Introduction
Total glucosides of peony (TGP) are the primary
active ingredients of Paeoniaceae paeonia and have been
widely used in the treatment of Sjögren's syndrome (SS) (1,2).
Previous studies have demonstrated that TGP is able to attenuate
the symptoms exhibited by patients with SS, including a dry mouth
and eyes, and the SS-associated decrease in erythrocyte
sedimentation rate (ESR) (3–5). However, the molecular mechanisms
underlying the activity of TGP in SS therapy remain unclear. The
levels of Th1-secreted interferon-γ (IFN-γ) and Th2-secreted
interleukin-4 (IL-4) are positively correlated with the extent of
lymphocytic infiltration in the labial glands of patients with SS
(6), and the ratio of IFN-γ to IL-4
indicates the Th1/Th2 cytokine balance (7).
The Fas and Fas ligand (FasL) system is an important
signaling pathway for inducing apoptosis in cells (8), and Fas/FasL-mediated apoptosis is
reported to be associated with cancer cell death (9). Fas antigen and its ligand FasL have an
important role in peripheral T cell clearance and the cytotoxicity
of cytotoxic T lymphocytes (CTLs) (10). It is established that activated T
cells and CTLs are critical for modulation of the immune response,
meaning that the function of Fas and FasL are also crucial to
immune reactions (11).
Furthermore, the levels of Fas and FasL in the
submandibular glands have been observed to be significantly
increased in SS mice compared with control mice (12). In the labial glands of patients with
SS, the levels of Fas and FasL are associated with IFN-γ and IL-4
levels and are positively correlated with lymphocytic infiltration
(13). Previous results thus
indicate that an increase in the levels of IFN-γ, IL-4, Fas and
FasL may be crucially involved in the pathogenesis of SS.
To clarify the molecular mechanism underlying the
efficacy of TGP for the treatment of SS, the present study
evaluated the effects of TGP on the levels of IFN-γ, IL-4, Fas and
FasL in serum and in the submandibular glands in an SS mouse
model.
Materials and methods
Animals
A total of 40 female, 8-week-old, non-obese (weight,
25–35 g) diabetic (NOD) mice with SS were provided by Shanghai SLAC
Laboratory Animal Co., Ltd (Shanghai, China). The diets of all mice
comprised 14% casein, 47% cornstarch, 15% gelatinized cornstarch,
10% sugar, 4% soybean oil, and 10% other (including fiber, vitamins
and minerals). All animals were maintained at 24°C in 60% humidity
in a pathogen-free environment. All the protocols in present study
were approved by ethical committee of The First Affiliated Hospital
of Zhejiang University School of Medicine (Hangzhou, China).
A total of 40 NOD mice with SS were randomly
assigned into 4 groups of 10 mice, as follows: i) Control group,
which was fed normally; ii) TGP group, receiving 1 mg TGP per day;
iii) HCQ group, receiving 0.25 mg hydroxychloroquine (HCQ) per day;
and the iv) combined group, receiving 1 mg TGP plus 0.25 mg HCQ per
day. After 8 weeks, blood samples were obtained from the femoral
artery, the mice were euthanized by intraperitoneal injection of
urethane (2.0 g/kg; Sigma-Aldrich, St. Louis, MO, USA) and their
submandibular glands were isolated for evaluation. All animal
experiments complied with the experimental animal ethics guidelines
of China Zoological Society.
Drugs
TGP tablets (Ningbo Lihua Pharmaceuticals Co. Ltd.,
Ningbo, China) were diluted in distilled water at 15 mg/ml. HCQ
tablets (Shanghai Zhongxi Pharmaceutical Co., Ltd., Shanghai,
China) were diluted in distilled water at 3 mg/ml.
Reagents
ELISA kits (Shanghai Langdun Biotechnology,
Shanghai, China) were used to detect mouse IFN-γ and IL-4 (cat. no.
BP-E60055). The following primary antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA): goat polyclonal
anti-IFN-γ (dilution, 1:1,000; cat. no. sc-9344), goat polyclonal
anti-IL-4 (dilution, 1:1,000; cat. no. sc-1260), rabbit polyclonal
anti-Fas (dilution, 1:2,000; cat. no. sc-716) and rabbit polyclonal
anti-FasL (dilution, 1:2,000; cat. no. sc-956). Biotinylated goat
anti-rabbit (cat. no. SP-9001) and biotinylated mouse anti-goat
(cat. no. PV-9003) secondary antibodies (dilution, 1:2,000; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China)
were used to visualize these. A DAB substrate kit (OriGene
Technologies, Inc., Beijing, China) was used to detect
chemiluminescence. In situ hybridization detection kits were
used in the detection of Fas and FasL (Wuhan Boshide Biological
Engineering Co., Ltd., Wuhan, China).
ELISA analysis of IFN-γ and IL-4
protein levels in serum
Blood samples were placed in serum separator tubes,
maintained at room temperature for 30 min then centrifuged for 15
min at 1,400 × g. Serum was transferred into a 1.5-ml centrifuge
tube and stored at 4°C. The levels of IFN-γ and IL-4 in the serum
samples were determined using EM1001 and EMIL4 ELISA kits,
respectively (Thermo Fisher Scientific, Inc., Waltham, MA, USA), in
accordance with the manufacturers instructions.
Immunohistochemical
streptavidin-peroxidase (S-P) assay to evaluate IFN-γ, IL-4, Fas
and FasL expression in submandibular glands
IFN-γ, IL-4, Fas and FasL protein levels in the
mouse submandibular glands were determined using an
immunohistochemical S-P kit (cat. no. 21124; Pierce Biotechnology,
Inc., Rockford, IL, USA), in accordance with the manufacturers
instructions. Image analysis was performed using a true color
multifunctional cell image analysis management system in Image-Pro
Plus 7.0 (Media Cybernetics, Inc., Rockville, MD, USA). The optical
densities of five randomly-selected fields were measured and their
average values were used for statistical analysis.
