Introduction
Hypoxia-hypercapnia (HH)-induced brain damage
(HHBD), which is commonly detected during the process of
asphyxiation and chronic obstructive pulmonary disease (COPD), has
previously been associated with nervous system disorders, mental
retardation and cerebral palsy (1).
In addition, HHBD is a type of brain injury that is associated with
high rates of neonatal and adult morbidity and mortality (2). Brain edema, which is defined as an
increase in brain water content, is a common pathological
characteristic of HHBD (3). Brain
edema is characterized by cell swelling, which alters the
concentrations of cellular metabolites and subsequently disturbs
normal cellular functions (4). In
addition, edema may lead to rapid increases in intracranial
pressure, which has previously been associated with headaches,
comas and life-threatening herniation (5). Therefore, the development of effective
therapeutic drugs that are able to limit the occurrence of
HHBD-induced brain edemia is required.
Various neuroprotective agents have been applied for
the treatment of brain edema, including oxygen free radical
scavengers, calcium channel blockers, excitatory amino acid
antagonists and neurotrophic factors. These drugs, which typically
have a single therapeutic target and defined underlying
pharmacological mechanisms, have demonstrated effectiveness in
various experimental studies; however, they have shown mixed
effectiveness and, in some cases serious side-effects, in human
clinical trials (6–8).
Previous studies have demonstrated that various
traditional Chinese medicines may exert neuroprotective effects,
including the prevention of neuronal cell death and the
proliferation of neural stem cells (9,10). In
addition, numerous herbal medicines have exhibited therapeutic
effectiveness against brain edema (11,12).
Therefore, traditional Chinese medicines may be considered a source
of potential drugs for the treatment of brain edema.
Curcumin (CU), which is a low molecular weight
spice, is isolated from the rhizome of the Curcumin longa
plant (13), and has been used in
the past for medicinal and food-coloring purposes (14). Previous studies demonstrated that CU
was able to attenuate neuroinflammation and neurological injury in
patients with Alzheimer's disease, ischemic stroke and subarachnoid
hemorrhage (15–17).
Our previous study investigated the effects of CU
against hypoxic-ischemic brain damage (HIBD) in a rat model
(18), and demonstrated that CU
treatment was able to partially attenuate HIBD-induced brain edema
and morphological tissue changes. These effects of CU were
demonstrated to occur as a result of nitric oxide synthase activity
inhibition and decreased expression levels of aquaporin (AQP)-4 in
the hippocampus of the rat. The present study established a novel
rat model of HHBD by exposing the rats to a low O2/high
CO2 environment, which simulated HH conditions. In
addition, the effects of CU against HHBD were investigated using
various techniques, including hematoxylin and eosin (HE) staining,
electron microscopy, streptravidin-biotin complex (SABC)
immunohistochemistry, western blotting and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR). In
particular, the present study focused on the association between CU
treatment and AQP4 expression levels in the rat model of HHBD, with
the intention of elucidating the underlying mechanism by which CU
may attenuate HHBD-induced brain edema.
Materials and methods
Rats and ethics statement
A total of 30 healthy male Sprague-Dawley rats (age,
6–7 weeks; weight, 200–310 g) were purchased from the Experimental
Animal Center of Wenzhou Medical College (Wenzhou, China). Rats
were housed under a 12 h light/dark cycle at a controlled
temperature of 22–25°C and a humidity of 55±5%, with ad
libitum access to water and food throughout the study. After
acclimatization to the laboratory environment for 1–2 days, the
rats were randomly divided into five groups, as follows (6
rats/group): i) Control (CK) group, in which the rats were raised
under normal laboratory conditions; ii) HH group, in which the rats
were exposed to HH conditions without drug treatment; iii) CU group
(Sigma-Aldrich, St. Louis, MO, USA), in which the rats were exposed
to HH conditions and were subsequently treated with CU; iv)
dimethyl sulfoxide (DMSO; Sigma-Aldrich) group, in which the rats
were exposed to HH conditions and were subsequently injected with
DMSO; and v) monosialoganglioside (GM1; Sigma-Aldrich) group, in
which the rats were exposed to HH conditions and were treated with
GM1. The present study was approved by the Medical Ethics Committee
of Wenzhou Medical College, and all procedures were in compliance
with the National Institute of Health Guide for the Care and Use of
Laboratory Animals (NIH Publications no. 80–23).
