Introduction
Subarachnoid hemorrhage (SAH)-induced cerebral
vasospasm (CVS) is the predominant cause of the high mortality and
disability rate associated with SAH (1). Numerous factors, such as bleeding
(location and volume), age, smoking, hypertension and operation
time, have previously been implicated in the pathogenesis of CVS.
In addition, increased levels of thrombin in catabolic processes
following SAH can cause strong and lasting CVS in the
cerebrovascular system of the SAH animal model (2). Thrombin and its receptor
protease-activated receptor 1 (PAR1) have previously been reported
to have an important role in vascular smooth muscle cell
proliferation, contraction and CVS pathogenesis (3).
The most common predisposing factor for vasospasm
onset following intracranial tumor resection is SAH (4). Blood accumulation in the basal cisterns
is thought to be an important factor in initiating the vasospasm
(5). Brain metabolism and blood flow
are regulated by the trigeminal system, which seems to be a
significant factor involved in cerebral vasospasm. Vasodilation and
upregulation in the cerebral blood flow are induced by stimulation
of the trigeminal nerve endings. It is considered that vasoactive
substances, direct mechanical trauma, tumor location and tumor
compression are the probable causes of vasospasm.
Tumor necrosis factor (TNF)-α has been demonstrated
to affect the incidence of CVS following SAH, and is closely
associated with a poor prognosis (6–8).
However, the role of TNF-α in promoting the upregulation of PAR1 in
cerebral vessels following SAH has rarely been reported in current
literature. In order to investigate the underlying mechanism of
SAH-induced CVS, the present study detected alterations in the
histological characteristics of the basilar artery in a rat model
of SAH-induced CVS. In addition, the expression of PAR1 and TNF-α
was analyzed by immunohistochemistry.
The present study aimed to investigate the
alterations in the expression of PAR1 and TNF-α in the basilar
artery of rats following a subarachnoid hemorrhage.
Materials and methods
Experimental rats and grouping
A total of 24 healthy male Sprague Dawley rats
(n=4/cage; weight, 280–320 g) were purchased from the Faculty of
Test Animals at Central South University (Changsha, China). The
rats were divided into four groups using a random number table: The
normal group, the SAH3d group, the SAH5d group and the SAH7d group.
Each group had ad libitum access to food and water, and the
rats were maintained at a temperature of 25°C and humidity of
66%.
Reagents and instruments
The PAR1 Monoclonal Antibody kit and the TNF-α
Immunohistochemical Detection kit were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The stereotaxic instrument
was obtained from RWD Life Science Co., Ltd. (Shenzhen, China), and
the epidural catheter was obtained from Xinghua Medical Equipment
Co., Ltd. (Shanghai, China). The microtome was purchased from Leica
Microsystems (Wetzlar, Germany). The DM3000 microscope was
purchased from Leica Microsystems, and the IX71 inverted microscope
was obtained from Olympus Corp. (Tokyo, Japan).
Preparation of the rat models of
SAH
The femoral artery blood collection tube was
prepared as follows: One end of a one-time epidural catheter was
pulled in order to generate a thin tube (~5 cm). The outer diameter
of the thin end was ~0.5 mm, and the thin end was trimmed to a 45°
slope. The rat model of SAH was established by administering double
injections of blood into the cisterna magna. Briefly, the rats were
anesthetized with an intraperitoneal injection of 10% chloral
hydrate (350 mg/kg body weight; Shanghai Jianglai Biotechnology
Co., Ltd., Shanghai, China), and were placed onto the operating
table in the prone position. Skin preparation and disinfection was
performed in the middle of the back of the neck, and the neck
muscle tissue was separated following incision, in order to expose
the atlanto-occipital fascia. Subsequently, in the supine position,
skin preparation and sterilization was performed on the right lower
extremity; followed by skin incision and exposure of the femoral
artery. Following femoral artery anterior incision, the thin end of
the femoral artery blood collection tube was inserted into the
femoral artery centripetally, using a 1 ml syringe to draw 0.3 ml
arterial blood. The rats were quickly placed onto the stereotaxic
instrument, and the cisterna magna was punctured.
Cerebrospinal fluid (0.05 ml) was drawn prior to
slow injection of 0.25 ml anticoagulated autologous arterial blood
into the subarachnoid space within 1 min (anticoagulant, heparin
sodium; Qilu Pharmaceutical Co., Ltd., Shandong, China). Following
the injection, the puncture point was pressed with a gelatin sponge
for ~30 sec, and the incision was sutured. The rats were placed
into the head-down (30°) prone position for ~30 min, in order to
facilitate the distribution of blood within the basilar artery.
After 48 h, blood was taken from the left femoral artery in the
same manner. The SAH3d, SAH5d and SAH7d groups were maintained and
observed, according to the grouping demand. The normal group did
not undergo treatment.