Quantitative polymerase chain reaction
(qPCR) analysis of Fas and FasL mRNA levels in submandibular
glands
Briefly, total RNA was extracted using TRIzol
(Beijing TransGen Biotech Co., Ltd., Beijing, China) and DNase
(final concentration, 10 mM; Takara Biotechnology Co., Ltd.), and
cDNA was synthesized from 1.5 µg of total RNA using a Takara RNA
PCR kit (AMV) v. 3.0 (Takara Biotechnology Co., Ltd.). This reverse
transcription was performed in one cycle, as follows: 30°C for 10
min; 42°C for 30 min; 95°C for 5 min; and 4°C for 5 min. qPCR was
performed using 10 µl Premix Ex Taq PCR probes (2X concentration;
cat. no. RR390A; Takara Biotechnology Co., Ltd.), 1 µl cDNA and 9
µl ddH2O in a 200-µl PCR tube, in accordance with the
manufacturers instructions, with GAPDH as an internal control.
Primers were synthesized as follows: Fas, forward
5′-AGGCCGCCCGCTGTTTTC-3 and reverse 5′-ACGAACCCGCCTCCTCAGC-3
(product length, 145 bp); FasL, forward 5′-GCCGCCACTGACCCCTCTAA-3
and reverse 5′-CCACACTCCTCGGCTCTTTT-3 (product length, 242 bp); and
GAPDH, forward 5′-AAATGGTGAAGGTCGGTGTG-3 and reverse
5′-TGAAGGGGTCGTTGATGG-3 (product length, 108 bp). PCR was conducted
as follows: Incubation at 94°C for 5 min, followed by 40 cycles at
94°C for 15 sec and at 60°C for 45 sec. The fluorescence value was
determined at 60°C. These reactions were all conducted using an
AriaMx Realtime PCR System (cat. no. G8830A; Agilent Technologies,
Inc., Santa Clara, CA, USA).
An identical quantity of control RNA, templates and
Premix Ex Taq PCR probes was used across all reactions. The average
mean threshold cycle (Cq value) was obtained and data
were analyzed using the relative quantitative analysis method
(14). The 2−ΔΔCq method
was used to calculate relative mRNA level, and ΔCq
values were used for statistical analysis. The amplification and
melting curves were analyzed using the AriaMx Realtime PCR System.
The control untreated group and was used as a basis of comparison
for the other groups.
In the present study, ΔCq = Cq
target gene - Cq internal reference gene, and
ΔΔCq = [(Cq target gene - Cq internal
reference gene) in the experimental group] - [(Cq target
gene - Cq internal reference gene) in the control
group]. A smaller ΔCq indicates a higher expression
level of Fas and FasL mRNA in the submandibular gland tissue. A
higher 2−ΔΔCq indicates a greater difference in Fas and
FasL mRNA expression between the experimental and control
groups.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 15.0 (SPSS, Inc., Chicago, IL, USA). Data are