Selection of CU dose
In order to determine the effective dose of CU, a
range of CU doses (0, 20, 40, 60 and 80 mg/kg) were analyzed in a
trial experiment, in which DMSO was used as the solvent, according
to our previous study (18). A 40
mg/kg CU dose was selected for further experiments, as doses >40
mg/kg resulted in similar results (data not shown).
Rat model of HHBD
All rats, except those in the CK group, were
maintained in an airtight container and were subjected to low
levels of O2 (9–11%) and high levels of CO2
(5–6%) for 8 h every day. The chronic intermittent hypoxia
treatment lasted for 2 weeks. Conversely, the rats in the CK group
were maintained in an open laboratory environment. The rats in all
groups were fed using an identical protocol, according to a
previous report (19). Prior to the
rats entering the container, equal volumes of CU (10 mg/ml; 40
mg/kg for rats in the CU group), DMSO (for rats in the DMSO group),
GM1 (10 mg/ml; 3 mg/kg for rats in the GM1 group) and water (for
rats in the HH and CU groups) were injected into the abdomen of the
rats.
Tissue collection and preparation
The rats were decapitated following 2 weeks of
chronic intermittent hypoxia treatment, and were maintained on ice.
The occiput was immediately cut open from the foramen magnum
forward along the midline. After the dura and pia were stripped,
the brain cortex, cerebellum and brain stem were removed completely
and rinsed with pre-cooled saline water. The brain tissues (~1/3)
were frozen and stored in liquid nitrogen for RT-qPCR analysis
(20,21), or they were used for water content
analysis (~1/3). The remaining brain tissue was prepared and
visualized using optical and electron microscopy, and SABC
immunohistochemical analysis.
Optical microscopy analysis
Tissue samples corresponding to the brain cortex,
cerebellum and brainstem were cut on a wax board and were
subsequently fixed at 4°C with 4% paraformaldehyde. Following
fixation, the tissues were washed and dehydrated with gradient
alcohol, after which the tissues were cleared with xylene, immersed
in paraffin and embedded. Subsequently, 4 µm tissue sections were
prepared for HE staining (Sigma-Aldrich) and immunohistochemistry.
Tissues were observed under an optical microscope (Eclipse E200;
Nikon Corporation, Tokyo, Japan) (22).
Electron microscopy analysis
Brain tissue samples were collected and fixed
initially with 2.5% glutaraldehyde and then with 1% osmium
tetroxide. After fixing, the tissues were dehydrated with gradient
mixtures of ethanol and acetone and embedded in Epon 812 (Electron
Microscopy Sciences, Hatfield, PA, USA). Subsequently, the tissues
were cut into 70 nm sections, and double-stained with uranyl
acetate and lead nitrate. Tissue samples were observed under a
transmission electron microscope (JEM-3010; JEOL, Ltd., Tokyo,
Japan) (18).
Water content measurement
The water levels in the brain tissues from the rats
in the various treatment groups were measured according to a
previous study (23). Briefly, brain
tissue (~0.15 g) from each rat was sliced, weighed and completely
dried in an oven at 110°C for 24 h. The dried tissue was weighed
again, and the water percentage of the brain tissue was calculated
using the following formula: Water (%) = [(wet weight-dried
weight)/wet weight] × 100%.
SABC immunohistochemical analysis of
AQP4 expression levels
AQP4 expression levels in the cortex, cerebellum,
and brainstem tissue samples were analyzed using the SABC
immunohistochemical method. The AQP4 antibody and the SABC kit were
purchased from OriGene Technologies, Inc. (Beijing, China). The
assay, image detection and analysis procedures were performed
according to our previous report (18).
