Introduction
Idiopathic inflammatory myopathies (IIM) are a group
of non-suppurative inflammatory diseases that have been identified
in skeletal muscle specimens. Although IIM are characterized by
proximal muscle weakness, the etiology of IIM remains to be
elucidated (1,2). The incidence rate of IIM in the general
population is high, with a range of 0.5–8.4 in every 100,000
individuals (3,4).
Dermatomyositis (DM) and polymyositis (PM) are the
most common IIM subtypes in clinical practice (2). The occurrence of these diseases often
presents with the impairment of multiple systems, with a poor
prognosis (1). It was previously
reported that the mortality rate of patients with IIM was 2- to
3-fold higher than that of the normal population, and that
infection, tumors and heart and lung impairment were the most
common causes of mortality (5,6).
However, due to the large timespans reported in prior literature,
in addition to differences in economic development and medical care
in the areas described in those studies, significant differences
exist in the mortality rate resulting from IIM (5–7).
The aim of the present study was to investigate and
analyze the main causes of infection and of complications that have
led to the mortality of patients with IIM in China since 2001.
Materials and methods
Review of patient information
The patients with IIM included in the current study
adhered to the 1975 Bohan/Peter Diagnostic Standard (8). The current analyses were performed on
676 patients with IIM who were diagnosed in the Department of
Rheumatism and Immunology at the Xiangya Hospital of Central South
University from January, 2001 to January, 2015. The medical records
of 49 patient mortalities (39 cases with DM and 10 cases with PM)
were retrospectively analyzed. These data included information
regarding clinical manifestations, laboratory examinations,
treatments and clinical outcomes.
Analysis of the cause of
mortality
The cause of mortality was determined following
analysis of the patient medical records and discussion with the
doctors responsible for a case. According to the clinical and
pathological symptoms, the primary causes of mortality included
infections, acute interstitial lung disease, ventilator weakness,
pneumothorax, pulmonary artery hypertension, acute myocardial
infarction, arrhythmia, gastrointestinal bleeding, liver failure,
renal failure and tumors. The majority of mortalities were
associated with infections and myositis. Infection-associated
mortality was caused by pathogenic infections and by
infection-associated complications resulting from IIM treatment.
Myositis-associated mortality was due to multi-organ failure
secondary to myositis and non-suppurative complications. If
patients succumbed to infection during treatment,
inflammatory-induced severe organ damage was considered to be an
infection-associated mortality.
Statistical analysis
Data analyses were conducted using SPSS v. 18.0
(SPSS, Inc., Chicago, IL, USA). Continuous distribution variables
are presented as mean ± standard deviation. For equal measurement
data, an independent samples t-test was used. Enumeration data were
evaluated using a χ2 test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Demographics
A total of 49 mortalities (7.2%) resulted from 676
IIM cases, with mortality occurring following DM and PM in 6.9%
(39/567) and 9.2% (10/109) of cases, respectively. No statistically
significant difference was observed between the DM and PM mortality
rates (χ2=0.717; P>0.05). Of the mortalities, 18 were
male (37.5%), after an average disease duration of 8.72±9.36 months
and at an average age of 48.83±12.19 years. Of the female
mortalities, the average disease duration was 10.09±9.02 months,
and the average age was 51.39±13.20 years. No significant
difference was observed between male and female in average disease
duration or age (t=0.507; 0.671; P>0.05).
Clinical manifestations
Of the 49 mortalities, the symptoms in 19 cases
(38.8%) began with skin lesions, while the symptoms of 17 cases
(34.7%) began with myalgia and muscle weakness. The remainder of
the patients (13 cases, 26.5%) reported joint pain, coughing,
gasping and edema as their initial symptoms. The majority of cases
resulting in mortality developed complications: 33 patients who
succumbed to the disease (67.3%) had a lung infection, 28 patients
(57.1%) had an interstitial lung disease and 1 patient (2.0%)
presented pulmonary hypertension. Three patients (6.1%) developed
diabetes secondary to glucocorticoid treatments, and 4 patients
(8.2%) developed a decubitus ulcer due to being bedridden.
Treatment
Patients were administered 1 mg/kg prednisone daily.
A number of immunosuppressants were administered to patients, as
follows: Hydroxychloroquine (25/49 cases), mycophenolate mofetil
(9/49 cases), thalidomide (27/49 cases), azathioprine (7/49 cases),
methotrexate (10/49 cases) and cyclophosphamide (6/49 cases). Two
patients with severe dysphagia were treated with intravenous
immunoglobulin. Patients with an infection were administered
anti-infective and symptom treatment.