expressed as the mean ± standard deviation. One-way analysis of
variance was used to compare the groups, and the
Student-Newman-Keuls method was used for post-hoc analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TGP reduces IFN-γ and IL-4 protein
levels in NOD mice
As indicated in Table
I, the levels of serum IFN-γ and IL-4 in NOD mice were
significantly higher in the control group compared with the other
groups (P<0.05). The levels of IFN-γ in the TGP and combined
groups were reduced compared with the HCQ group (P<0.05). The
levels of IFN-γ and IL-4 in the submandibular glands were increased
in the control group compared with the other groups (P<0.05).
Furthermore, the levels of IL-4 were significantly reduced in the
serum and the submandibular glands in the combined group compared
with the HCQ group (P<0.05).
 | Table I.Protein levels of IFN-γ and IL-4 in
NOD mice, expressed as the mean ± standard deviation. |
Table I.
Protein levels of IFN-γ and IL-4 in
NOD mice, expressed as the mean ± standard deviation.
|
| IFN-γ, pg/ml | IL-4, pg/ml |
|---|
|
|
|
|
|---|
| Groups | Serum | SG | Serum | SG |
|---|
| Control |
393.62±10.16 |
478.23±12.35 |
66.36±3.85 |
367.67±6.13 |
| TGP |
230.50±23.65a,b |
240.24±11.67a |
48.79±5.26a |
180.21±7.96a |
| HCQ |
282.87±9.54a |
205.18±12.63a |
53.55±6.04a |
210.44±8.74a |
| Combined |
200.68±21.64a,b |
112.71±15.31a |
44.16±5.88a,b |
133.65±6.05a,b |
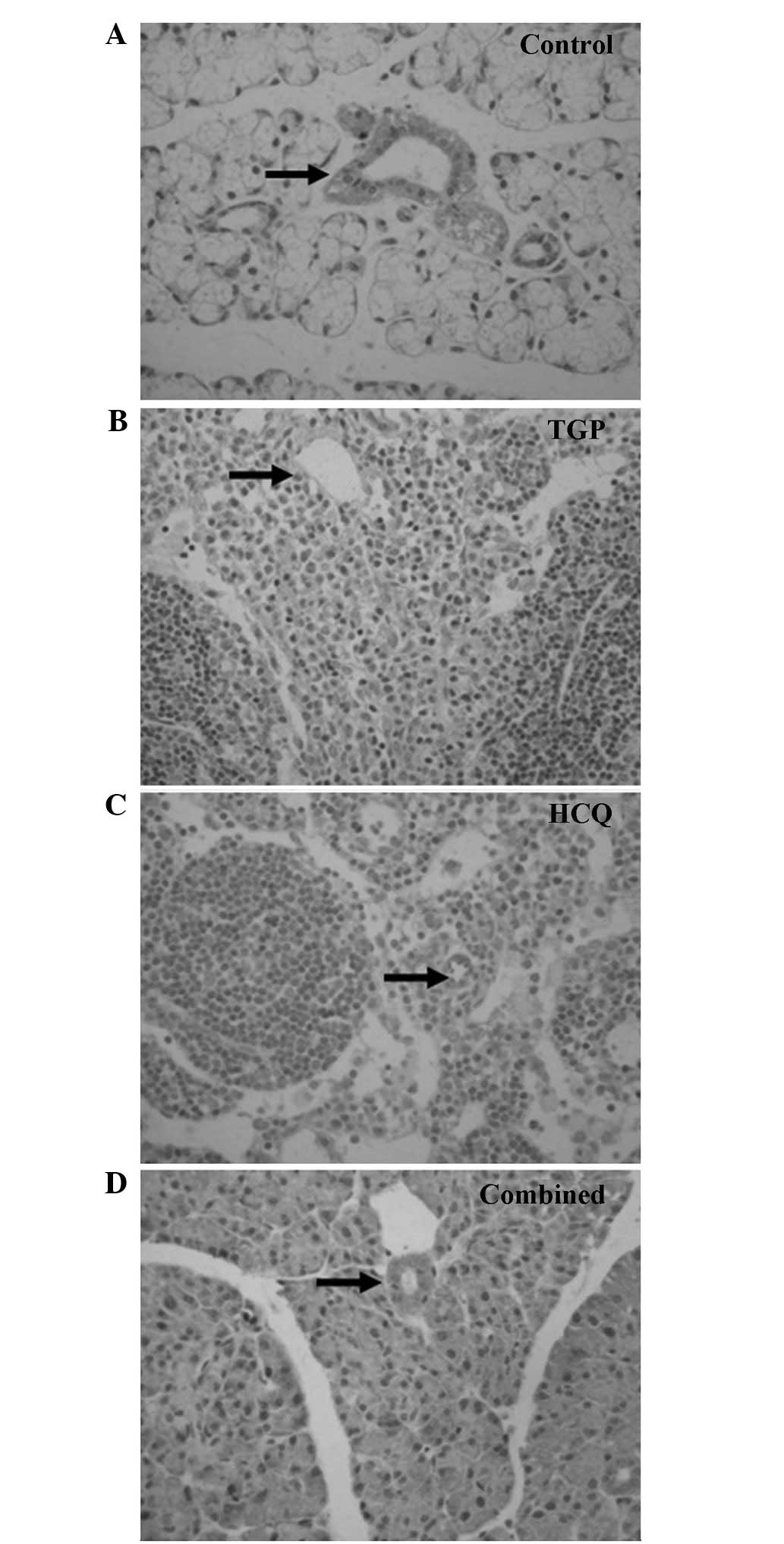
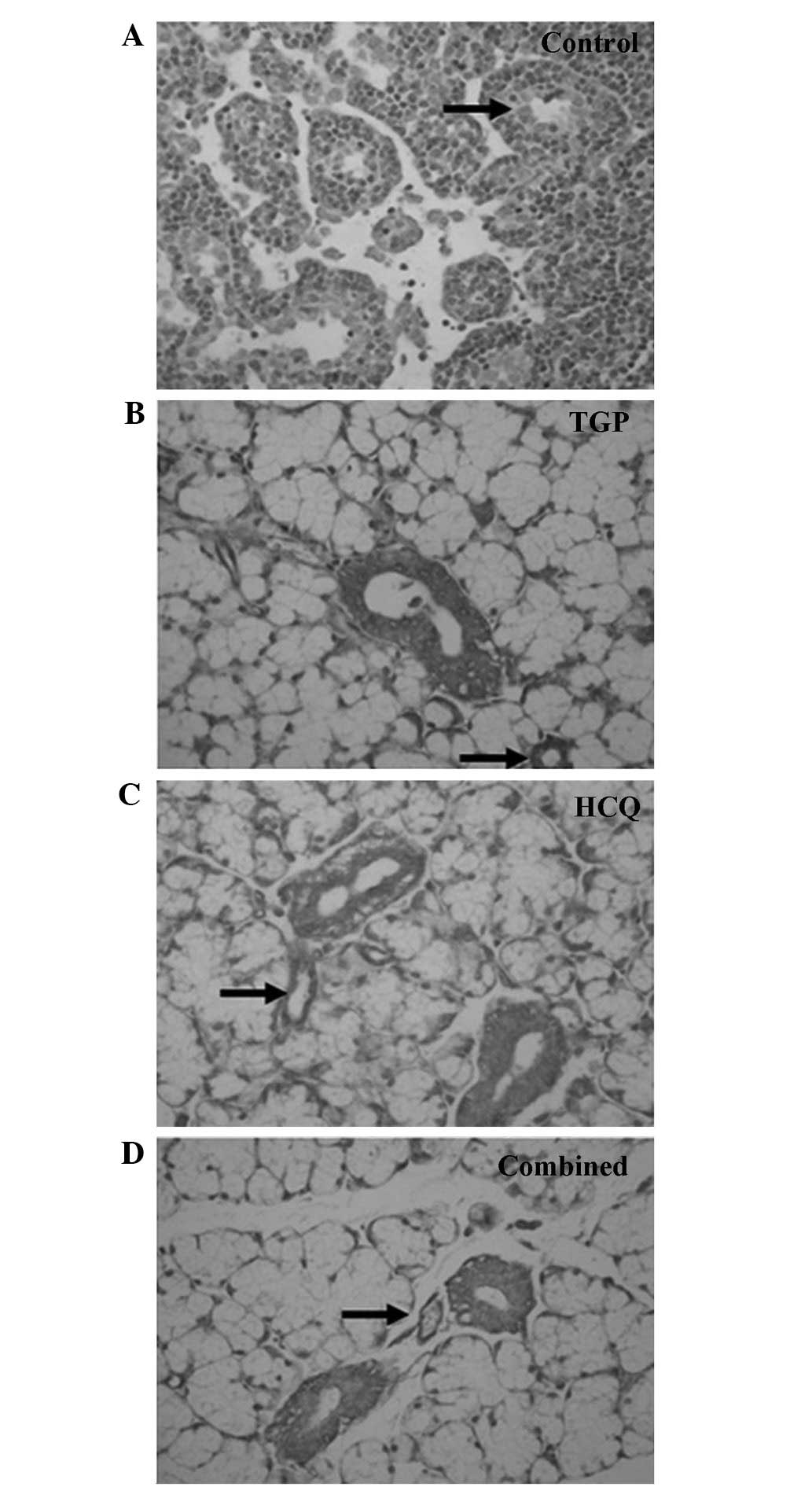
As indicated by the immunohistochemical S-P assay
(Figs. 1 and 2), particles of intense IFN-γ staining were
present in the apical and basolateral membranes of the
submandibular gland acini, the intercalated ducts and the duct
epithelia in the control NOD mice. An increased number of these
IFN-γ particles were present in the submandibular glands of the
control group compared with the other groups, and fewer particles
were present in a number of the intercalated ducts, duct epithelia
and gland cells of the HCQ group. However, the IFN-γ staining in
the HCQ-treated group was more intense compared with the combined
group. Marked expression of IL-4 particles was detected in the
basement membrane of the submandibular gland acini and ducts in the
control group. Diffuse staining was observed in the basement
membrane of submandibular gland acini and ducts in all groups, with
less intense expression observed in the combined and TGP
groups.
