Introduction
Juvenile idiopathic arthritis (JIA) is defined as a
chronic inflammatory disease associated with arthritis of unknown
etiology. It is a form of arthritis that is observed in childhood,
occurring in 16–150 out of 100,000 children, and thus is the most
common chronic rheumatic disease affecting children worldwide
(1). JIA is diagnosed in cases where
the age at onset is <16 years, the disease duration is 6 weeks
or greater, and other known conditions are excluded (2,3). All
presentations of JIA share the common characteristic of chronic
joint inflammation. Based on its pathology, JIA is classified into
several categories, including systemic, polyarticular,
oligoarticular, psoriatic, enthesitis-associated and
undifferentiated JIA (4,5). Although systemic JIA is the rarest form
of juvenile arthritis (~10% of all types of JIA) (6), it is the most difficult to treat and
has a disproportionately high morbidity compared with the other
subtypes (7). Additionally, systemic
JIA frequently results in the worst outcome compared with the other
subtypes, as it affects the entire body. It is generally considered
that JIA severity increases with the number of joints affected. The
possibility that a patient will eventually experience a total
remission of symptoms decreases with increasing disease severity
(8).
The present study described the typical case of a
patient who was diagnosed with systemic JIA associated with notably
elevated plasma levels of D-dimer. Following 6 months of medical
treatment, the patient's joint swelling and pain were controlled,
and the D-dimer levels were considerably decreased. The treatment
and outcome of the present case were also discussed.
Case report
A 5-year-old Chinese girl presented at the
Department of Rheumatology and Immunology (The First Affiliated
Hospital of Guangzhou University of Chinese Medicine, Guangzhou,
China) with severe pain in multiple joints in August 2013. The
patient had progressive swellings at the neck, waist, elbows,
wrists, knees, ankles and knuckles. The patient's medical history
was normal, with up-to-date immunizations and no notable family
history of autoimmune diseases, including JIA. At the first
examination following admission, the patient was noted to be tired
and pale, and the highest axillary temperature was 39.2°C
(102.5°F).
The patient had felt unwell for >2 months, and
initially presented with joint swelling and pain in the left knee.
She had been previously diagnosed in a local clinic and received
external medical treatment (10 days earlier, in August 2013;
treatment details unknown); however, the condition was not improve
noticeably. Subsequently, 12 days after treatment at the local
clinic, the patient presented recurrent fever and was administered
a fever-reducing medication (ibuprofen) when the fever reached
38.5°C (101.3°F). Following administration of antipyretic, the
temperature returned to normal. However, 2 weeks before admission
to our hospital, the patient experienced severe swelling and pain
in the medial sides of her neck, waist, elbows, wrists, knees,
ankles and knuckles. The severe pain was accompanied by a fever,
which usually occurred once daily in the evening. The patient was
unable to stand or ambulate without assistance.
A physical examination revealed that the patient had
no erythrasma or hemorrhagic spots on the skin (rash), and the
superficial lymph nodes were palpable in the size range between
0.5×0.5 and 0.5×1 cm. No adhesions with the surrounding tissues and
no tenderness were observed. In addition, the patient displayed
throat congestion with blood and bilateral amygdala enlargement
with degree I swelling (9), but no
evidence of pyosis. Further physical examination demonstrated that
the heart and lung functions were normal, while the abdomen was
soft, non-tender and without evidence of abdominal masses. The
liver was palpable at 1.5 cm below the right costal margin at the
right midclavicular line and 1 cm below the xiphoid process, but
not below the spleen rib. The patient experienced tenderness (mild
to moderate) over the sternum, but not over the cervical and lumbar
vertebrae. Joint tenderness and swelling involved the shoulders,
elbows, wrists, bilateral metacarpophalangeal 1–5, proximal
interphalangeal joints 1–5, bilateral hip, knees and ankles, while
the patient presented limited range of motion in the aforementioned
joints. Restricted ranges of flexion and extension were observed in
the neck and lumbar regions.
