
Fasting
All rats in group 1 received normal food and water
up to 8 h prior to surgery. Conversely, the rats in group 2 and the
EEN group had ad libitum access to glucose water (5%) during
the fasting period.
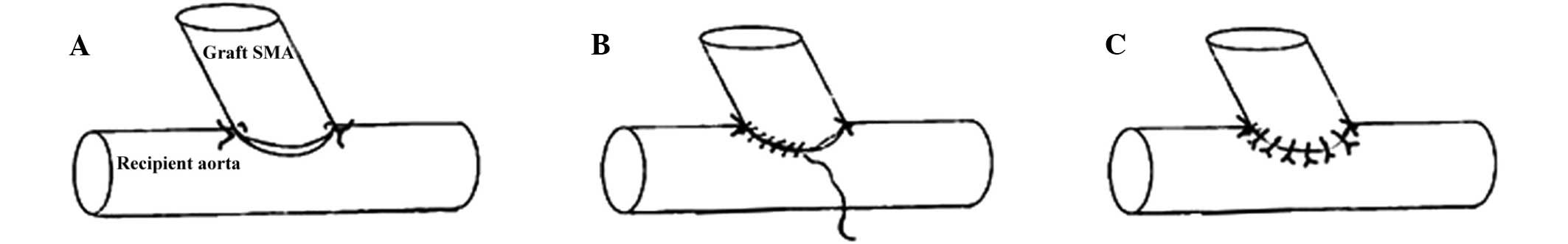
Suturing technique
An end-to-side anastomosis was performed between the
aortic patch of the donor rat and the abdominal aorta of the
recipient rat. In group 1, a conventional continuous suture was
used, as outlined in a previous study (5). Conversely, in group 2 and the EEN
group, the arteries of the donor and recipient rats were initially
anastomosed using a 10-0 suture (Double Arrow; Yiling Medical
Device Sales Co. Ltd., Shanghai, China) consisting of two knots,
one placed at the proximal apex and the other at the distal apex of
the anastomosis. Subsequently, continuous 10-0 suturing was
performed at the posterior and anterior walls, using an
‘out-in/in-out’ technique, from the graft to the recipient. Without
tightening the suture, each part of the suture was cut and knotted
(~5–6 knots were needed for each side and additional knots were
added when required; Fig. 2).
HIT technique
In group 2 and the EEN group, the recipient rats
were prepared prior to completing the final irrigation step to
obtain the donor graft. Briefly, following dissection of the left
renal vein of the recipient rat, the intrarenal abdominal aorta was
carefully handled, and a moist gauze was used to cover the surgical
area in preparation for the graft. Subsequently, the donor rat was
returned to for the final irrigation step.
Early EEN
In the EEN group, the recipient rats underwent EEN
by gavage, during which the rats received the standard total
calories per day for rats (836.8 kJ; 200 kcal)/(kg × day). The
enteral formula used in the present study was the commercially
available Peptisorb Liquid (Nutricia Research, Amsterdam,
Netherlands), which consists of maltodextrin, hydrolyzed whey
protein peptide, fat, vitamins and trace elements. A summary of the
composition of Peptisorb Liquid is presented in Table I (6).
The dosage and gavage speed were gradually increased from half (6 h
post-surgery) to full strength (at postoperative day 1), in order
to reduce side effects, including diarrhea and vomiting. Throughout
this period, the rats in the EEN group were not allowed to consume
other dietary items, with the exception of water. Groups 1 and 2
rats received a normal diet of chow.
 | Table I.Constituents of Peptisorb liquid
(energy, 500 kcal per 500 ml). |
Table I.
Constituents of Peptisorb liquid
(energy, 500 kcal per 500 ml).