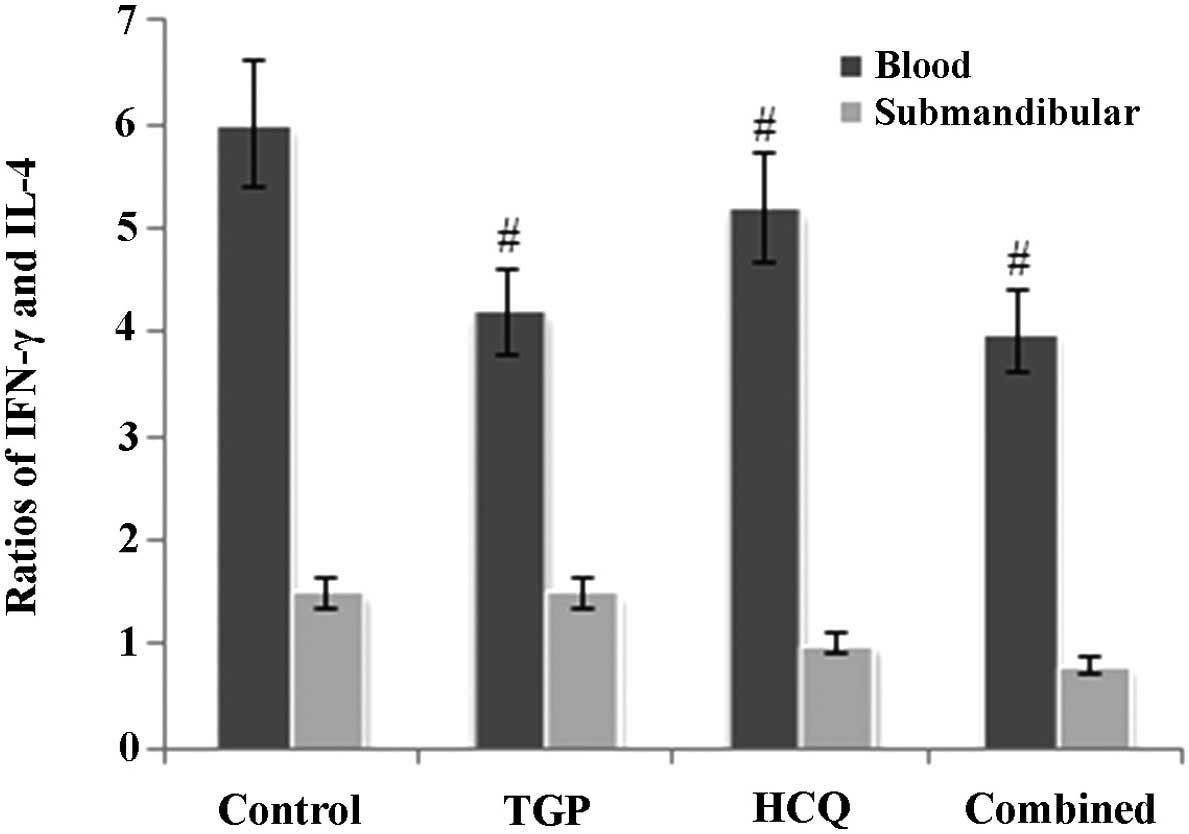
TGP reduces the ratio of IFN-γ to IL-4
in NOD mice
As reported in Fig.
3, the ratio of IFN-γ to IL-4 in mouse serum was higher in the
control group compared with the other groups (P<0.05). In
addition, the ratio of IFN-γ to IL-4 in the submandibular glands of
the control group mice was increased compared with the other
groups; however, this difference was not statistically significant
(P>0.05).
TGP reduces Fas and FasL protein
levels in mouse submandibular glands
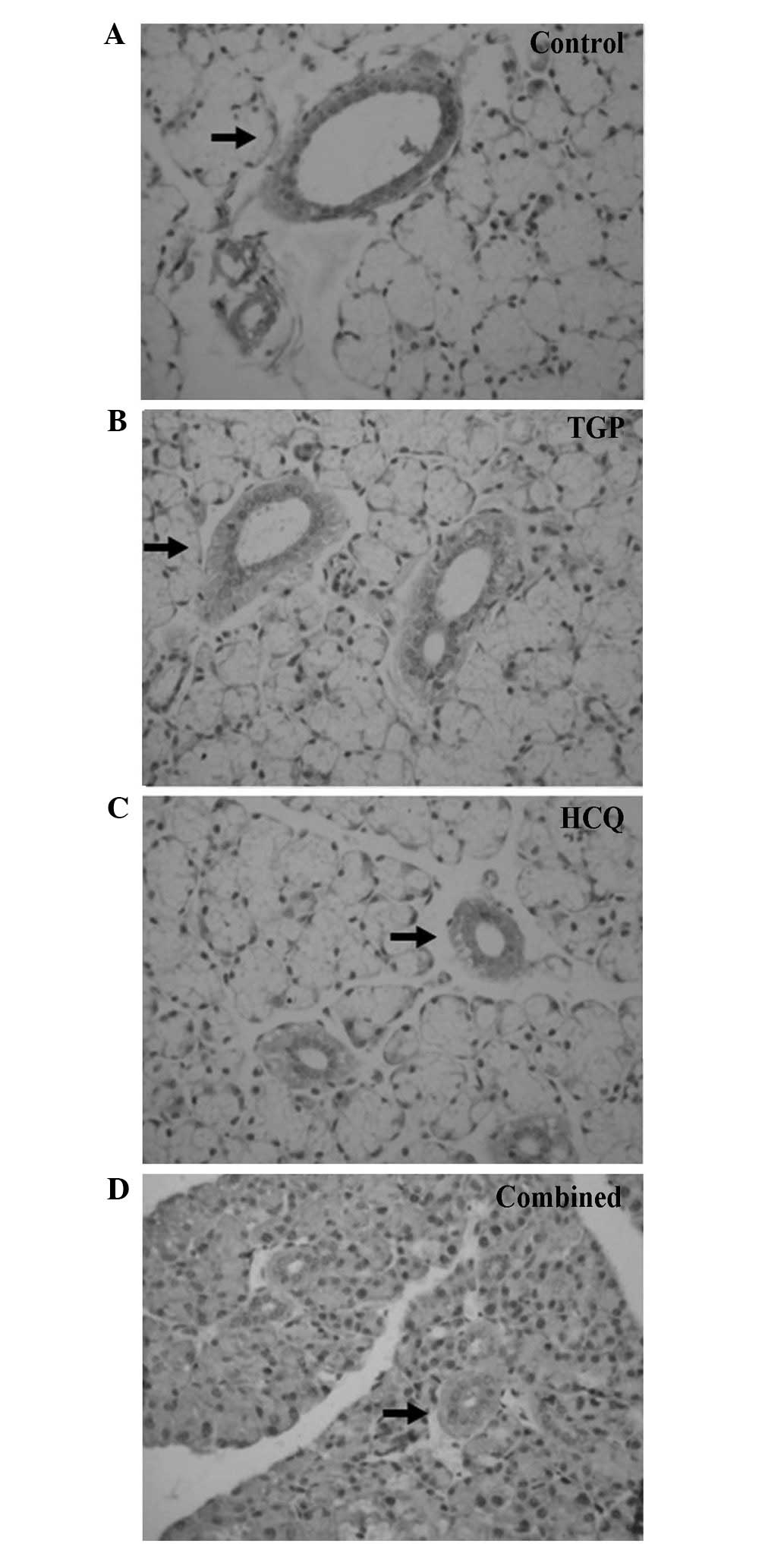