Laboratory tests (results presented in Table I) performed at the initial visit of
the patient in our hospital showed a white blood cell (WBC) count
of 11,710/µl (normal range, 4–10×109/l), platelet count
of 471,000/µl (normal range, 100–300×109/l), hemoglobin
level of 77 g/l (normal range, 120–160 g/l), C-reactive protein
(CRP) level of 128 mg/l (normal range, 0–8 mg/l), an erythrocyte
sedimentation rate (ESR) of 75 mm/h (normal range, 0–15 mm/h) and a
noticeably elevated plasma level of D-dimer (10,600 ng/ml; normal
range, 0–324 ng/ml). Tests for antinuclear antibodies (ANAs) and
double-stranded DNA were negative, while the complement C3 and C4
levels and the results of an anti-extractable nuclear antigen
antibody test were normal. Furthermore, the levels of anti-cyclic
citrullinated peptide antibody and rheumatoid factor (RF) were
found to be <0.5 U/ml (normal range, 0–20 U/ml) and <20 U/ml
(normal range, 0–5 U/ml), respectively, while a test for glucose
phosphate isomerase was negative. The patient was suspected to
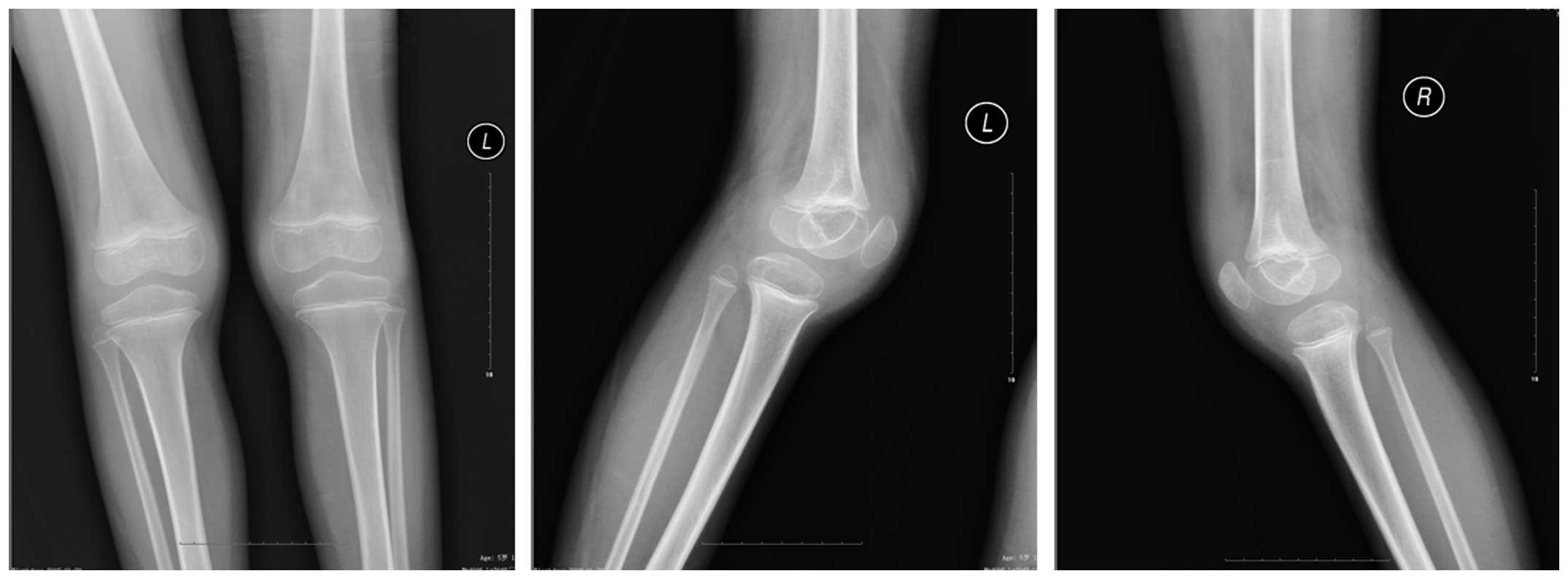
suffer from systemic JIA, thus, X-ray examination was performed in
order to rule out other potential causes of symptoms (such as
fracture). A radiography examination revealed no abnormalities in
the chest; however, the knee joint capsules of the patient were
swollen (Fig. 1). Furthermore, a
color Doppler ultrasound showed hepatomegaly, but no abnormal
findings were observed for the gallbladder, spleen, pancreas,
kidneys, ureter or urinary bladder. Echocardiography revealed no
abnormalities.
 | Table I.Clinical laboratory test results. |
Table I.