Key causes of mortality
The cause of mortality in 49 patients, and their
survival time, are shown in Table I.
The most frequent causes of mortality were infection, heart and
lung dysfunction and malignancy. The malignancy cases included
breast cancer, lung cancer, nasopharyngeal carcinoma and ovarian
cancer. Patients in which mortality was associated with an
infection had a lower survival time than patients succumbing to
myositis-associated mortality (t=−2.819; P<0.05). In the cases
leading to mortality, the proportion of patients with a survival
time of <1 year due to an infection was higher than in patients
succumbing to myositis-induced mortality (χ2=6.110;
P<0.05).
 | Table I.Causes of mortality and survival time
of 49 patients with idiopathic inflammatory myopathy. |
Table I.
Causes of mortality and survival time
of 49 patients with idiopathic inflammatory myopathy.
| Cause of
mortality | Cases | Mean survival time
(months) | Patients surviving
<1 year | Patients surviving
>1 year |
|---|
|
Infection-associated | 34 (69.4) |
7.3a | 26b | 8 |
| Bacterial or fungal
infection | 31 (63.3) | 6.9 | 24 | 7 |
| Viral hepatitis | 3 (6.1) | 11.7 | 2 | 1 |
|
Myositis-associated | 15 (30.6) | 14.7 | 6 | 9 |
| Acute interstitial
lung disease | 2 (4.1) | 7.0 | 1 | 1 |
| Ventilator
weakness | 1 (2.0) | 3.0 | 1 | 0 |
| Pneumothorax | 1 (2.0) | 3.0 | 1 | 0 |
| Liver failure | 1 (2.0) | 11.0 | 1 | 0 |
| Pulmonary artery
hypertension | 1 (2.0) | 22.0 | 0 | 1 |
| Acute myocardial
infarction | 2 (4.1) | 12.5 | 1 | 1 |
| Arrhythmia | 1 (2.0) | 21.0 | 0 | 1 |
| Gastrointestinal
bleeding | 1 (2.0) | 13.0 | 0 | 1 |
| Renal failure | 1 (2.0) | 16.0 | 0 | 1 |
| Tumor | 4 (8.2) | 23.3 | 1 | 3 |
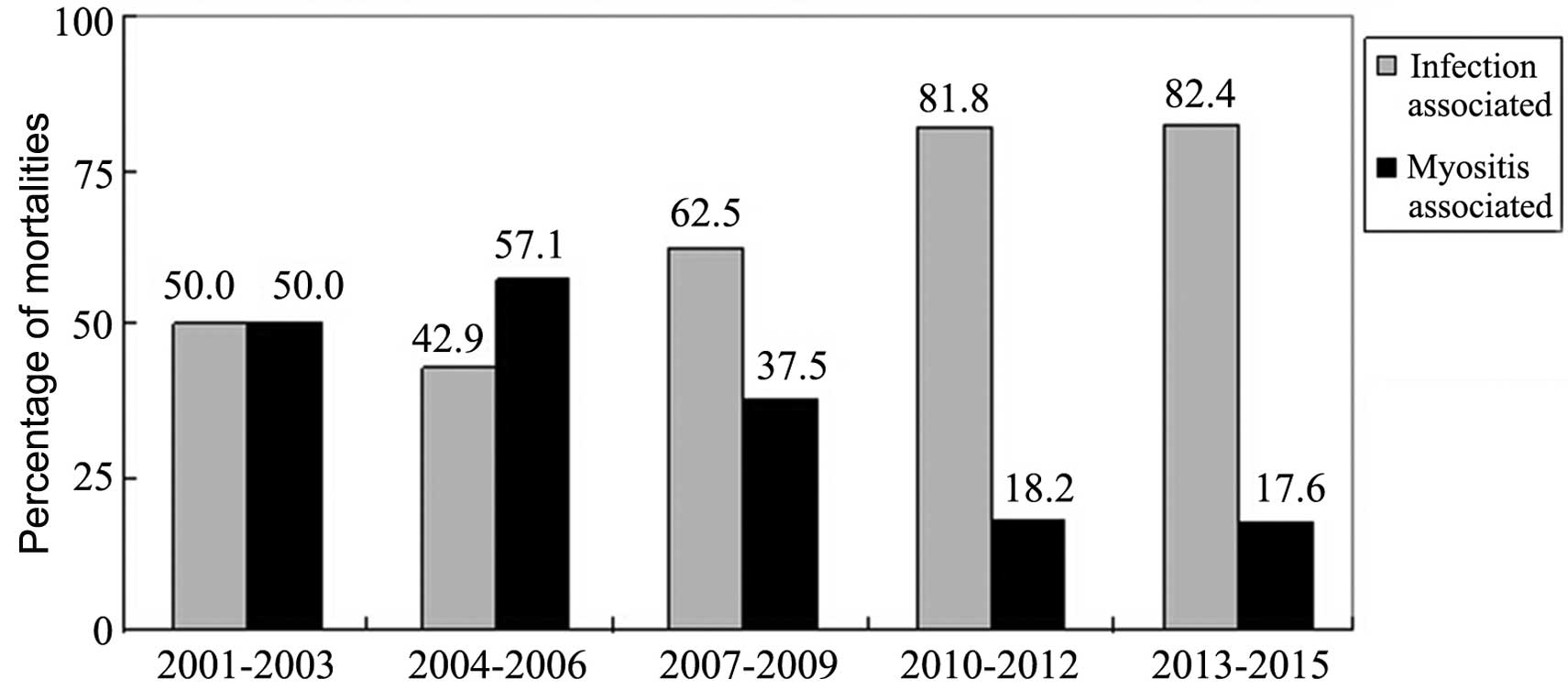
The mortality rate of IIM patients admitted to the
Xiangya Hospital was assessed in 3-year groupings from 2001 to
2015, together with the proportion of infection- or
myositis-associated mortalities (Table
II). The mortality rate decreased in the period of 2013–2015
compared with the previous time periods, but this was not
identified as statistically significant (P>0.05). Although the
proportion of infection-associated mortalities within the total
number of patients with IIM was not significantly different among
the time groupings, the proportion of mortalities secondary to