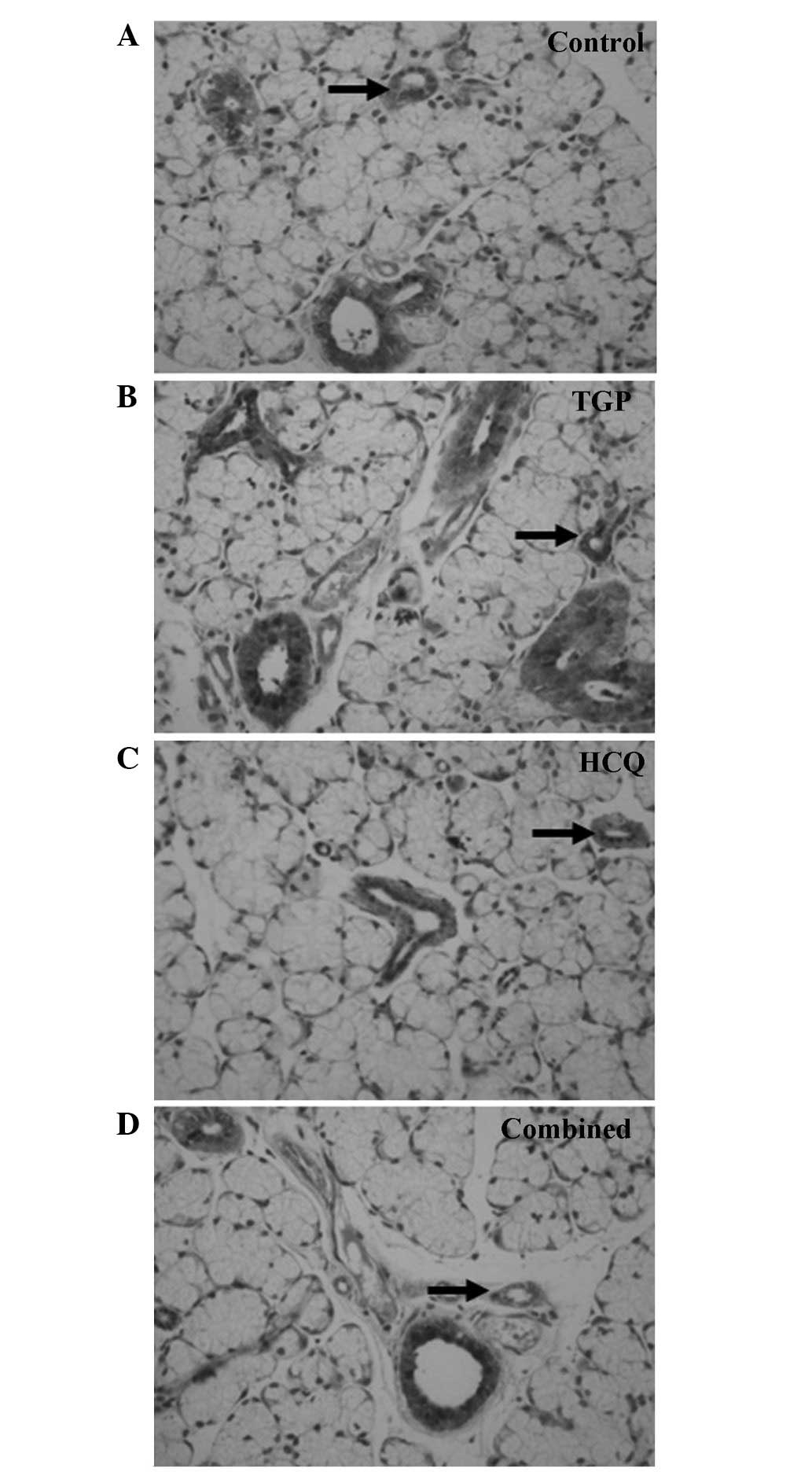
Fas and FasL expression was detected using
immunohistochemical staining (Figs.
4 and 5). High levels of Fas and
FasL were observed in the cytoplasm and membrane of submandibular
gland cells and in the duct epithelia of the control mice. Diffuse
expression of Fas and FasL in the cytoplasm and on the membrane of
submandibular gland cells and in the duct epithelia of the mice was
also observed in the TGP, HCQ and combined groups; however, FasL
expression in these groups was lower compared with the control
group.