Preparation of tissue samples
The rats in the SAH3d, SAH5d and SAH7d groups were
perfused with 4% paraformaldehyde on day 3, 5 and 7, respectively,
following model establishment. The rats were sacrificed by cervical
dislocation, and the brains were immediately removed and fixed in
4% neutral paraformaldehyde at 4°C for 24 h. The basilar artery was
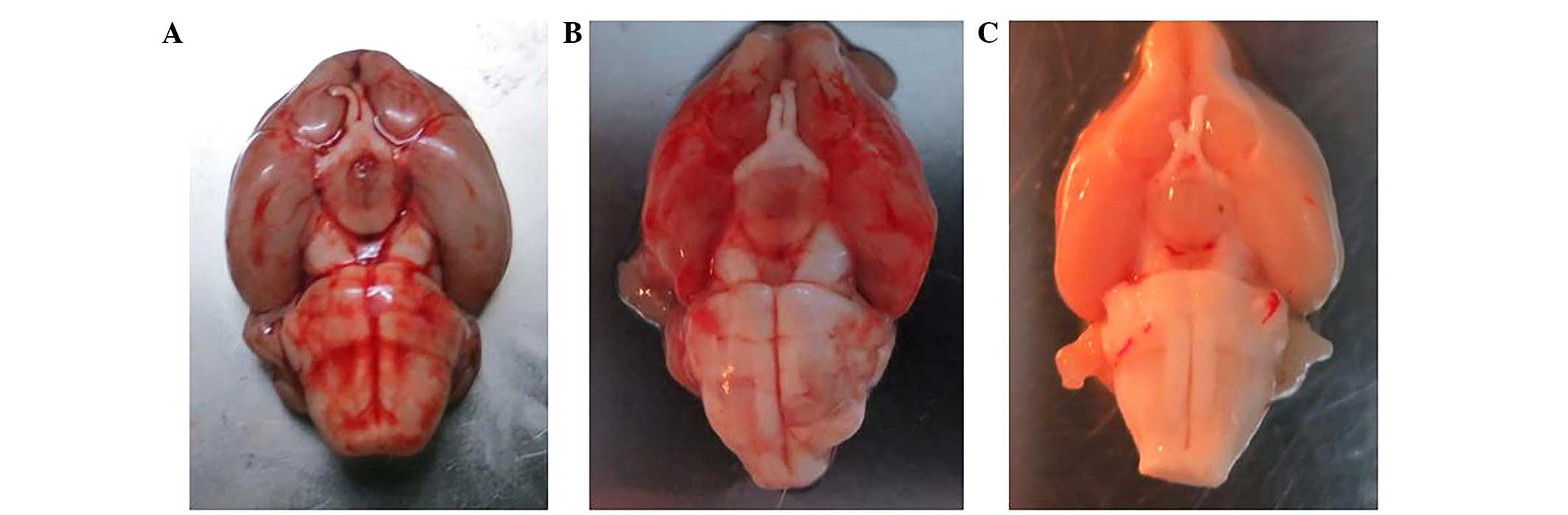
isolated, together with the brainstem (Fig. 1).
Hematoxylin and eosin (HE) staining of
the basilar artery and evaluation of spasm degree
The basilar artery was stained using HE (Shanghai
Jianglai Biotechnology Co., Ltd.), and the lumen cross-sectional
area was measured as an indication of spasticity. Tissue slices
(0.2 mm) were obtained from blood vessels of the following four
breakpoints: 0.2 mm below the superior cerebellar artery; upper
breakpoint of anterior inferior cerebellar artery; lower breakpoint
of anterior inferior cerebellar artery; and the intersection of
basilar artery and vertebral artery (9). The histological characteristics of the
basilar artery were observed under a microscope by a single
pathologist blinded to the treatment group, and Image-Pro Plus 6.0
image analysis software (Media Cybernetics, Inc., Rockville, MD,
USA) was used to analyze the average cross-sectional area of the
basilar artery lumen from each slice (10).
Immunohistochemical detection of PAR1
and TNF-α
Immunohistochemical detection of PAR1 and TNF-α
protein expression in the basilar artery was conducted, according
to the manufacturer's protocol. Brown coloration was indicative of
positive expression. A total of five horizons per slice were
randomly selected using a light microscope, in order to measure the
average optical density (OD) of positive reactions, and statistical
analyses were conducted using the mean OD of each slice.
Statistical analysis
All data were analyzed using the SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ±
standard deviation. Statistical inference was evaluated using
one-way analysis of variance (ANOVA) and inter-group comparison was
assessed using the least significant difference (LSD) method.
Heterogeneity of variance was investigated using the Brown-Forsythe
method and Kruskal-Wallis nonparametric test. Pair wise comparisons
between groups were evaluated using Tamhane's T2 method.