organ failure due to myositis were significantly reduced over time
(P<0.05). However, infection was the primary cause of mortality
in patients with IIM. The proportion of infection-associated
mortalities in patients with IIM increased, while the proportion of
myositis-associated mortalities decreased (Fig. 1).
 | Table II.Rates of mortality caused by infection
and myositis in idiopathic inflammatory myopathy patients, reported
by year. |
Table II.
Rates of mortality caused by infection
and myositis in idiopathic inflammatory myopathy patients, reported
by year.
|
| Cases |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Cause of
mortality | 2001–2003 | 2004–2006 | 2007–2009 | 2010–2012 | 2013–2015 | Subtotal | χ2 | P-value |
|---|
|
Infection-associated | 3 (4.4) | 3 (3.1) | 5 (4.2) | 9 (6.0) | 14 (5.8)a | 34 (5.0) | 1.053 | 0.305 |
|
Myositis-associated | 3 (4.4) | 4 (4.1) | 3 (2.5) | 2 (1.3) | 3 (1.2) | 15 (2.2) | 4.339 | 0.037 |
| Subtotal | 6 (8.8) | 7 (7.1) | 8 (6.8) | 11 (7.3) | 17 (7.1) | 49 (7.2) | 0.101 | 0.750 |
| Total
hospitalizations | 68 | 98 | 118 | 151 | 241 | 676 |
|
|
Infection sites and pathogens
In the 34 infection-associated mortality cases, the
lungs were the primary site of infection, with the blood and
intracalvarium as the second and third most common sites,
respectively (Table III). Limb
gangrene, an abdominal abscess, a decubitus ulcer on the buttocks
and other foci of infection were also observed in a number of
cases. Etiological diagnoses were conducted for 16 cases from lung,
blood and urinary tract infections and a decubitus ulcer of the
buttocks, based on sputum, blood, urine and secretion cultures and
clinical manifestations. Intracranial infection was demonstrated by
cerebrospinal fluid gram stain, revealing gram-positive cocci.
Bacterial and fungal infections were identified in 14 and 3 cases,
respectively. Co-infection with cytomegalovirus was also identified
by serum antibody detection in 2 cases. Three viral
hepatitis-associated mortalities were identified, with 2 of these
being hepatitis B virus (HBV) cases and 1 being caused by hepatitis
C virus (HCV).
 | Table III.Infection sites and identifiable
pathogens present in cases of infection-associated mortality
associated with idiopathic inflammatory myopathy. |
Table III.