Clinical laboratory test results.
| Date | WBC
(109/l) | HGB (g/l) | PLT
(109/l) | ESR (mm/H) | CRP (mg/l) | RF (U/ml) | D-dimer (ng/ml) |
|---|
| Aug 25, 2013 | 11.71 | 77 | 471 | 75 | 128.0 | <20 | – |
| Aug 28, 2013 | 7.05 | 87 | 509 | 90 | 56.2 | – | 10,600 |
| Sep 1, 2013 | 6.31 | 83 | 460 | 93 | 26.8 | <20 |
7,320 |
| Sep 6, 2013 | 7.28 | 89 | 580 | 60 |
9.6 | <20 |
2,460 |
| Nov 23, 2013 | 8.40 | 129 | 328 | 5 |
1.2 | <20 |
717 |
| Dec 21, 2013 | 6.40 | 131 | 368 | 4 |
2.2 | <20 |
351 |
| Feb 11, 2014 | 7.80 | 132 | 378 | 3 |
<1.0 | <20 |
336 |
Based on the JIA classification system proposed by
the International League of Associations for Rheumatology, the
patient was diagnosed with systemic JIA (4). The patient was treated with
methotrexate (MTX; 5 mg per week; Sine Pharmaceutical, Co., Ltd.,
Shanghai, China), hydroxychloroquine (HCQ; 100 mg, twice a day;
Zhongxi Pharmaceutical Co., Ltd.,Shanghai, China) and
methylprednisolone tablets (Medrol, 4 mg per day; Pfizer, Inc., New
York, NY, USA) for 7 days. Low doses of folic acid (5 mg per week;
Changzhou Pharmaceutical Factory, Changzhou, China) and vitamin D
(chewable tablets; Xingsha Pharmaceutical Co., Ltd., Xiamen, China)
were also prescribed to minimize the treatment side effects.
However, although the patient's D-dimer level decreased from 10,600
to 2,460 ng/ml, the fever could not be controlled. Therefore,
etanercept (12.5 mg per week, subcutaneous injection; Pfizer, Inc.)
was added at the beginning of week 2. Following the administration
of etanercept, clinical remission was rapidly obtained, accompanied
by an absence of joint swelling and pain, and no recurrence of
fever. After 6-months of treatment, the D-dimer levels, CRP levels
and ESR values of the patient were considerably decreased. No
adverse events occurred during the treatment.
The most recent follow-up was conduced in August
2015, at which the patient continued the treatment with MTX, HCQ
and etanercept, but not with methylprednisolone. Written informed
consent was obtained from the patient's guardian prior to the
publication of this study.
Discussion
JIA is a form of chronic arthritis that occurs in
children and has no apparent cause. Systemic JIA may initially
present as a swollen knuckle, a spiking fever or an unexplained
rash (4). The present study
described a case of JIA in a 5-year-old Chinese girl, in whom the
triggers for the disease were unknown. The patient was initially
diagnosed and treated at a local clinic; however, the symptoms were
not alleviated. The initial treatment details are unknown, thus it
is unclear whether it exacerbated the disease. The patient
presented daily fevers for >2 weeks and displayed lymph node
enlargement. She also showed symptoms of swelling, pain and
stiffness in numerous joints. No family history of
HLA-B27-associated diseases was identified, and the RF of the
patient was <20 IU/ml, while the ANA test gave negative results;
these are characteristic features of systemic JIA as compared with
other types of JIA (4). After
excluding infection, leukemia, malignancy and other rheumatic
diseases, the patient was diagnosed with systemic JIA.
The various forms of JIA are a heterogeneous group
of disorders presenting with certain common first symptoms,
including joint pain or swelling, and reddened or warm joints
(10). Although systemic JIA is the
least prevalent JIA subtype, it is often associated with the worst
outcomes, and has disproportionately high morbidity compared with
the other subtypes (11). Due to its
distinct clinical and epidemiological features, systemic JIA has
been recognized as a unique condition among childhood arthritis
diseases (12). Systemic JIA poses a
formidable challenge to pediatric rheumatologists, since prediction
of its long-term clinical course and outcome based on its initial
presentation and early course is difficult. In addition, there is
no single definitive laboratory test for the diagnosis of systemic
JIA.
Studies have shown that endothelial activation is a
key component in the pathogenesis of JIA, and that the levels of
biomarkers for endothelial activation are usually elevated in JIA
patients with the systemic subtype (13,14).