Correlation analysis between two groups was conducted using Pearson
correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
General condition and neurological
score of the SAH rat models
After successfully modeling for 24 h, the rats
exhibited listlessness, a loss of appetite and poor self-cleaning
behavior. Neurological scores were given, according to the four
point method of Endo et al (11): 1 point corresponded to no
neurological deficit; 2 points corresponded to mild neurological
deficit (lethargy, activity reduction); 3 points was indicative of
a moderate nerve deficit (limb weakness, lameness); and 4 points
corresponded to a severe neurological deficit (circling movement or
difficulty in walking). The neurological scores were as follows: In
the SAH3d group, two rats were given 2 points (33.3%) and four rats
were given 3 points (66.7%); in the SAH5d group, three rats were
given 1 point (50%) and three rats were given 2 points (50%); and
in the SAH7d group, four rats were given 1 point (66.7%) and two
rats were given 2 points (33.3%). The rats in the normal group were
all given 1 point. Following SAH modeling, the nerve dysfunction of
the rats presented remission, which may have been associated with
abundant collateral circulation in the rats, as described in a
previous study (9).
Histological examination
Rats were perfused using 4% paraformaldehyde in
order to isolate tissue samples. The SAH model rats exhibited a
diffuse SAH, predominantly located around the basilar artery and
the cisterna ambiens (Fig. 2).
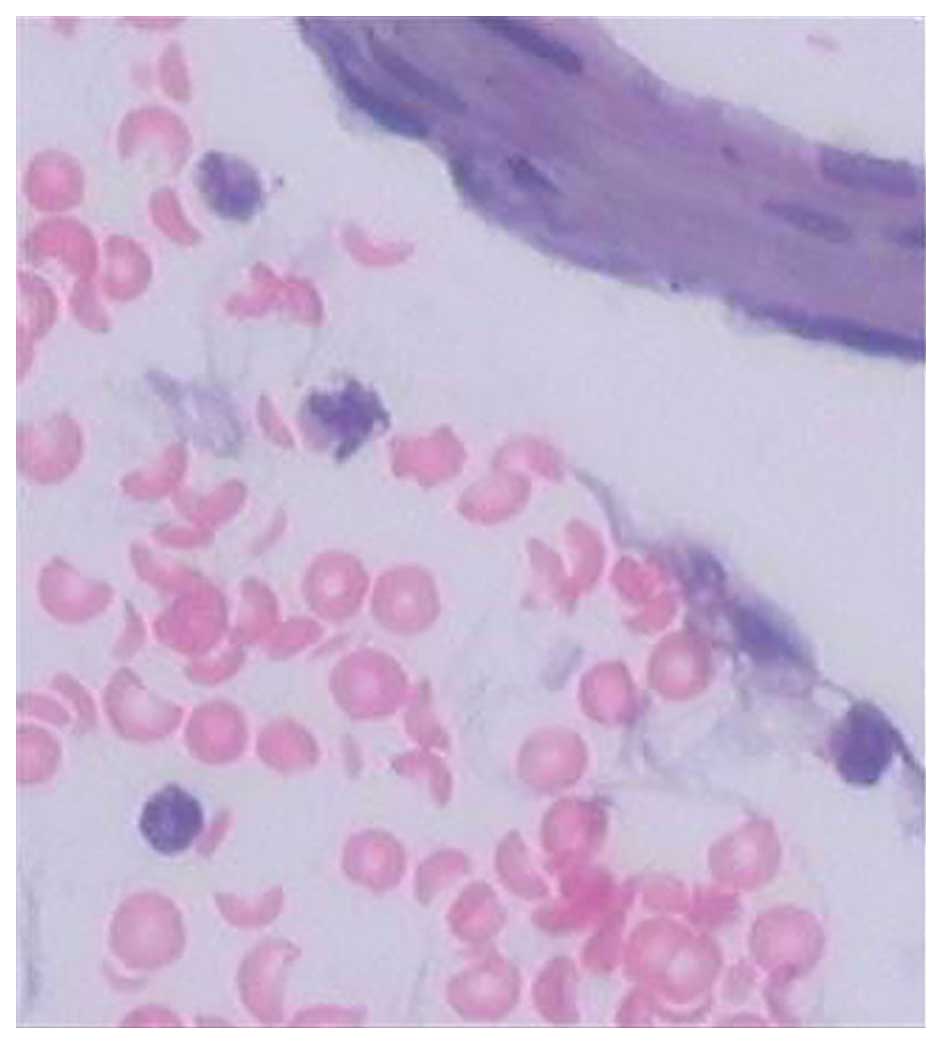
Basilar artery HE staining demonstrated that there was a large
number of erythrocytes and inflammatory cells surrounding the