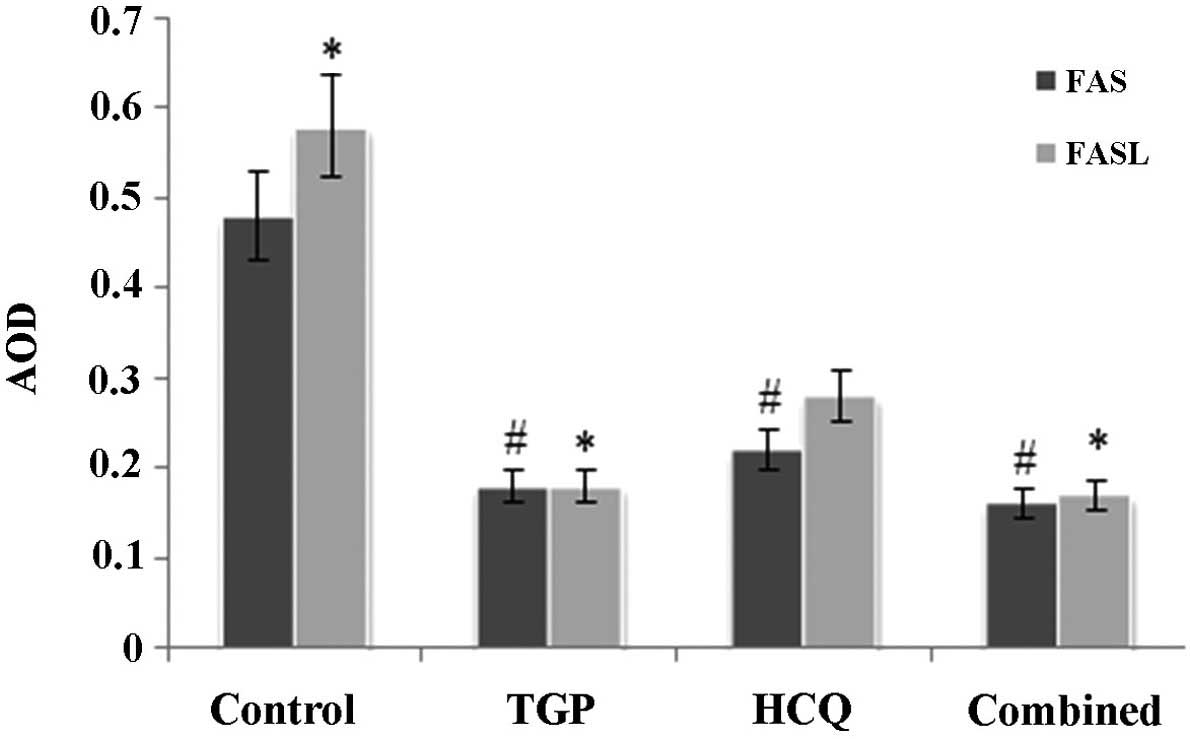
As shown in Fig. 6,
the expression of Fas and FasL proteins in the submandibular glands
was significantly increased in the control group compared with the
other groups (P<0.05). There was no statistical difference in
the expression of Fas protein amongst the 3 treatment groups,
whilst FasL protein expression was significantly lower in the TGP
group compared with the HCQ group (P<0.05). FasL protein
expression was significantly lower in the combined group compared
with the HCQ group (P<0.05).
TGP reduces the levels of Fas and FasL
mRNA in mouse submandibular glands
The amplification curves of Fas and FasL mRNA in all
samples indicated exponential growth, eventually reaching a
plateau, indicative of its high-efficiency amplification. The
melting curves for Fas mRNA and FasL PCR products presented with a
single peak, indicating a single amplification product (data not
shown).
As demonstrated in Table
II, the levels of Fas mRNA in mouse submandibular glands were
significantly increased in the control group compared with the
combined and TGP groups (P<0.05). Furthermore, Fas mRNA levels
were lower in the TGP and combined groups compared with the HCQ
group (P<0.05).
 | Table II.Relative mRNA levels of Fas and FasL
in mouse submandibular glands |
Table II.
Relative mRNA levels of Fas and FasL
in mouse submandibular glands
|
| ΔCq, mean
± SD | ΔΔCq, mean
± SD |
2−ΔΔCq |
|---|
|
|
|
|
|
|---|
| Group | Fas | FasL | Fas | FasL | Fas | FasL |
|---|
| Combined |
6.91±1.23a,b |
10.13±1.03a,b | 0.00±1.74 | 0.00±1.46 | 1.000 | 1.000 |
| HCQ |
12.53±1.87 |
13.93±2.36 |
5.62±2.24 |
3.80±2.57 | 0.020 | 0.072 |
| TGP |
9.23±1.35a,b |
11.38±1.69a |
2.32±1.83 |
1.25±1098 | 0.200 | 0.420 |
| Control |
13.26±1.68 |
15.25±3.18 |
6.35±2.08 |
5.12±3.34 | 0.012 | 0.029 |
The levels of FasL mRNA in mouse submandibular
glands were significantly higher in the control group compared with
the combined and TGP groups (P<0.05). The relative mRNA levels
of FasL mRNA were significantly lower in the combined group
compared with the HCQ group (P<0.05).
Discussion
Previous pharmacological studies have demonstrated
that TGP is able to exert bidirectional immunomodulatory,
anti-inflammatory, anti-oxidative and analgesic effects (15,16). TGP
has been repeatedly used in the treatment of SS, with effective
clinical results (3). The combined
use of TGP and HCQ, however, may have more significant therapeutic
effects in the treatment of SS. A combined therapy may reduce ESR,
improve symptoms including a dry mouth and eyes and can increase
saliva flow with reduced side effects (17). Therefore, the present study aimed to
elucidate the molecular mechanism underlying this combination
treatment. SS has complex clinical manifestations, and there is
currently no uniform classification criterion for SS (18). Previous studies have primarily relied
on animal models to investigate the pathogenesis and treatment of
SS (19). The NOD mouse frequently
develops insulin-dependent diabetes and lymphocytic infiltration in
the submandibular and lacrimal glands and other organs (20). These types of pathological damage are
similar to those observed in SS. The NOD mouse is therefore an
appropriate animal model for the study of SS (21).
SS is a chronic systemic autoimmune disease that
affects the exocrine glands, including salivary and lacrimal
glands; and the majority of patients with SS are women aged 40–50
years (22). Currently, the etiology
and pathogenesis of SS is unclear, and there is no specific
treatment for SS. There has been increased attention directed
towards the immune effects of the helper T cells (Th) and cytokines
secreted by Th cells in patients with SS and in animal models.
During the onset of SS, Th1- and Th2-type cytokines are in a state
of dynamic equilibrium. Th2-type cytokines are dominant during the
early stages of lymphocytic infiltration into the exocrine glands
in patients with SS (23). However,
Th1-type cytokines gradually become more involved during the late
stage of SS, when infiltration becomes more severe (24). A previous study revealed that the
levels of Th1-type cytokine IFN-γ and tumor necrosis factor-α
(TNF-α) in the salivary glands are significantly higher in patients
with SS compared with normal control patients (25). IFN-γ is able to reduce the growth and
development of salivary gland cells, thus serving an crucial
function in early SS onset (26).