| Component | Mass (mg) |
|---|
| Maltodextrin | 88,000 |
| Hydrolyzed whey
protein peptide | 20,000 |
| Lipid (vegetable
oil) | 8,500 |
| Potassium | 750 |
| Sodium | 500 |
| Calcium | 400 |
| Magnesium | 115 |
| Phosphorus | 360 |
| Chlorine | 625 |
| Iron | 8.0 |
| Zinc | 6.0 |
| Copper | 0.9 |
| Manganese | 1.65 |
| Fluorine | 0.5 |
| Iodine | 0.065 |
| Molybdenum | 0.05 |
| Chromium | 0.0335 |
| Selenium | 0.0285 |
| Vitamin A | 0.41 |
| Vitamin B1 | 0.75 |
| Vitamin B2 | 0.8 |
| Vitamin B6 | 0.85 |
| Vitamin B12 | 0.00105 |
| Vitamin C | 50.0 |
| Vitamin D | 0.0035 |
| Vitamin E | 6.5 |
| Vitamin K | 0.0265 |
| Niacin | 9.0 |
| Pantotenic acid | 2.65 |
| Folic acid | 0.135 |
| Biotin | 0.02 |
| Choline | 185 |
| Carotenoids
mixture | 1.0 |
| Taurine | 0.05 |
Morphological alterations to the intestinal
mucosa
Morphological alterations to the graft intestinal
mucosa were detected using light microscopy (BX60; Olympus
Corporation, Tokyo, Japan), according to a common protocol
(6). The length of the intestinal
villus was measured using cartographic software at various time
points (12, 24 and 48 h) (7). Graft
pathological examination was conducted on postoperative day 7. The
allografts were collected from a stoma each time. The rats were
sacrificed by cervical dislocation.
Statistical analysis
Statistical analyses were conducted using SPSS
software 12.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as
the mean ± standard deviation. Comparisons between groups were
conducted using one-way analysis of variance, followed by Dunnett's
multiple comparisons test. Comparisons between two groups were
conducted using paired and unpaired t-tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
Response of rats to HIT
A total of 96 pairs of rats underwent HIT by a
single group of surgeons between September 2012 and May 2013. The
majority of procedures were successful and the rats appeared to
tolerate HIT well. The rats exhibited a good appetite upon
awakening from anesthesia and were in good health. No significant
differences in the overall conditions of the rats, including body
weight and baseline characteristics, were detected among the three
groups prior to surgery.
Effects of interventions on HIT
procedure
The surgical duration for each group is presented in
Table II. As the procedure order
was altered in group 2 and the EEN group, only the total surgery
time was calculated. The surgical time was significantly decreased
in group 2 and the EEN group rats (137.44±16.03 and 139.67±15.25
min, respectively), as compared with the group 1 rats (169.36±13.72
min; P<0.05). In group 1, the average recovery time from
anesthesia was ~25.64±9.45 min, which was significantly increased,
as compared with the group 2 and EEN group rats (P<0.05).
 | Table II.Duration of surgery (min) for each
group. |
Table II.
Duration of surgery (min) for each
group.
| Group | Donor part | Recipient part | Total surgery
time | Awakening time |
|---|
| 1 | 59.45±8.16 | 109.91±8.21 |
169.36±13.72a |
25.64±9.45a |
| 2 | N/A | N/A | 137.44±16.03 | 14.81±4.12 |
| EEN | N/A | N/A | 139.67±15.25 | 15.11±4.06 |
The incidence of complications frequently associated
with HIT was compared among the groups, and are presented in
Table III. Hemorrhagic shock, in
particular uncontrollable bleeding in the arterial anastomotic
area, was the complication most frequently associated with HIT
failure, and the rats typically succumbed to surgery. Two cases of
hemorrhagic shock occurred in group 1, but were absent from the
other groups. One case of an anesthetic accident occurred in group
2, and was associated with excessive inhalation of sevoflurane. The
surgical success rate at 3 days post-surgery was 84.4% for group 1,
93.8% for group 2 and 96.9% for the EEN group rats. In addition,
comparing the 14-day survival rate indicated that there was a
statistically significant difference between the EEN group and
group 1 (P<0.05); the 14-day survival rate was 68.7% for group
1, 87.5% for group 2 and 90.6% for the EEN group rats.
 | Table III.Perioperative complications leading to
mortality. |
Table III.