basilar artery, and the inflammatory cells were predominantly
neutrophils. In addition, the majority of erythrocytes had a
complete morphology and only a few had disintegrated. The basilar
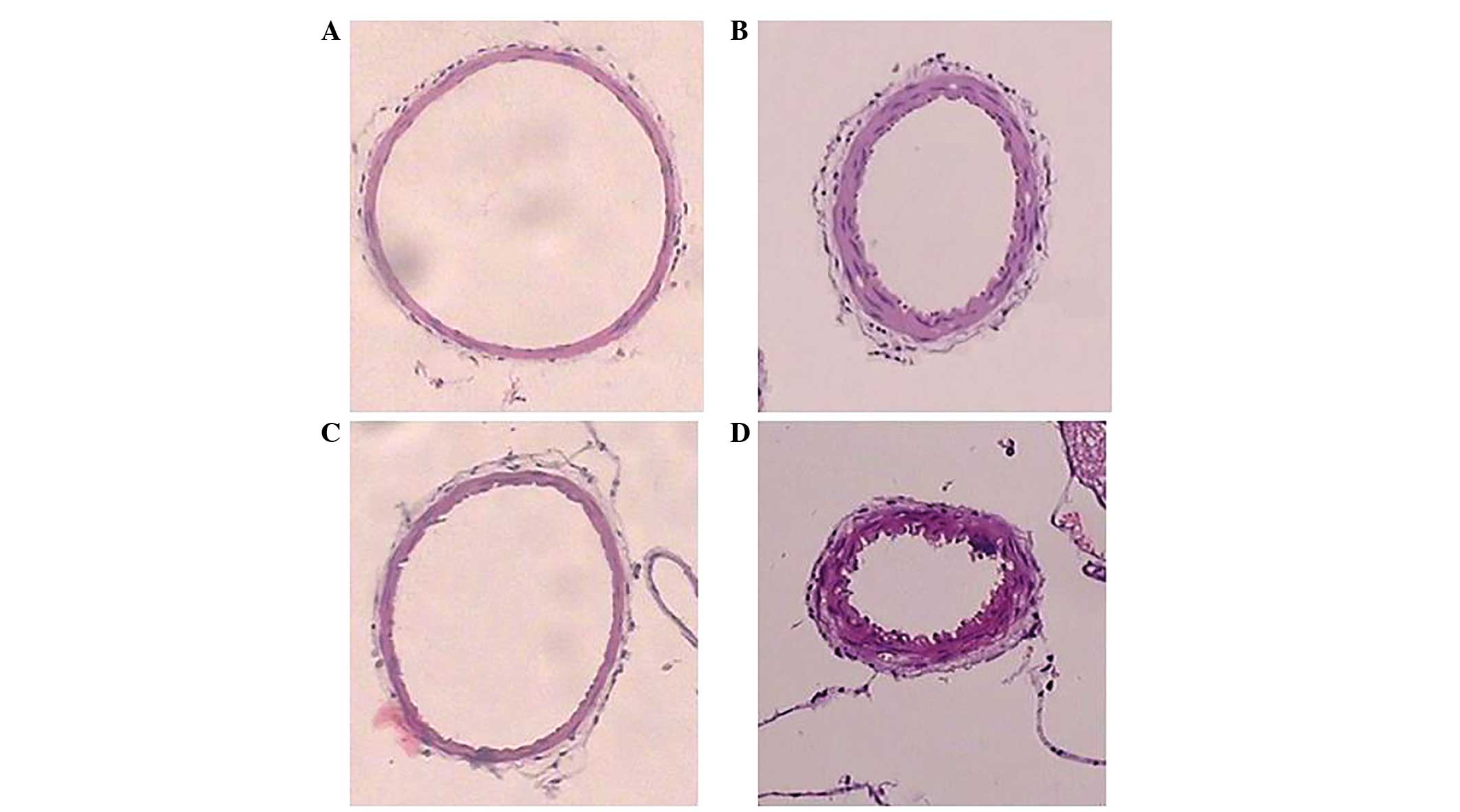
artery lumen was at a maximum size in the normal group, and
appeared rounded and oval-shaped, with a thin wall and smooth
intima without wrinkles. Conversely, the basilar artery lumen of
the SAH rats was narrow, with a thickened wall and wrinkled intima.
The SAH7d group rats exhibited the most severe histological
characteristics, followed by the SAH3d group, whereas basilar
artery spasms in the SAH5d group had eased (Fig. 3).
Analysis of the cross-sectional area
of the basilar artery between the groups
ANOVA demonstrated that the cross-sectional area of
the basilar artery among the four groups was significantly
different (P<0.05). LSD comparisons between two groups
demonstrated that the cross-sectional area of the basilar artery
was significantly decreased in the SAH3d, SAH5d and SAH7d groups,
as compared with the normal group (7.3438±0.0612×10−2
mm2; P<0.01). In addition, the cross-sectional area
of the basilar artery lumen of the SAH3d rats (3.6003±0.1034×10–2
mm2) was significantly smaller, as compared with the
SAH5d group rats (3.9195±0.0481×10−2 mm2;
P<0.05). Furthermore, the cross-sectional area of the basilar
artery lumen of the SAH3d rats was significantly larger, as
compared with the SAH7d group rats (2.2945±0.0995×10−2
mm2; P<0.05), and the cross-sectional area of the
basilar artery lumen of the SAH5d group rats was significantly
larger, as compared with the SAH7d group rats (data not shown).