Western blot analysis of AQP4 protein
expression levels
AQP4 protein expression levels were also analyzed by
western blotting. Briefly, total protein was extracted according to
a previous study (24), and the
total protein concentration was measured using the bicinchoninic
acid assay (Pierce Biotechnology, Inc., Rockford, IL, USA).
Subsequently, 50 µg protein samples were separated by 12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and transferred
to nitrocellulose membranes (Schleicher & Schuell BioScience,
Inc., Keene, NH, USA). The membranes were washed with Tris-buffered
saline containing 0.1% Tween 20 (TBST; Sigma-Aldrich) and blocked
with non-fat dry milk, after which they were incubated with rabbit
polyclonal anti-AQP4 (1:500; TA326513, OriGene Technologies, Inc.,
Beijing, China) and mouse monoclonal anti-glyceraldehyde
3-phosphate dehydrogenase (GAPDH; loading control; 1:5,000;
sc-365062, Kangchen Biotechnology Inc., Shanghai, China)
antibodies, at 4°C overnight. Following repeated washes with TBST,
the membranes were incubated with goat anti-mouse horseradish
peroxidase-conjugated secondary antibodies (1:5,000; sc-2005;
Jackson Immunoresearch Laboratories, Inc., West Grove, PA, USA) for
2 h at room temperature. Subsequently, target bands were detected
using Hyperfilm Enhanced Chemiluminescence (RPN3103 K) and X-ray
exposure (GE Healthcare Life Sciences, Chalfont, UK). Optical
density (OD) values were quantitatively analyzed using a laser
densitometer (UltroScan XL; GE Healthcare Life Sciences), and the
results of AQP4 expression were reported as percentage changes over
GAPDH (25).
Total RNA extraction and RT-qPCR
In order to validate the AQP4 protein expression
levels detected in the brain tissues of the CK, CU, HH, DMSO and
GM1 groups at the transcriptional level in vivo, RT-qPCR was
performed using the StepOneTM RT-PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). Total RNA was
extracted from tissues, which were homogenized using an electrical
homogenizer and an RNeasy Plant Mini kit (Qiagen GmbH, Hilden,
Germany), according to the manufacturer's protocol. Subsequently,
extracted RNA was treated with the RNase-Free DNase kit (Qiagen
GmbH), after which cDNA (1 µg) was obtained by reverse
transcription using the PrimeScript RT-PCR kit (Takara Bio, Inc.,
Otsu, Japan). The primer pairs were designed for amplification of
the GAPDH and AQP4 genes with the use of Premier 5.0 software
(Premier Biosoft International, Palo Alto, CA, USA), and were
synthesized by Shanghai Sunred Biological Technology Co., Ltd.
(Shanghai, China). The sequences of the primer pairs were as
follows: AQP4 (110 bp) forward, 5′-ACCCTGGACAGCTGTAAGTGTGGA-3′ and
reverse, 5′-AGGAACTCTGCTGTGACCGCCT-3′; and GAPDH (93 bp) forward,
5′-GGGAAATCGTGCGTGACATT-3′ and reverse, 5′-GCGGCAGTGGCCATCTC-3′.
The cycling conditions were as follows: 10 min at 95°C, followed by
40 cycles of 95°C for 20 sec, 56°C for 30 sec and 72°C for 30 sec,
and a final extension step at 72°C for 10 min. Three independent
replicates were performed for each sample. The comparative
quantification cycle (Cq) method was used to determine relative
levels of gene expression. The GADPH gene was used as an internal
control. mRNA transcriptional abundance value of the AQP4 gene was
expressed as 2-ΔΔCq (26).
Statistical analysis
SPSS 17.0 statistical software (SPSS Inc., Chicago,
IL, USA) was applied for the processing and analyzing of all data.
Data are presented as the mean ± standard error of the mean.