Perioperative complications leading to
mortality.
| Complications | Group 1 | Group 2 | EEN group |
|---|
| Hemorrhagic
shock | 2 during surgery | – | – |
| Anesthetic
accident | – | 1 during surgery | – |
| Portal vein
thrombosis or stenosis | 2 in 3 days | 1 in 3 days | 1 in 3 days |
| Intestinal
obstruction | 1 in 3 days | – | – |
| Survival at 3
days | 27 (84.4%) | 30 (93.8%) | 31 (96.9%) |
| Infection of
abdominal cavity | 2 in 14 days | 1 in 14 days | – |
| Other
complications | 3 in 3 days | 1 in 14 days | 2 in 14 days |
| Survival at 14
days | 24 (68.7%) | 28 (87.5%) | 29
(90.6%)a |
Morphological alterations of the
intestinal mucosa
Grafts were obtained from all rats in order to
evaluate histological alterations at specific time points. Light
microscopy demonstrated that the intestinal villi of the grafts
from all three groups gradually became eroded, necrotic and were
decomposed following HIT, and these lesions were most severe at
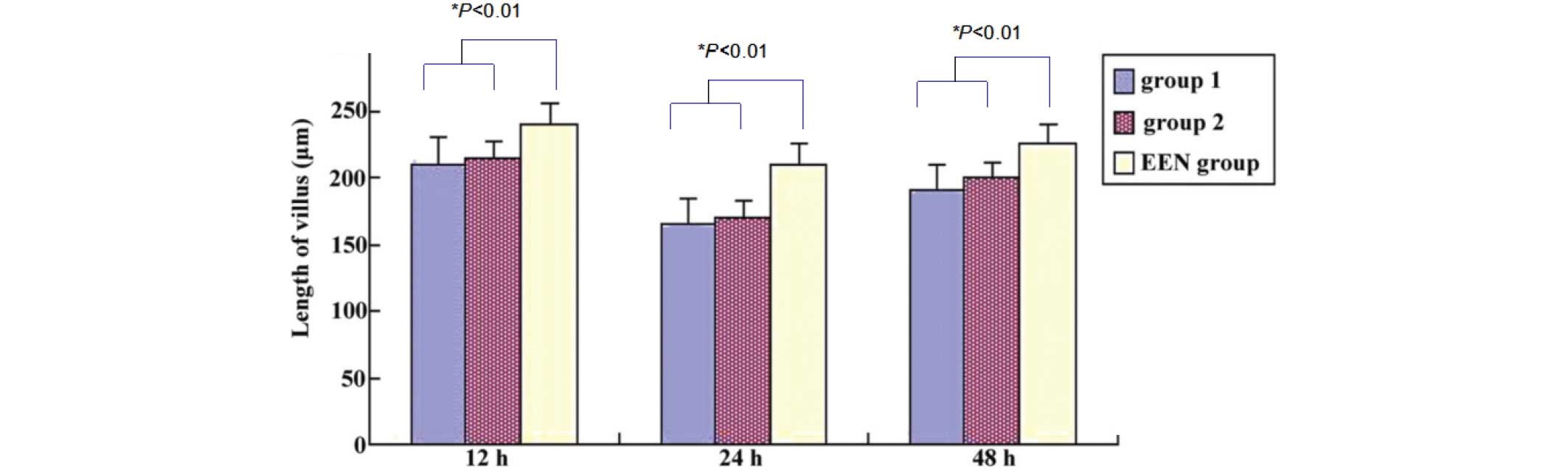
postoperative day 1. By the end of the study, the villi from the
EEN group rats exhibited the least severe morphological alterations
under a transmission electron microscope, and the villi were
significantly longer and exhibited narrower interspaces, as
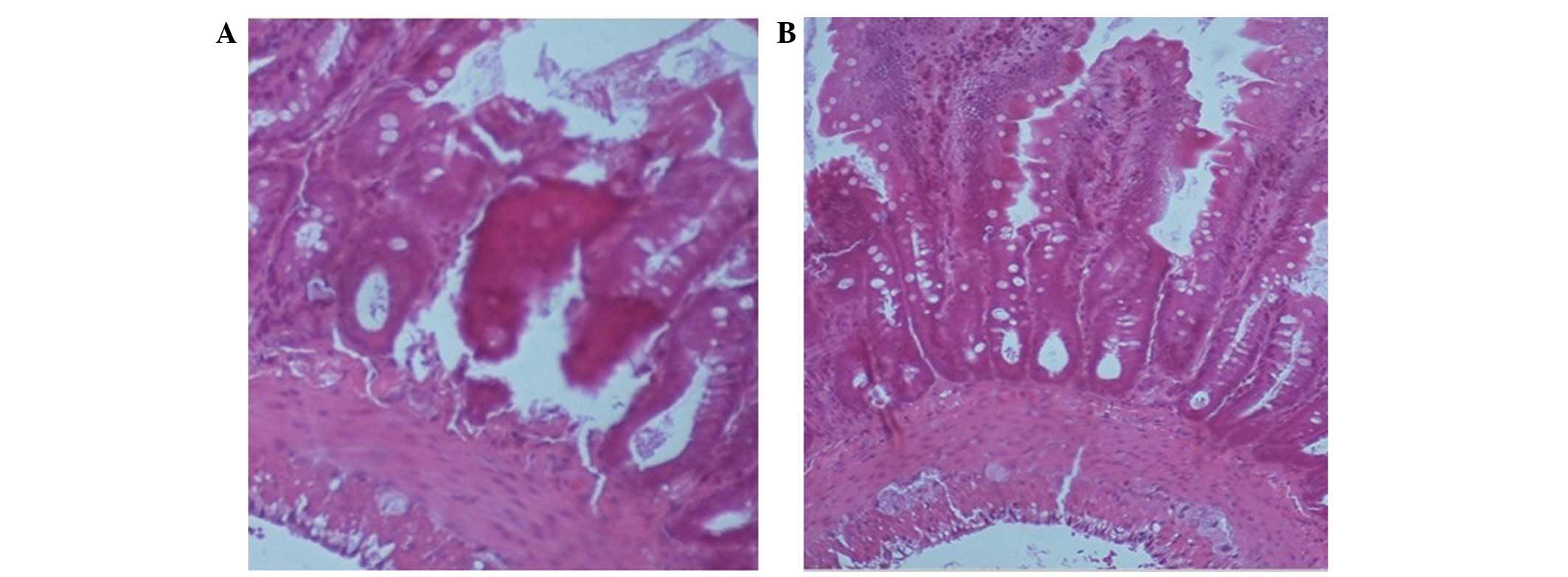
compared with the group 1 and 2 rats (P<0.01; Fig. 3). Histological examination of the
grafts using hematoxylin and eosin staining on day 7 detected less
severe ischemia-reperfusion injury and markedly decreased
mononuclear cell infiltration, cryptitis and rupture in the tips of
the villi from the EEN group grafts, as compared with the group 1
and 2 grafts (Fig. 4).
Discussion
Intestinal transplantation has emerged as a
potential strategy for the treatment of end-stage intestinal
failure, and remains essential for the investigation of immunology
and the underlying mechanisms of graft rejection in animal models
(8). Complications exist regarding