Infection sites and identifiable
pathogens present in cases of infection-associated mortality
associated with idiopathic inflammatory myopathy.
| Infection site | Cases | Pathogen | Cases |
|---|
| Lung infection | 25 | Candida
parapsilosis | 1 |
|
|
| Candida
glabrata | 1 |
|
|
| Candida
albicans + cytomegalovirus | 1 |
|
|
| Streptococcus
pneumoniae | 1 |
|
|
| Klebsiella
pneumoniae | 2 |
|
|
| Acinetobacter
baumannii | 2 |
|
|
| Pseudomonas
alcaligenes + Acinetobacter baumannii | 1 |
|
|
| Enterobacter
cloacae + Stenotrophomonas maltophilia | 1 |
|
|
| Mycobacterium
tuberculosis | 1 |
|
|
| Acinetobacter
baumannii + cytomegalovirus | 1 |
| Urinary tract
infections | 3 | Escherichia
coli | 1 |
| Intracranial
infection | 1 | Gram-positive
cocci | 1 |
| Bloodstream
infection | 6 |
Methicillin-resistant Staphylococcus
aureus | 1 |
|
|
| Proteus
mirabilis | 1 |
| Right upper limb
gangrene | 1 |
|
|
| Abdominal
abscess | 1 |
|
|
| Decubitus ulcer of
the buttocks | 3 | Pseudomonas
aeruginosa | 1 |
Discussion
IIM is a form of autoimmune disease that is
characterized by progressive muscle weakness of the symmetrical
proximal limb muscles, periorbital edema, purple spots and a purple
mound on the extensor side of the joint (2). IIM may also result in the damage of
multiple organs. It has previously been reported that although the
administration of glucocorticoids and immunosuppressants decreases
the risk of mortality from systemic lupus erythematosus, it may
increase the risk of infections and associated complications
(9). The present study showed that
the proportion of infection-associated mortalities increased while
the proportion of myositis-associated mortalities decreased in
patients with IIM following treatment with glucocorticoids and
immunosuppressants.
Infection is considered to be the primary cause of
mortality in IIM patients. Based on the analysis of 15,407 patients
with DM/PM from the HCUP Nationwide Inpatient Sample, Murray et
al (10) reported that the
mortality rate was significantly higher in patients with IIM who
also had an infection. In the current study, the lungs and the
blood were the primary infection sites, and bacteria and fungi were
the primary pathogens. Patients with IIM with a pathogenic
co-infection typically demonstrated a number of the following
characteristics: Long-term glucocorticoid and immunosuppressant
treatments; also suffering from diabetes, chronic viral hepatitis
or kidney disease; interstitial lung disease causing pulmonary
infection; damage in respiratory muscles, leading to increased
difficulty removing secretions and resulting in ventilatory
disorders; damage in the gastrointestinal muscles, resulting in
dysphagia and pulmonary aspiration defects; and decubitus ulcers
and delayed healing, secondary to nutritional deficiencies and
being bedridden. The most common bacteria causing infections in IIM
cases included Klebsiella pneumoniae, Acinetobacter
baumannii, Streptococcus pneumoniae, Staphylococcus
aureus and Candida spp. Co-infections, opportunistic
infections, invasive fungal infections and viral infections were
common. During IIM therapy, pneumococcal and influenza vaccinations
should be administered for prevention (1). Additionally, glucocorticoid dosages
should be gradually reduced when the condition is controlled.
It can be concluded from the present study that
physicians should consider HBV and HCV infections in patients with
IIM as long-term glucocorticoid and immunosuppressant treatments
that may cause the reactivation of viral hepatitis. In the current
study, 2 patients were HBV surface antigen-positive, HBV antibody
e-positive and hepatitis B core antibody-positive following
diagnosis with IIM and 1 patient was anti-HCV antibody-positive but
all 3 patients demonstrated normal liver function. However,
following glucocorticoid and methotrexate administration, viral
replication was reactivated in the patients presenting with viral
resistance to the antiviral therapy. The levels of aspartate
aminotransferase and alanine aminotransferase in these patients was
elevated and they were jaundiced, with an outcome of liver failure
and mortality in all 3 cases. Screening for HBV and HCV should
therefore be conducted prior to the initiation of IIM therapy. If
patients with IIM are positive for HBV and HCV, anti-viral therapy
(such as entecavir or tenofovir disoproxil) should be administered
2–4 weeks prior to immunosuppressive therapy and until 6 months
after the immunosuppressive therapy has been ceased. This
administration should occur even if HBV-DNA and HCV-RNA are not
detectable and the liver enzyme level is normal in these patients.