Cytokine IL-4, secreted by Th2 cells, has a key role in the
adaptive immune response during clinical onset of SS, and the
knocking out of IL-4 genes has been demonstrated to restore gland
secretory functions in SS animal models (6). Previous experiments by the authors of
the current study have demonstrated that the levels of Th1- and
Th2-specific cytokines in serum and submandibular glands of NOD
mice were significantly increased compared with normal BALB/C mice,
indicating a Th1/Th2 immune imbalance (8).
Fas and its ligand FasL are crucially involved in
the regulation of apoptosis, immune privilege and the maintenance
of homeostasis in the body (27).
Previous studies have demonstrated that Fas expression in SS
exocrine epithelial cells and the classical Fas/FasL system, induce
apoptosis, causing human placental and gestational trophoblastic
disease (28). Saegusa et al
(29) reported CD4+ T
cell infiltration in salivary gland tissue and large ratio of Fas
to FasL ligands in an SS animal model, but that salivary duct
epithelial cells continue to secrete Fas. These results suggested
that Fas/FasL pathway-mediated apoptosis may be partially
responsible for labial gland tissue destruction and dysfunction in
SS.
Thl cells are able to induce apoptosis of target
cells by expressing FasL (30). Fas
mRNA is predominantly expressed in Th2 cells, and FasL mRNA in Thl
cells. Thl cells are able to downregulate Th2 and Th0 cells by
Fas/FasL-mediated apoptosis; however, imbalance of Thl to Th2 cells
causes changes to cytokine secretion and inhibits the normal
apoptosis of immune cells, leading to the accumulation of
nucleosomes in cells (31). These
nucleosomes subsequently stimulate the production of antibodies by
autoreactive T-lymphocytes, thereby damaging the body. The combined
effects of Th1 and Th2 cells regulate the onset of SS. Previous
studies have suggested that the Fas to FasL ratio is correlated
with the IFN-γ to IL-4 ratio in SS labial gland lymphocytes, and is
positively correlated with lymphocyte infiltration in labial glands
(32). These results suggest that
the increase in IFN-γ to IL-4 and Fas to FasL ratio is crucially
involved in the destruction of SS labial glands and pathogenesis of
SS.
The present study demonstrated that the levels of
IFN-γ and IL-4 in serum and in submandibular glands were
significantly higher in the control NOD mice compared with the
other groups. However, IFN-γ levels in serum were lower in the TGP
and combined groups compared with the HCQ group. The ratio of IFN-γ
to IL-4 in serum was higher in the control group compared with the
TGP, HCQ and combined groups. Following TGP intervention, the
expression of Fas and FasL in the submandibular glands reduced, and
the expression of Fas and FasL mRNA in the submandibular glands was
significantly lower in the TGP group compared with the control
group. These results suggest that TGP is able to reduce cytokine
levels in serum and submandibular glands in SS, and decrease the
ratio of IFN-γ to IL-4 in submandibular glands. Furthermore, TGP
appears to be able to downregulate the levels of Fas, FasL and
their mRNA expression, thereby mediating the Th1/Th2 immune balance
and reducing cell apoptosis, thus achieving its therapeutic effect
on SS. Future studies are required to aid understanding of the
mechanism by which TGP affects cell apoptosis.
References
|
1
|
Wang Y and Wang Y: Pharmacological study
and clinical application of total glucosides of peony in autoimmune
diseases. Zhe Jiang Zhong Yi Yao Da Xue Xue Bao. 2:240–241, 244.
2007.(In Chinese).
|
|
2
|
Zhang HF, Hou P and Xiao WG: Clinical
observation on effect of total glucosides of paeony in treating
patients with non-systemic involved Sjogren syndrome. Zhong Guo
Zhong Xi Yi Jie He Za Zhi. 27:596–598. 2007.(In Chinese).
|
|
3
|
Li X, Li X, Wang G, Qian L and Wang Z:
Effectiveness and safety of total glucosides of peony in the
treatment of patients with Sjogren syndrome. An Hui Yi Xue.
5:370–371. 2006.(In Chinese).
|
|
4
|
Wu GL, Pu XH, Yu GY and Li TY: Effects of
total glucosides of peony on AQP-5 and its mRNA expression in
submandibular glands of NOD mice with Sjogren's syndrome. Eur Rev
Med Pharmacol Sci. 19:173–178. 2015.PubMed/NCBI
|
|
5
|
Carsons SE: Issues related to clinical
trials of oral and biologic disease-modifying agents for Sjogren's
syndrome. Rheum Dis Clin North Am. 34:1011–1023. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nguyen CQ, Gao JH, Kim H, Saban DR,
Cornelius JG and Peck AB: IL-4-STAT6 signal transduction-dependent
induction of the clinical phase of Sjögren's syndrome-like disease
of the nonobese diabetic mouse. J Immunol. 179:382–390. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Munder M, Eichmann K and Modolell M:
Alternative metabolic states in murine macrophages reflected by the
nitric oxide synthase/arginase balance: Competitive regulation by
CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol.
160:5347–5354. 1998.PubMed/NCBI
|
|
8
|
Choi YJ, Saez B, Anders L, Hydbring P,
Stefano J, Bacon NA, Cook C, Kalaszczynska I, Signoretti S, Young
RA, Scadden DT, et al: D-cyclins repress apoptosis in hematopoietic
cells by controlling death receptor Fas and its ligand FasL. Dev
Cell. 30:255–267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elmansy H, Kotb A, Hammam O, Abdelraouf H,
Salem H, Onsi M and Elleithy T: Prognostic impact of apoptosis
marker Fas (CD95) and its ligand (FasL) on bladder cancer in Egypt:
Study of the effect of schistosomiasis. Ecancermedicalscience.
6:2782012.PubMed/NCBI
|
|
10
|
Nakajima H and Oka T: Analysis of
biochemical and biological functions of Fas-ligand (FasL) and Fas
on activated T cells in allo-immune response. Transplant Proc.