PAR1 and TNF-α immunohistochemical
analysis
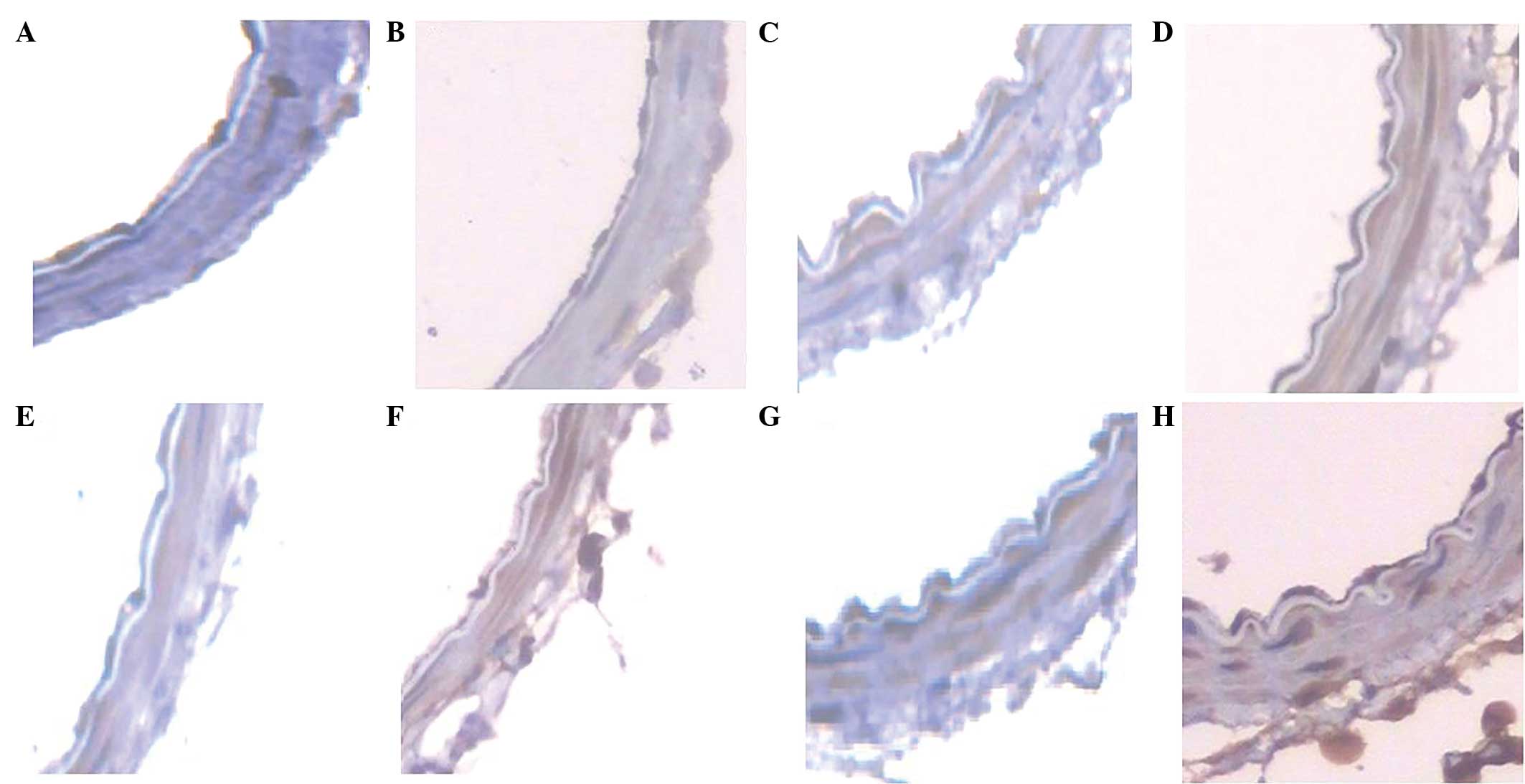
PAR1 and TNF-α protein expression in the basilar
artery of the SAH3d, SAH5d and SAH7d group rats increased in a
time-dependent manner, as demonstrated by immunohistochemistry. The
normal group was negative for the expression of both proteins
(Fig. 4).
Differences in the average OD of PAR1
between the four groups
The data were tested using the Brown-Forsythe method
and Kruskal-Wallis nonparametric test analysis (variance
nonhomogeneity in four groups, P<0.05); and the difference among
the four groups was statistically significant (P<0.01). In
addition, Tamhane's T2 comparisons between two groups demonstrated
that the mean OD of PAR1 in the basilar artery of the SAH3d
(0.1180±0.0042), SAH5d (0.2081±0.0016) and SAH7d (0.2382±0.0103)
groups was significantly increased, as compared with the OD of PAR1
in the basilar artery of the normal group (0.0851±0.0035;
P<0.01). Furthermore, the mean OD of PAR1 in the SAH5d and SAH7d
groups was significantly increased, as compared with the SAH3d
group rats (P<0.01). There was no significant difference in the
mean PAR1 OD between the SAH5d and SAH7d groups (P>0.05; data
not shown).
Differences in the average OD of TNF-α
between the four groups
ANOVA demonstrated that the average OD of TNF-α
between the four groups was significantly different (P<0.05).