If anti-viral therapy is administered following liver enzyme
elevation, recurrence of viral hepatitis may result in liver
failure (11).
Impaired heart function was observed in 9–72% of the
IIM patients, predominantly presenting as sinus tachycardia and
atrioventricular block, and a number of cases also presented with
associated symptoms (12). In the
present study, 1 patient succumbed to complete heart failure, and 2
patients succumbed to heart failure induced by an acute myocardial
infarction. Impaired lung function secondary to IIM typically
presented as interstitial lung disease, aspiration pneumonia and
hypoventilation. Previous studies indicated that interstitial lung
disease may occur in 36–65% of patients with IIM (13). In the present study, 1 patient
succumbed to right-side heart failure that was associated with
pulmonary hypertension resulting from interstitial lung disease.
Pulmonary hypertension may occur in 5.2% of IIM patients and reduce
the quality of life of patients (14). Without treatment, pulmonary
hypertension in IIM may cause right-side heart failure, arrhythmia
and sudden mortality (15).
Furthermore, a previous study of 150 patients with IIM suggested
that impaired renal function occurred in 23.3% of patients, and
that 10.7 and 20.7% had acute kidney disease and chronic kidney
disease, respectively (16). Acute
kidney disease predominantly resulted from drug- and
myoglobin-induced renal papillary necrosis; 12.5% of patients then
progressed to end-stage renal disease (17). In the present study, 1 patient
succumbed to renal disease due to hemodialysis cessation. However,
as therapy for renal function replacement was widely used, few
patients succumbed to chronic renal failure. Gastrointestinal
bleeding is rare in patients with IIM, and only few cases have been
reported worldwide, but visceral vasculitis may be a potential
cause (18–20). A number of reports have indicated
that intravenous immunoglobulin may be an effective treatment for
patients with IIM with severe gastrointestinal disease (21). Furthermore, patients with IIM
presenting with a skin rash should be mindful of the risk of
malignant atrophic papulosis due to the subsequent poor prognosis,
even with passive therapy (22).
Previous studies have reported that the prognosis of
IIM may be affected by gender, age, proximal muscle weakness,
dysphagia, hypoventilation, interstitial lung disease, serum
creatine kinase level and delayed diagnosis or treatment (23). Glucocorticoids remain the primary
choice in treating PM and DM. A number of clinicians immediately
administer immunosuppressants following confirmation of an IIM
diagnosis (24). However, there is a
lack of large-scale supporting evidence for this approach, thus
that doctors typically depend on their experience in the treatment
of patients with IIM.
The limitations of the present study are the absence
of postmortem autopsy, and its retrospective nature as it is
difficult to differentiate between DM/PM and IBM without an
autopsy. Causes of these mortalities may therefore be substantially
different from those succumbing to this disease outside of a
hospital.
The mortality rate of patients with IIM has
decreased in recent years, but is primarily associated with
infection. In addition to the occurrence of bacterial infections,
viral hepatitis necessitates increased consideration. Although
myositis-associated mortality and concurrent complications
decreased over time, infection-associated mortality increased. As a
result, the present study concludes that infection in IIM cases
necessitates increased focus to decrease the mortality rate.
References
|
1
|
Ernste FC and Reed AM: Idiopathic
inflammatory myopathies: Current trends in pathogenesis, clinical
features, and up-to-date treatment recommendations. Mayo Clin Proc.
88:83–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rider LG and Miller FW: Deciphering the
clinical presentations, pathogenesis, and treatment of the
idiopathic inflammatory myopathies. JAMA. 305:183–190. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Furst DE, Amato AA, Iorga ŞR, Gajria K and
Fernandes AW: Epidemiology of adult idiopathic inflammatory
myopathies in a U.S. managed care plan. Muscle Nerve. 45:676–683.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oddis CV, Conte CG, Steen VD and Medsger
TA Jr: Incidence of polymyositis-dermatomyositis: A 20-year study
of hospital diagnosed cases in Allegheny County, PA 1963–1982. J
Rheumatol. 17:1329–1334. 1990.PubMed/NCBI
|
|
5
|
Woo JH, Kim YJ, Kim JJ, Choi CB, Sung YK,
Kim TH, Jun JB, Bae SC and Yoo DH: Mortality factors in idiopathic
inflammatory myopathy: Focusing on malignancy and interstitial lung
disease. Mod Rheumatol. 23:503–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maldonado F, Patel RR, Iyer VN, Yi ES and
Ryu JH: Are respiratory complications common causes of death in
inflammatory myopathies? An autopsy study. Respirology. 17:455–460.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Torres C, Belmonte R, Carmona L,
Gómez-Reino FJ, Galindo M, Ramos B, Cabello A and Carreira PE:
Survival, mortality and causes of death in inflammatory myopathies.