Normality testing was performed and Student-Newman-Keuls-q tests
were conducted for the examination of the overall mean of the
samples. Variance homogeneity tests were used in order to compare
the mean of multiple-sample groups. In order analyze the variance
between two groups, one-way analysis of variance was conducted.
P<0.05 was considered to indicate a statistically significant
difference.
Results
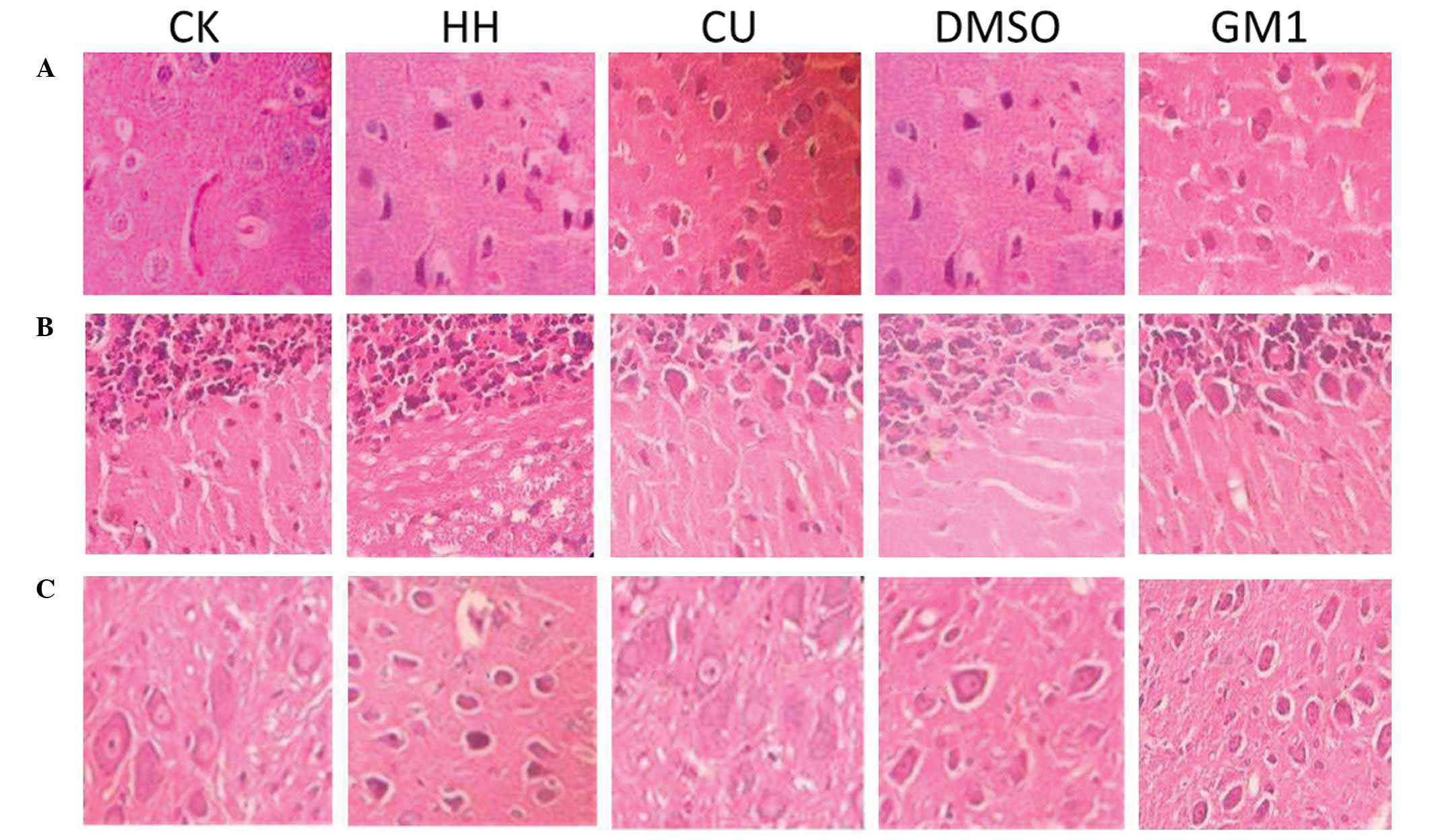
Morphological observations using light
microscopy
Morphological characteristics of the rat brain
tissue samples from the CK, HH, CU, DMSO and GM1 groups were
investigated under a light microscope (Fig. 1). In the CK group, HE staining
demonstrated that the area surrounding the brain capillaries was
slightly widened and the nerve cells were arranged neatly. In
addition, normal cell borders, nuclei, nucleoli and cell extensions
were observed. Conversely, in the HH group tissue samples, signs of
edema were detected, including a condensed cytoplasm, shrunken
membrane, dwindled cell body and pyknotic or fragmented nuclei.