the complex microvascular techniques and high mortality rates
associated with HIT. The present study reports the use of a novel
HIT protocol, in which FTS concepts and postoperative EEN gavage
were applied. These have previously been associated with beneficial
effects in recovering patients in numerous clinical trials
(9–14). In particular, the present study
compared the success rate, graft conditions and survival outcomes
of the novel vs. traditional methods in a rat model of HIT.
In a pre-test (data not shown), controlling the dose
of perioperatively administered ketamine was shown to be
challenging, and was frequently associated with respiratory failure
or cardiac arrest. Therefore, sevoflurane anesthesia, which is
widely used in clinical practice due to its safe induction, rapid
emergence, hemodynamic stability and nonirritating airway
properties, was selected for the present study. In addition,
decreasing the amount of ketamine decreased the frequency of
writhing times and improved the sleeping state of the rats.
Additional ketamine has previously been shown to decrease the
incidence of emergence agitation, and exert analgesic and hypnotic
effects following sevoflurane general anesthesia (15). In the novel HIT method established in
the present study, the rats who awoke perioperatively returned to
sleep immediately upon additional treatment with sevoflurane, and
each successive treatment lasted for ~20 min. The rats in group 2
and the EEN group exhibited rapid recovery from surgery and
improved flexibility, as compared with the rats in group 1, and
this was not associated with adverse effects on the long-term
survival rates of the rats. A short recovery time following
anesthesia is beneficial for the survival rate, since it may help
to avoid low body temperature. These results suggested that the
combined use of sevoflurane and ketamine was an ideal strategy for
anesthetizing recipient rats for HIT.