29:1096–1100. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Riddell SR, Elliott M, Lewinsohn DA,
Gilbert MJ, Wilson L, Manley SA, Lupton SD, Overell RW, Reynolds
TC, Corey L and Greenberg PD: T cell mediated rejection of gene
modified HIV specific cytotoxic T lymphocytes in HIV infected
patients. Nat Med. 2:216–223. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu GL, Li TY, Lu WW, Yu GY and Fan YS:
Effect of nourishing Yin, strengthening Qi and activating blood
decoction on Fas/FasL in salivary glands of NOD mice with Sjogren's
syndrome and their mRNA expression. Zhong Guo Zhong Yao Za Zhi.
38:4148–4151. 2013.(In Chinese).
|
|
13
|
Tzioufas AG, Kapsogeorgou EK and
Moutsopoulo HM: Pathogenesis of Sjögren's syndrome: What we know
and what we should learn. J Autoimmun. 39:4–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin L, Lloyd RV, Nassar A, Lappinga PJ,
Sebo TJ, Swartz K, Seys AR, Erickson-Johnson MR, Roth CW, Evers BR,
et al: HMGA2 expression analysis in cytological and
paraffin-embedded tissue specimens of thyroid tumors by relative
quantitative RT-PCR. Diagn Mol Pathol. 20:71–80. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim SH, Lee MK, Lee KY, Sung SH, Kim J and
Kim YC: Chemical constituents isolated from Paeonia
lactiflora roots and their neuroprotective activity against
oxidative stress in vitro. J Enzyme Inhib Med Chem. 24:1138–1140.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou X, Ding J, Zhu M, Mou C, Wang H, Wang
Y, He J, Chen R, Gao X and Yang Z: The effects on TNF-α and sICAM-1
in the adjuvant arthritis rat model by the total glucosides of
peony. Xin Jiang Yi Ke Da Xue Xue Bao. 12:1677–1679. 2009.(In
Chinese).
|
|
17
|
Pijpe J, van Imhoff GW, Spijkervet FK,
Roodenburg JL, Wolbink GJ, Mansour K, Vissink A, Kallenberg CG and
Bootsma H: Rituximab treatment in patients with primary Sjögren's
syndrome: An open-label phase II study. Arthritis Rheum.
52:2740–2750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Novljan Plešivčnik M, Rotar Z, Ambrožič A,
Vidmar G and Tomšič M: Comparison of the performance of the
different classification criteria for primary Sjögren's syndrome: A
prospective cohort study. Clin Rheumatol. 33:1657–1664. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi H, Yu CQ, Xie LS, Wang ZJ, Zhang P and
Zheng LY: Activation of TLR9-dependent p38MAPK pathway in the
pathogenesis of primary Sjögren's syndrome in NOD/Ltj mouse. J Oral
Pathol Med. 43:785–791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Asamoto H, Akazawa Y, Tashiro S, Oishi M,
Azuma T, Koide S, Sudo K, Yokota H and Tochino Y: Infiltration of
lymphocytes in various organs of the NOD (non-obese diabetic)
mouse. J Jpn Diabetes Soc. 27:775–781. 1984.
|
|
21
|
Wang D, Xue L, Yang Y, Hu J, Li G and Piao
X: Temporal gene expression analysis of Sjögren's syndrome in
C57BL/6.NOD-Aec1Aec2 mice based on microarray time-series data
using an improved empirical Bayes approach. Mol Biol Rep.
41:5953–5960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vale DL: Recognition and management of
Sjögren's syndrome: Strategies for the advanced practice nurse.
Nurs Clin North Am. 35:267–278. 2000.PubMed/NCBI
|
|
23
|
Ramos-Casals M, Garcia-Carrasco M, Cervera
R, Filella X, Trejo O, de la Red G, Gil V, Sánchez-Tapias JM, Font
J and Ingelmo M: Th1/Th2 cytokine imbalance in patients with
Sjögren syndrome secondary to hepatitis C virus infection. Semin
Arthritis Rheum. 32:56–63. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mitsias DI, Tzioufas AG, Veiopoulou C,
Zintzaras E, Tassios IK, Kogopoulou O, Moutsopoulos HM and
Thyphronitis G: The Th1/Th2 cytokine balance changes with the
progress of the immunopathological lesion of Sjogren's syndrome.
Clin Exp Immunol. 128:562–568. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang EH, Lee YJ, Hyon JY, Yun PY and Song
YW: Salivary cytokine profiles in primary Sjögren's syndrome differ
from those in non-Sjögren sicca in terms of TNF-α levels and
Th-1/Th-2 ratios. Clin Exp Rheumatol. 29:970–976. 2011.PubMed/NCBI
|
|
26
|
Mariette X: Pathophysiology of Sjogren's
syndrome. Ann Med Interne (Paris). 154:157–168. 2003.(In French).
PubMed/NCBI
|
|
27
|
Nagarkatti N: Tumor-derived Fas ligand
induces toxicity in lymphoid organs and plays an important role in
successful chemotherapy. Cancer Immunol Immunother. 49:46–55. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mor G, Gutierrez LS, Eliza M, Kahyaoglu F
and Arici A: Fas-fas ligand system-induced apoptosis in human
placenta and gestational trophoblastic disease. Am J Reprod
Immunol. 40:89–94. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Saegusa K, Ishimaru N, Yanagi K, Mishima
K, Arakaki R, Suda T, Saito I and Hayashi Y: Prevention and
induction of autoimmune exocrinopathy is dependent on pathogenic
autoantigen cleavage in murine Sjögren's syndrome. J Immunol.
169:1050–1057. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Brunner T, Carter L, Dutton RW,
Rogers P, Bradley L, Sato T, Reed JC, Green D and Swain SL: Unequal
death in T helper cell (Th)1 and Th2 effectors: Th1, but not Th2,
effectors undergo rapid Fas/FasL-mediated apoptosis. J Exp Med.
185:1837–1849. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yung R, Kaplan M, Ray D, Schneider K, Mo
RR, Johnson K and Richardson B: Autoreactive murine Th1 and Th2
cells kill syngeneic macrophages and induce autoantibodies. Lupus.
10:539–546. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou Q, Bai T and Wu H: Expression of
Th1/Th2 and Fas/FasL in the labial salivary gland of patients with
Sjögren's syndrome and its significance. Xu Zhou Yi Xue Yuan Xue
Bao. 28:28–30. 2008.(In Chinese).
|