LSD comparison between two groups demonstrated that the mean OD of
TNF-α in the basilar artery of the SAH3d (0.0872±0.0024), SAH5d
(0.1170±0.0019) and SAH7d (0.1587±0.0016) groups was significantly
increased, as compared with the OD of TNF-α in the basilar artery
of the normal group (0.0383±0.0014; P<0.01). In addition, there
was a significant difference in the OD of TNF-α between the SAH3d
and SAH5d group rats (P<0.01), and there was a significant
difference between the SAH3d and SAH7d group rats (P<0.01).
Furthermore, there was a significant difference between the SAH5d
and SAH7d group rats (P<0.01; data not shown).
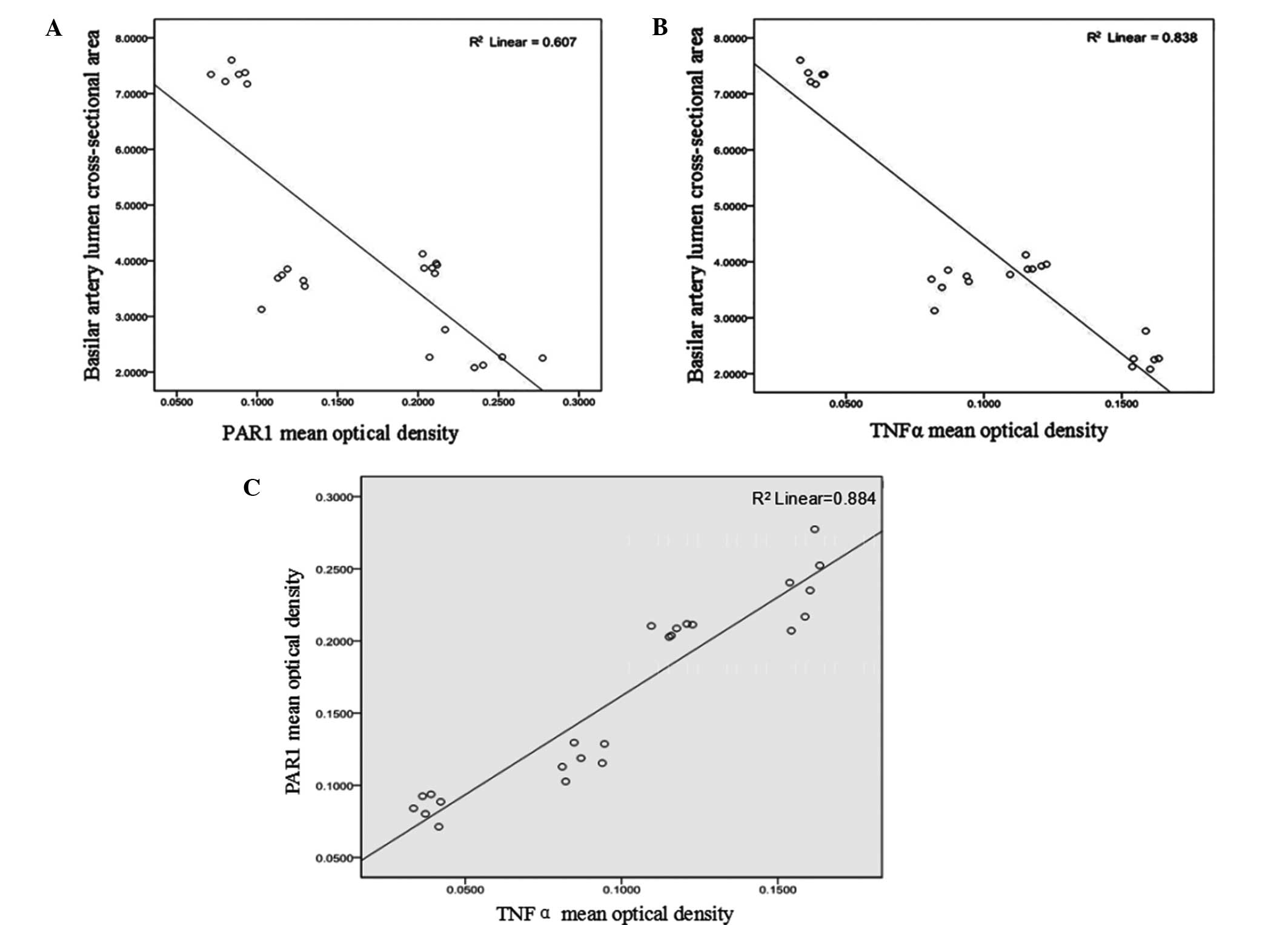
Correlation analysis of PAR1
expression, TNF-α expression and the cross-sectional area of the
basilar artery lumen
Pearson correlation analysis demonstrated that the
correlation coefficient (r) between the mean OD of PAR1 and
the cross-sectional area of the basilar artery following SAH was
−0.779 (P<0.01; Fig. 5A); thus
suggesting that there was a negative correlation between these
factors. In addition, the mean OD of TNF-α and the cross-sectional
area of the basilar artery following SAH were negatively correlated
(r=−0.915; P<0.01; Fig.
5B).