Furthermore, HE staining demonstrated that the area surrounding the
nerve cells was widened, and the nerve cells were shrunken. Cells
in the brain tissue of the DMSO group rats were similar to those of
the HH group. Conversely, CU treatment markedly attenuated edema in
the brain. In the CU group tissues, the brain cells were arranged
neatly and the area surrounding the capillaries was only slightly
expanded. In addition, vascular endothelial cells and astrocytes
surrounding the capillaries did not exhibit obvious swelling. The
morphological characteristics observed in the brain of the GM1
group rats were similar to those detected for the CU group.
Comparison of the ultrastructures of
the brain tissue samples from the CK, HH and CU groups
The ultrastructures of the rat brain tissue samples
derived from the CK, HH and CU groups were investigated using
electron microscopy. In the CK group, the nuclei of neuronal cells
appeared large and round, and the nucleolus exhibited a clear
boundary with double-layer envelopes. In addition, the nuclei of
the astrocytes appeared normal and their extensions did not exhibit
swelling. No edema was detected in the area surrounding the
capillaries and intraluminal red cells, and the endothelial cells
were closely interconnected (Fig.
2A). Conversely, in the HH group, the neurons were markedly
swollen, and contained enlarged rough endoplasmic reticuli and
swollen mitochondria. Furthermore, some neurons and astrocytes
exhibited signs of apoptosis, including nuclear envelope shrinkage
and nuclear pyknosis, the capillaries were shrunken and deformed,
and the endothelial cells protruded towards the cavity and the
interconnections of the endothelium were enlarged. The extension of
the astrocytes in the blood-brain barrier (BBB) swelled and
vesicles were formed (Fig. 2B). CU
treatment markedly attenuated HHBD-induced ultrastructure
destruction in the brain tissue, as demonstrated by marked
improvements in ultrastructural integrity. In addition, the number
of cells exhibiting nuclear envelope shrinkage and nuclear pyknosis
were markedly decreased, and edema of the neurons, astrocytes and
the area surrounding the capillaries was improved (Fig. 2C).