Autoimmunity. 39:205–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bohan A and Peter JB: Polymyositis and
dermatomyositis (first of two parts). N Engl J Med. 292:344–347.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fei Y, Shi X, Gan F, Li X, Zhang W, Li M,
Hou Y, Zhang X, Zhao Y, Zeng X and Zhang F: Death causes and
pathogens analysis of systemic lupus erythematosus during the past
26 years. Clin Rheumatol. 33:57–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murray SG, Schmajuk G, Trupin L, Lawson E,
Cascino M, Barton J, Margaretten M, Katz PP, Yelin EH and Yazdany
J: A population-based study of infection-related hospital mortality
in patients with dermatomyositis/polymyositis. Arthritis Care Res.
67:673–680. 2015. View Article : Google Scholar
|
|
11
|
Martin P, Lau DT, Nguyen MH, Janssen HL,
Dieterich DT, Peters MG and Jacobson IM: A treatment algorithm for
the management of chronic hepatitis B virus infection in the United
States: 2015 update. Clin Gastroenterol Hepatol. 13:2071–2087.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Van Gelder H and Charles-Schoeman C: The
heart in inflammatory myopathies. Rheum Dis Clin North Am. 40:1–10.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fathi M, Lundberg IE and Tornling G:
Pulmonary complications of polymyositis and dermatomyositis. Semin
Respir Crit Care Med. 28:451–458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Wang GC, Ma L and Zu N: Cardiac
involvement in adult polymyositis or dermatomyositis: A systematic
review. Clin Cardiol. 35:686–691. 2012.PubMed/NCBI
|
|
15
|
Minai OA: Pulmonary hypertension in
polymyositis-dermatomyositis: Clinical and hemodynamic
characteristics and response to vasoactive therapy. Lupus.
18:1006–1010. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Couvrat-Desvergnes G, Masseau A,
Benveniste O, Bruel A, Hervier B, Mussini JM, Buob D, Hachulla E,
Rémy P, Azar R, et al: The spectrum of renal involvement in
patients with inflammatory myopathies. Medicine (Baltimore).
93:33–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qian Y, Ren H, Chen X, Zhang W, Li X, Shi
H and Chen N: Renal injury in patients with polymyositis and
dermatomyositis. Shang Hai Jiao Tong Da Xue. 31:451–454. 2011.(In
Chinese).
|
|
18
|
Chen GY, Liu MF, Lee JY and Chen W:
Combination of massive mucinosis, dermatomyositis, pyoderma
gangrenosum-like ulcer, bullae and fatal intestinal vasculopathy in
a young female. Eur J Dermatol. 15:396–400. 2005.PubMed/NCBI
|
|
19
|
Marie I, Levesque H, Cailleux N, Courtois
H and Guédon C: Intravenous immunoglobulin for treatment of
gastro-intestinal haemorrhage in dermatomyositis. Ann Rheum Dis.
60:723–724. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tweezer-Zaks N, Ben-Horin S, Schiby G,
Bank I, Levi Y, Livneh A and Langevitz P: Severe gastrointestinal
inflammation in adult dermatomyositis: Characterization of a novel
clinical association. Am J Med Sci. 332:308–313. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang DX, Shu XM, Tian XL, Chen F, Zu N, Ma
L and Wang GC: Intravenous immunoglobulin therapy in adult patients
with polymyositis/dermatomyositis: A systematic literature review.
Clin Rheumatol. 31:801–806. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Burgin S, Stone JH, Shenoy-Bhangle AS and
McGuone D: Case records of the Massachusetts General Hospital. Case
18–2014. A 32-year-old man with a rash, myalgia, and weakness. N
Engl J Med. 370:2327–2337. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brandão M and Marinho A: Idiopathic
inflammatory myopathies: Definition and management of refractory
disease. Autoimmun Rev. 10:720–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Carstens PO and Schmidt J: Diagnosis,
pathogenesis and treatment of myositis: Recent advances. Clin Exp
Immunol. 175:349–358. 2014. View Article : Google Scholar : PubMed/NCBI
|