Correlation between mean OD of PAR1
and TNF-α
Pearson correlation analysis demonstrated that the
average ODs of PAR1 and TNF-α were positively correlated (r=0.940;
P<0.01; Fig. 5C).
Discussion
Numerous factors have previously been implicated in
the pathophysiology of CVS (12). An
intracranial hemorrhage may initiate the production of large
amounts of thrombin, which is detected by PAR1 in vascular smooth
muscle. In addition, the expression levels of TNF-α have previously
been shown to be upregulated during a cerebral hemorrhage (13). The present study aimed to investigate
the association between PAR1, TNF-α and CVS in a rat model of
SAH.
Hirano (3) reported
that upregulation of PAR1 in vascular smooth muscle following SAH
may be associated with the proliferation and contraction of
vascular smooth muscle cells. Kai et al (14) demonstrated that PAR1 was able to
regulate thrombin-induced convulsions involved in the pathogenesis
of CVS, and increased cerebrovascular reactivity was associated
with the upregulation of PAR1. In addition, intrathecal injection
of a PAR1 antagonist was able to attenuate the extent of CVS and
the upregulation of PAR1 expression levels in the basilar artery of
a rabbit double hemorrhage SAH model, in a dose-dependent
manner.
Inflammatory factors have an important role in the
formation of SAH-induced CVS. TNF-α is a classic inflammatory
cytokine, which is able to promote the vascular oxidative stress
response. The aggregation of various other inflammatory mediators
involved in the pathogenesis of CVS was shown to be dependent on
the expression levels of TNF-α in TNF-α transduction system
knockout mice (15). In addition,
the basilar artery spasm was attenuated by blocking TNF-α
transduction in a rabbit model of SAH (6,15–17).
Mbebi et al (18)
demonstrated that TNF-α was able to promote the mRNA and protein
expression of PAR1 in myoblast cells in vitro. Chieng-Yane
et al (19) reported that the
application of PAR1 antagonists following angioplasty was able to
protect against vascular restenosis by reducing the expression
levels and migration of TNF-α and matrix metalloproteinase 7, and
by promoting the amplification of vascular smooth muscle cells.
Furthermore, Liu and Tang (20)
detected TNF-α-induced renal vascular spasm in models of severe
pancreatitis and in rat kidney damage experiments.
The present study detected neurological impairments
in the rats following SAH modeling, and erythrocytes and
inflammatory cells were shown to be highly concentrated in the
subarachnoid space, which was associated with basilar artery spasm.
The average OD of PAR1 was calculated in order to assess the
protein expression of PAR1 in the SAH rats. Notably, protein
expression of PAR1 was significantly upregulated in the SAH rats in
a time-dependent manner, which was consistent with a previous study
(21). In addition, there was a
negative correlation (r=−0.779; P<0.01) between the
protein expression of PAR1 and the cross-sectional area of the
basilar artery lumen, thus suggesting that PAR1 may have an
important role in the pathological mechanism underlying CVS.
Positive expression of TNF-α was detected in the
basilar artery of the SAH rats, and the expression was
significantly increased over time (P<0.01). Furthermore, a
correlation analysis demonstrated that the protein expression of
TNF-α was negatively correlated (r=−0.915; P<0.01) with
the cross-sectional area of the basilar artery lumen, thus
suggesting that TNF-α may be associated with SAH-induced CVS. TNF-α
was previously shown to promote dose-dependent vasoconstriction in
the rat basilar artery (6), and
TNF-α in the cerebrospinal fluid is used as a sensitive marker of
the severity of CVS (8), thus
suggesting that it may have an important role in the development
and poor prognosis of SAH-induced CVS (7).
The results of the present study suggested that
there was a strong positive correlation (r=0.940; P<0.01)
between the positive expression levels of TNF-α and PAR1, while
there was a negative correlation between the average OD and the
upregulation of TNF-α and PAR1 expression levels. Furthermore, the
cross-sectional area of the rat basilar artery, which was an
indication of the degree of vascular spasm, was negatively
correlated with the upregulation of these factors, thus suggesting
that the expression of PAR1 and TNF-α following SAH may influence
the severity of CVS. Notably, thrombin may also be associated with
the incidence and development of CVS, since it is able to initiate
the upregulation of PAR1 and induce inflammatory responses. Future
studies should investigate whether inhibition of PAR1 and TNF-α may
attenuate SAH-induced CVS.
Acknowledgements
The present study was supported by the Scientific
Research Subject from the Health Department of Hainan Province
(grant no. 2010-64).
References
|
1
|
Komotar RJ, Schmidt JM, Starke RM,
Claassen J, Wartenberg KE, Lee K, Badjatia N, Connolly ES Jr and
Mayer SA: Resuscitation and critical care of poor-grade
subarachnoid hemorrhage. Neurosurgery. 64:397–410. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kameda K, Kikkawa Y, Hirano M, Matsuo S,
Sasaki T and Hirano K: Combined argatroban and anti-oxidative
agents prevents increased vascular contractility to thrombin and
other ligands after subarachnoid haemorrhage. Br J Pharmacol.