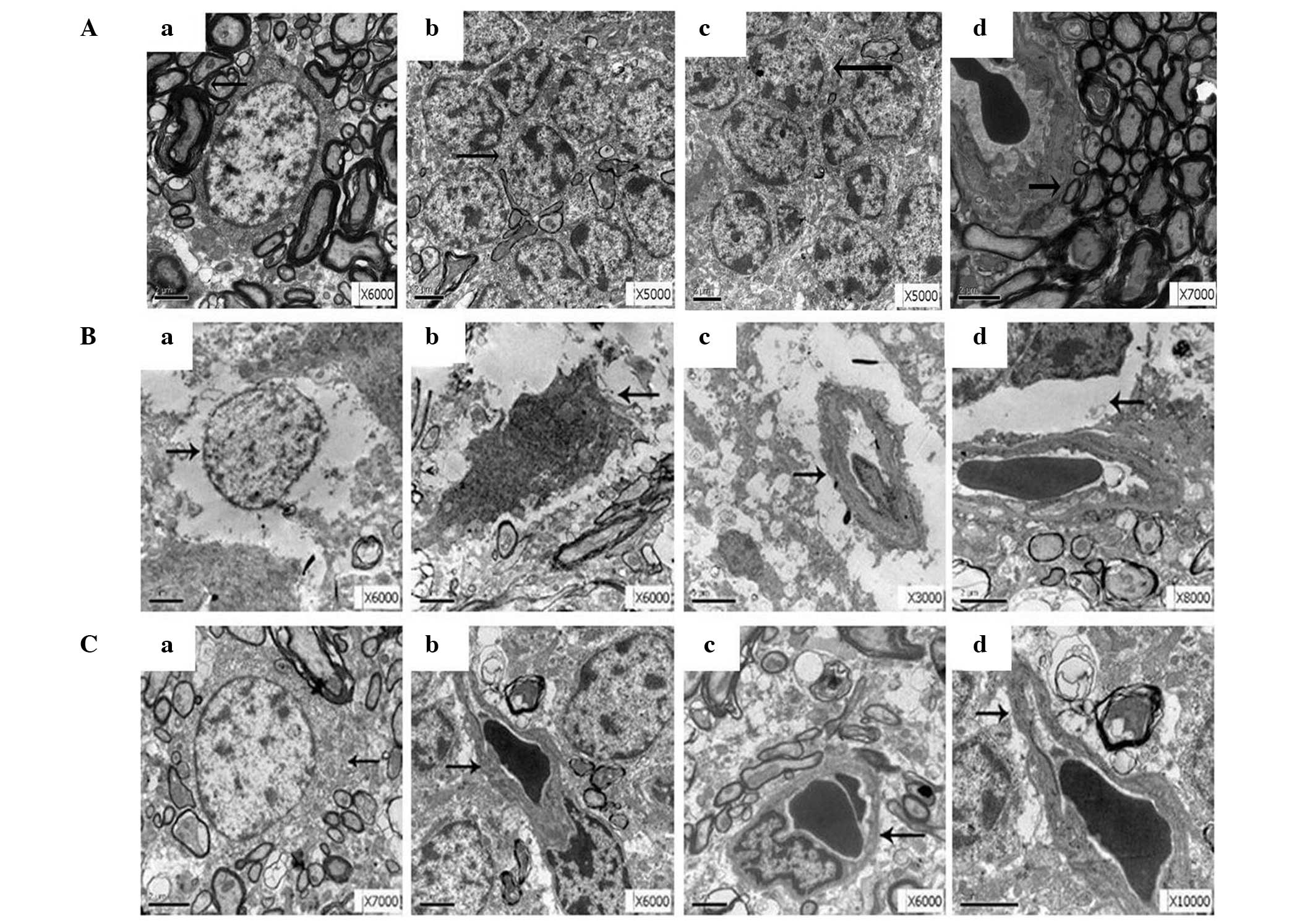 | Figure 2.Electron microscopy analysis of the
ultrastructure alterations in rat brain tissues derived from the
various treatment groups. At least five fields with positive
results were identified in each case. (A) Brain tissue
ultrastructure of the control group, showing (a) normal neurons, (b
and c) neuroglial cells, and (d) capillary structure. (B) Brain
tissue ultrastructure of the hypoxic-hypercapnia group, showing (a)
neural edema, (b) neuronal cell apoptosis, (c) endothelial cells
protruding towards the cavity, and (d) regions of swelling
surrounding the capillaries. (C) Brain tissue ultrastructure of the
curcumin group, showing reduced levels of edema in (a and b) the
neurons and (c and d) in the area surrounding the capillaries.