Among these biomarkers, soluble intercellular adhesion molecule-1
(sICAM-1), E-selectin and D-dimer have been reported as being
predictive for JIA disease activity (15,16).
Elevated D-dimer levels have been correlated with the elevation of
sICAM-1 levels. Bloom et al found that levels of fibrin
D-dimer were correlated with short-term outcomes and the response
to immunomodulatory therapies in patients with systemic JIA
(13,16,17). A
normal plasma D-dimer level is <250 ng/ml; however, in the
current case, a significantly elevated level of D-dimer (10,600
ng/ml) was observed at the first examination upon admission. In
addition, abnormally high levels of WBC, ESR and CRP were observed.
Elevated plasma D-dimer levels typically reflect the activation of
the procoagulant and fibrinolytic systems; however, the abnormal
D-dimer level in the patient of the present study was much higher
compared with the initial median value (~1,335 ng/ml) that is
commonly detected in children with thrombosis (18). Thrombosis was also excluded based on
the patient's other clinical information. Bloom et al
reported that, among 24 patients with systemic JIA, 23 (96%) were
found to have elevated D-dimer levels (16). D-dimer levels are correlated with
fever and total leukocyte count, but not with the ESR, the duration
of morning stiffness or swollen joint counts. In the current case,
we observed abnormally high levels of WBC, ESR and CRP, in addition
to elevated D-dimer levels.
After 1 week of treatment with MTX, HCQ and
methylprednisolone, joint pain was alleviated and the patient's
D-dimer level was reduced from 10,600 to 2,460 ng/ml, suggesting an
association of D-dimer levels with joint pain. However, the
patient's fever could not be controlled, suggesting that these
medications were not sufficient to alleviate all symptoms.
Etanercept administration is recommended for children aged 4–17
years who have active JIA in at least five joints, and whose
condition has not adequately responded to MTX treatment (11). In the present case, a clinical
remission was quickly achieved after addition of etanercept to the
treatment regimen from week 2. Fever, joint swelling and pain were
less severe, and the results of laboratory tests showed a
continuous decline in D-dimer levels, reaching 717 ng/ml after 2
months of treatment. Recent studies conducted in adult RA patients
have observed encouraging results concerning the use of MTX in
combination with the new anti-tumor necrosis factor drugs,
etanercept and infliximab (19).
Those studies reported near normal D-dimer levels (~330 ng/ml)
following 4–6 months of treatment. This treatment duration is
consistent with that expected for MTX therapy, where the maximum
therapeutic effect is usually achieved 4–6 months after initiation
of the treatment (20). The
reduction in D-dimer levels was correlated with the patient's level
of disease activity, and was associated with reductions in ESR and
CRP levels. During the constant follow-up, no signs of
complications were observed.
In the case reported in the present study, reduced
D-dimer levels were accompanied by rapid clinical remission, as
well as reduction and later absence of joint swelling and pain. At
present, there are no guidelines for making clinical decisions
regarding for how long MTX treatment should be continued following
disease remission. Currently, the patient of the present study
continues to be treated with MTX, HCQ and etanercept, but not
methylprednisolone. At the time of the most recent visit (August
2015), the patient presented no joint swelling, joint pain or
incidence of recurrent fever, and continued to receive treatment
with MTX, HCQ and etanercept, but not with methylprednisolone. Due
to the high relapse rate in JIA patients following MTX withdrawal,
a weekly regimen of MTX, HCQ and etanercept using the previous
dosages was continued for an additional 6 months, until a sustained
remission was achieved.
In conclusion, the present study reported the case
of a 5-year-old girl who suffered from swelling and pain at
multiple joints. The patient was diagnosed with systemic JIA and
was treated with a combination therapy. Disease remission
accompanied by decreased plasma D-dimer levels was observed after 6
months of treatment. In future studies, D-dimer may be used to
evaluate the degree of disease activity in JIA patients.
References
|
1
|
Foster H, Rapley T and May C: Juvenile
idiopathic arthritis: Improved outcome requires improved access to
care. Rheumatology (Oxford). 49:401–403. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prakken B, Albani S and Martini A:
Juvenile idiopathic arthritis. Lancet. 377:2138–2149. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Espinosa M and Gottlieb BS: Juvenile
Idiopathic Arthritis. Pediatr Rev. 33:303–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Petty RE, Southwood TR, Manners P, Baum J,
Glass DN, Goldenberg J, He X, Maldonado-Cocco J, Orozco-Alcala J,
Prieur AM, et al: International League of Associations for
Rheumatology classification of Juvenile Idiopathic Arthritis:
Second revision, Edmonton, 2001. J Rheumatol. 31:390–392.