Arterial anastomosis is the major procedure in HIT
and several months of training is required in order to gain
competence in the technique. Previous studies have reported
conflicting results regarding the use of an interrupted or
continuous suturing technique in end-to-side anastomosis (16). The novel suturing technique used in
the present study combined the advantages of both techniques. In
particular, it was time-saving without increasing the possibility
of bleeding. Wang et al (16)
reported that bleeding typically occurs from within the
inter-stitch space during the suturing process, particularly near
to the proximal and distal points; thus suggesting that controlling
the size of the inter-stitch space is required in order to reduce
blood loss. This was easy to achieve using the novel suturing
method, in which each part of the continuous suture was cut and
knotted. Therefore, the alteration to the suturing procedure may
benefit the training process and save time.
Ideally, the cold ischemia time should be as short
as possible: Kato et al (17)
demonstrated that prolonged cold ischemia/reperfusion enhanced the
rate of immune rejection and affected the recovery of bowel
function. In the group 2 and EEN group rats, the procedure order
was altered in an attempt to reduce the impact of cold ischemia. In
particular, various steps of the recipient preparation were
completed ahead of the graft lavage with the aid of an assistant,
including isolation of the recipient artery and renal vein.
Initially, there was concern regarding whether there would be an
increased risk of rat mortality associated with the extended
duration of anesthesia; however, the altered protocol effectively
reduced cold ischemia time when using the novel method of
sevoflurane inhalation anesthesia.
Nutritional support is necessary for HIT patients,
as it improves nutrient absorption and limits food antigen
overload, which may trigger immune stimulation and a subsequent
increased risk of acute graft rejection (18). Glucose water (5%) is rich in sugar
and energy, and its preoperative use in the present study helped
the recipient rats to tolerate the long surgical duration. Previous
studies suggested that enteral nutrition may also promote the
reconstruction of mucosa, improve impaired intestinal transit and
regulate immune functions (19). In
addition, Zhang et al (20)
reported that enteral rehabilitative therapy was able to induce
potent trophic effects on the graft structure and recipient
metabolism. The underlying mechanism by which the diet may exert
such beneficial effects is currently unclear; however, an arginine
and glutamine deficiency has previously been shown to initiate
immune function decline and inflammatory responses (6,7). The
Nutrison Fibre used in the present study contained numerous amino
acids, which served as an essential supplement and protected the
intestinal barrier function. The European Society for Clinical
Nutrition and Metabolism guidelines for enteral nutrition
postoperatively recommend that enteral feeding is initiated within
24 h following surgery, since a previous study demonstrated that
early enteral nutrition decreased the rate of postoperative
complications due to infection, and the duration of hospitalization
(21). In the EEN group, EEN gavage
was conducted immediately post-surgery and lasted for >3 days,
and was associated with an increased survival rate and improved
graft conditions.
In conclusion, the results of the present study
suggested that the application of FTS concepts and EEN gavage to a
rat model of HIT was able to improve the recovery and graft
function of the recipient rats. Future studies should investigate
the molecular mechanisms underlying EEN therapy, in order to reduce
the risk of graft rejection, improve gut trophicity and optimize
nutrient absorption. The perioperative interventions applied in the
present study may improve HIT modeling and reduce the suffering of
the experimental rats.
Acknowledgements
The authors of the present study would like to thank
B Shen, Y Xu and Q Zhao (Jinling Hospital, Nanjing University,
Jiangsu, China) for their helpful suggestions and assistance
throughout the present study and for preparing the manuscript. The
present study was supported by grants from the Natural Science
Foundation of Jiangsu Province (grant no. BK2008237) and the
Nanjing Science and Technology Development Project (grant no.
201104027).
References
|
1
|
Monchik GJ and Russell PS: Transplantation
of small bowel in the rat: Technical and immunological
considerations. Surgery. 70:693–702. 1971.PubMed/NCBI
|
|
2
|
Serclová Z, Dytrych P, Marvan J, Nová K,
Hankeová Z, Ryska O, Slégrová Z, Buresová L, Trávníková L and Antos
F: Fast-track in open intestinal surgery: Prospective randomized
study (Clinical Trials Gov Identifier no. NCT00123456). Clin Nutr.
28:618–624. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slim K: Fast-track surgery: The next
revolution in surgical care following laparoscopy. Colorectal Dis.