165:106–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirano K: The roles of
proteinase-activated receptors in the vascular physiology and
pathophysiology. Arterioscler Thromb Vasc Biol. 27:27–36. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qi J, Zhang L, Jia W, Zhang J and Wu Z:
Diffuse cerebral vasospasm after resection of schwannoma: A case
report. Neuropsychiatr Dis Treat. 11:317–320. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eisenhut M: The evidence for a role of
vasospasm in the pathogenesis of cerebral malaria. Malar J.
14:4052015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vecchione C, Frati A, Di Pardo A, Cifelli
G, Carnevale D, Gentile MT, Carangi R, Landolfi A, Carullo P,
Bettarini U, et al: Tumor necrosis factor-alpha mediates
hemolysis-induced vasoconstriction and the cerebral vasospasm
evoked by subarachnoid hemorrhage. Hypertension. 54:150–156. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jayaraman T, Paget A, Shin YS, Li X, Mayer
J, Chaudhry H, Niimi Y, Silane M and Berenstein A:
TNF-alpha-mediated inflammation in cerebral aneurysms: A potential
link to growth and rupture. Vasc Health Risk Manag. 4:805–817.
2008.PubMed/NCBI
|
|
8
|
Hanafy KA, Stuart RM, Khandji AG, Connolly
ES, Badjatia N, Mayer SA and Schindler C: Relationship between
brain interstitial fluid tumor necrosis factor-α and cerebral
vasospasm after aneurysmal subarachnoid hemorrhage. J Clin
Neurosci. 17:853–856. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Raslan F, Albert-Weißenberger C,
Westermaier T, Saker S, Kleinschnitz C and Lee JY: A modified
double injection model of cisterna magna for the study of delayed
cerebral vasospasm following subarachnoid hemorrhage in rats. Exp
Transl Stroke Med. 4:232012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raslan F, Schwarz T, Meuth SG, Austinat M,
Bader M, Renné T, Roosen K, Stoll G, Sirén AL and Kleinschnitz C:
Inhibition of bradykinin receptor B1 protects mice from focal brain
injury by reducing blood-brain barrier leakage and inflammation. J
Cereb Blood Flow Metab. 30:1477–1486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Endo S, Branson PJ and Alksne JF:
Experimental model of symptomatic vasospasm in rabbits. Stroke.
19:1420–1425. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang QS and Li G: Recent advance in
pathogenesis of cerebral vasospasm after subarachnoid hemorrhage.
Chinese Journal of Neuromedicine. 4:422–425. 2013.
|
|
13
|
Ma Y, Liu AM, Cai WQ and Hu Z: Granulocyte
colony stimulating factor for hemorrhage rats after the influence
of the expression of tumor necrosis factor alpha and nerve
protective effect. Chinese Journal of Experimental Surgery.
28:3132011.
|
|
14
|
Kai Y, Maeda Y, Sasaki T, Kanaide H and
Hirano K: Basic and translational research on proteinase-activated
receptors: The role of thrombin receptor in cerebral vasospasm in
subarachnoid hemorrhage. J Pharmacol Sci. 108:426–432. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aoki T, Kataoka H, Shimamura M, Nakagami
H, Wakayama K, Moriwaki T, Ishibashi R, Nozaki K, Morishita R and
Hashimoto N: NF-kappaB is a key mediator of cerebral aneurysm
formation. Circulation. 116:2830–2840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cahill J, Calvert JW and Zhang JH:
Mechanisms of early brain injury after subarachnoid hemorrhage. J
Cereb Blood Flow Metab. 26:1341–1353. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou ML, Shi JX, Hang CH, Cheng HL, Qi XP,
Mao L, Chen KF and Yin HX: Potential contribution of nuclear
factor-kappaB to cerebral vasospasm after experimental subarachnoid
hemorrhage in rabbits. J Cereb Blood Flow Metab. 27:1583–1592.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mbebi C, Rohn T, Doyennette MA, Chevessier
F, Jandrot-Perrus M, Hantaï D and Verdière-Sahuqué M: Thrombin
receptor induction by injury-related factors in human skeletal
muscle cells. Exp Cell Res. 263:77–87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chieng-Yane P, Bocquet A, Létienne R,
Bourbon T, Sablayrolles S, Perez M, Hatem SN, Lompré AM, Le Grand B
and David-Dufilho M: Protease-activated receptor-1 antagonist F
16618 reduces arterial restenosis by down-regulation of tumor
necrosis factor α and matrix metalloproteinase 7 expression,
migration and proliferation of vascular smooth muscle cells. J
Pharmacol Exp Ther. 336:643–651. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu C and Tang JW: Expression of tumor
necrosis factor-α in rats with severe acute pancreatitis
complicated with acute renal injury. Chinese Journal of
Experimental Surgery. 30:26–28. 2013.
|
|
21
|
Hirano K and Hirano M: Current perspective
on the role of the thrombin receptor in cerebral vasospasm after
subarachnoid hemorrhage. J Pharmacol Sci. 114:127–133. 2010.
View Article : Google Scholar : PubMed/NCBI
|