2004.PubMed/NCBI
|
|
5
|
Kahn P: Juvenile idiopathic arthritis: An
update for the clinician. Bull NYU Hosp Jt Dis. 70:152–166.
2012.PubMed/NCBI
|
|
6
|
Schneider R and Laxer RM: Systemic onset
juvenile rheumatoid arthritis. Bailliere's Clin Rheumatol.
12:245–271. 1998. View Article : Google Scholar
|
|
7
|
Wallace CA and Levinson JE: Juvenile
rheumatoid arthritis: Outcome and treatment for the 1990s. Rheum
Dis Clin North Am. 17:891–905. 1991.PubMed/NCBI
|
|
8
|
Prince FH, Otten MH and van Suijlekom-Smit
LW: Diagnosis and management of juvenile idiopathic arthritis. BMJ.
341:c64342010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watson C, Nielsen SL, Cobb C, Burgerman R
and Williamson B: Pathological grading system for hippocampal
sclerosis: Correlation with magnetic resonance imaging-based volume
measurements of the hippocampus. J Epilepsy. 9:56–64. 1996.
View Article : Google Scholar
|
|
10
|
Martini A: Are the number of joints
involved or the presence of psoriasis still useful tools to
identify homogeneous disease entities in juvenile idiopathic
arthritis? J Rheumatol. 30:1900–1903. 2003.PubMed/NCBI
|
|
11
|
Quartier P, Taupin P, Bourdeaut F, Lemelle
I, Pillet P, Bost M, Sibilia J, Koné-Paut I, Gandon-Laloum S,
LeBideau M, et al: Efficacy of etanercept for the treatment of
juvenile idiopathic arthritis according to the onset type.
Arthritis Rheum. 48:1093–1101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mellins ED, Macaubas C and Grom AA:
Pathogenesis of systemic juvenile idiopathic arthritis: Some
answers, more questions. Nat Rev Rheumatol. 7:416–426. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bloom BJ, Nelson SM, Eisenberg D and
Alario AJ: Soluble intercellular adhesion molecule-1 and E-selectin
as markers of disease activity and endothelial activation in
juvenile idiopathic arthritis. J Rheumatol. 32:366–372.
2005.PubMed/NCBI
|
|
14
|
Turhan H, Erbay AR, Yasar AS, Aksoy Y,
Bicer A, Yetkin G and Yetkin E: Plasma soluble adhesion molecules;
intercellular adhesion molecule-1, vascular cell adhesion
molecule-1 and E-selectin levels in patients with isolated coronary
artery ectasia. Coron Artery Dis. 16:45–50. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dolezalova P, Telekesová P, Nemcová D and
Hoza J: Soluble adhesion molecules ICAM-1 and E-selectin in
juvenile arthritis: Clinical and laboratory correlations. Clin Exp
Rheumatol. 20:249–254. 2002.PubMed/NCBI
|
|
16
|
Bloom BJ, Tucker LB, Miller LC and
Schaller JG: Fibrin D-dimer as a marker of disease activity in
systemic onset juvenile rheumatoid arthritis. J Rheumatol.
25:1620–1625. 1998.PubMed/NCBI
|
|
17
|
Bloom BJ, Alario AJ and Miller LC:
Persistent elevation of fibrin D-dimer predicts longterm outcome in
systemic juvenile idiopathic arthritis. J Rheumatol. 36:422–426.
2009.PubMed/NCBI
|
|
18
|
Goldenberg NA, Knapp-Clevenger R and
Manco-Johnson MJ: Mountain States Regional Thrombophilia Group:
Elevated plasma factor VIII and D-dimer levels as predictors of
poor outcomes of thrombosis in children. N Engl J Med.
351:1081–1088. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keyser FD: Choice of Biologic Therapy for
Patients with Rheumatoid Arthritis: The Infection Perspective. Curr
Rheumatol Rev. 7:77–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
American College of Rheumatology
Subcommittee on Rheumatoid Arthritis Guidelines: Guidelines for the
management of rheumatoid arthritis: 2002 Update. Arthritis Rheum.
46:328–346. 2002. View Article : Google Scholar : PubMed/NCBI
|