13:478–480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lassen K, Soop M, Nygren J, Cox PB, Hendry
PO, Spies C, von Meyenfeldt MF, Fearon KC, Revhaug A, Norderval S,
et al: Enhanced Recovery After Surgery (ERAS) Group: Consensus
review of optimal perioperative care in colorectal surgery:
Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch
Surg. 144:961–969. 2011. View Article : Google Scholar
|
|
5
|
Wu XT, Li JS, Zhao XF, Zhuang W and Feng
XL: Modified techniques of heterotopic total small intestinal
transplantation in rats. World J Gastroenterol. 8:758–762. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Feng Y, Li Y, Mei S, Zhang L, Gong J, Gu
L, Zhang W, Zhu W, Li N and Li J: Exclusive enteral nutrition
ameliorates mesenteric adipose tissue alterations in patients with
active Crohn's disease. Clin Nutr. 33:850–858. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Chen Y, Zhang J, Zhu JF, Liu ZJ,
Liang SY, Sun K, Liao WY and Gong JP: Protective effect of
glutamine-enriched early enteral nutrition on intestinal mucosal
barrier injury after liver transplantation in rats. Am J Surg.
199:35–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grant D, Abu-Elmagd K, Reyes J, Tzakis A,
Langnas A, Fishbein T, Goulet O and Farmer D: Intestine Transplant
Registry: 2003 report of the intestine transplant registry: A new
era has dawned. Ann Surg. 241:607–613. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Basse L, Jakobsen DH, Bardram L,
Billesbølle P, Lund C, Mogensen T, Rosenberg J and Kehlet H:
Functional recovery after open versus laparoscopic colonic
resection: A randomized, blinded study. Ann Surg. 241:416–423.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
King PM, Blazeby JM and Ewings P: The
influence of an enhanced recovery programme on clinical outcomes,
costs and quality of life after surgery for colorectal cancer.
Colorectal Dis. 8:506–513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gatt M, Anderson AD, Reddy BS,
Hayward-Sampson P, Tring IC and MacFie J: Randomized clinical trial
of multimodal optimization of surgical care in patients undergoing
major colonic resection. Br J Surg. 92:1354–1362. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khoo CK, Vickery CJ, Forsyth N, Vinall NS
and Eyre-Brook IA: A prospective randomized controlled trial of
multimodal perioperative management protocol in patients undergoing
elective colorectal resection for cancer. Ann Surg. 245:867–872.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muller S, Zalunardo MP, Hubner M, Clavien
PA and Demartines N: Zurich Fast Track Study Group: A fast-track
program reduces complications and length of hospital stay after
open colonic surgery. Gastroenterology. 136:842–847. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Serclová Z, Dytrych P, Marvan J, Nová K,
Hankeová Z, Ryska O, Slégrová Z, Buresová L, Trávníková L and Antos
F: Fast-track in open intestinal surgery: prospective randomized
study (Clinical Trials Gov Identifier no. NCT00123456). Clin Nutr.
28:618–624. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abu-Shahwan I and Chowdary K: Ketamine is
effective in decreasing the incidence of emergence agitation in
children undergoing dental repair under sevoflurane general
anesthesia. Paediatr Anaesth. 17:846–850. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Ma H, Zhang H, Lu B, Wang J, Wang
Z, Li Y and Li J: Continuous locked suture technique for arterial
anastomosis in rat small bowel transplantation. Microsurgery.
27:112–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato T, Tzakis AG, Selvaggi G, Gaynor JJ,
David AI, Bussotti A, Moon JI, Ueno T, DeFaria W, Santiago S, et
al: Intestinal and multivisceral transplantation in children. Ann
Surg. 243:756–766. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colomb V and Goulet O: Nutrition support
after intestinal transplantation: How important is enteral feeding?
Curr Opin Clin Nutr Metab Care. 12:186–189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hegazi RA and O'Keefe SJ: Nutritional
immunomodulation of acute pancreatitis. Curr Gastroenterol Rep.
9:99–106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang XQ, Li JS, Li N, Li YS and Fan XH:
Trophic effect of enteral rehabilitative therapy in rat small bowel
transplantation. Transplant Proc. 37:2351–2353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weimann A, Braga M, Harsanyi L, Laviano A,
Ljungqvist O and Soeters P: DGEM (German Society for Nutritional
Medicine). Jauch KW, Kemen M, Hiesmayr JM, et al: ESPEN (European
Society for Parenteral and Enteral Nutrition): ESPEN Guidelines on
Enteral Nutrition: Surgery including organ transplantation. Clin
Nutr. 25:224–244. 2006.
|