Arrows indicate (a) neuron, (b and c) neuroglial cells or
endothelial cells, and (d) surrounding capillaries. |
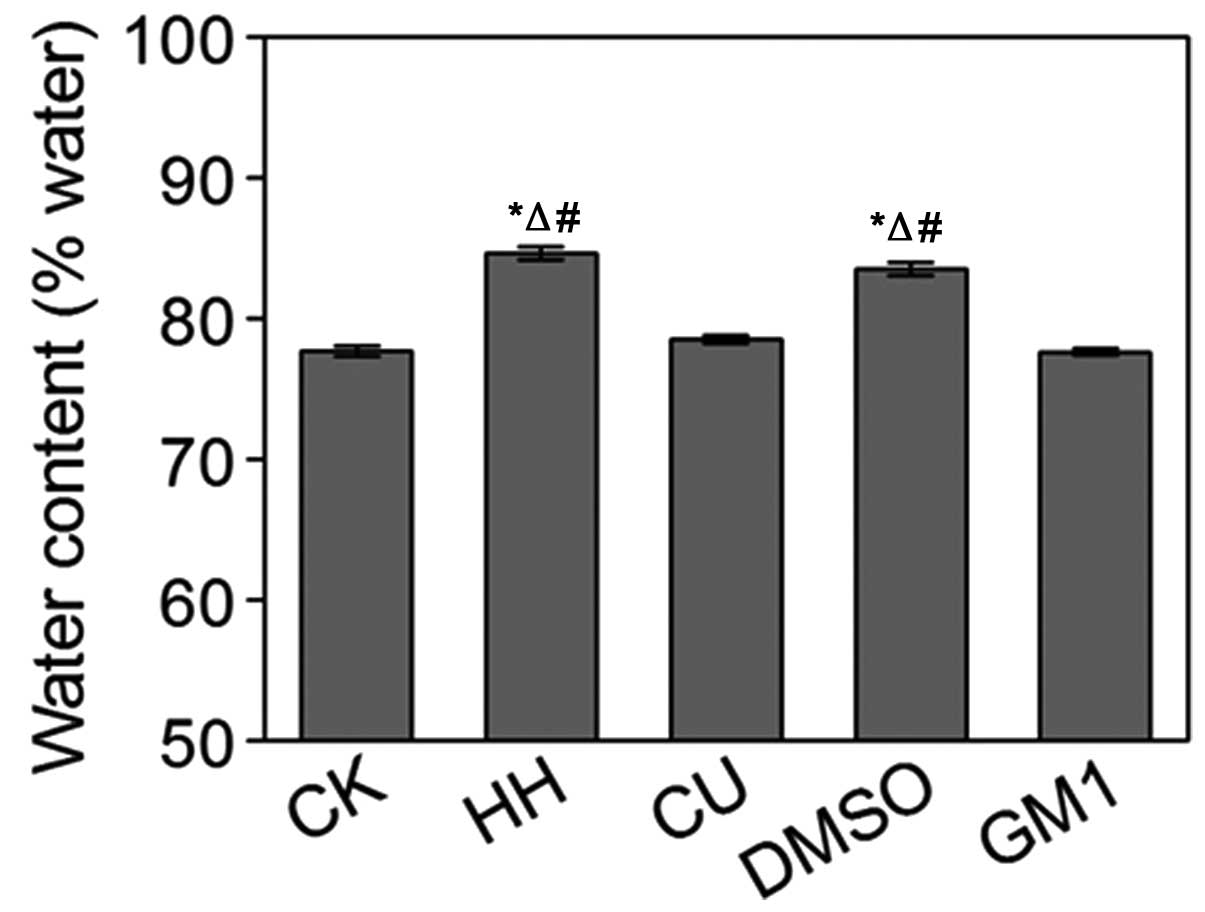
Water content measurement
Water content in the brain tissues from the various
treatment groups was analyzed. Water levels in the HH group rats
were significantly increased, as compared with the CK group
(P<0.05; Fig. 3). No significant
differences were detected between the HH and DMSO groups
(P>0.05; Fig. 3). Conversely,
water levels in the CU group were significantly decreased, as
compared with the HH group (P<0.05; Fig. 3). Notably, GM1 treatment had the same
effects as CU treatment (P>0.05; Fig.
3).
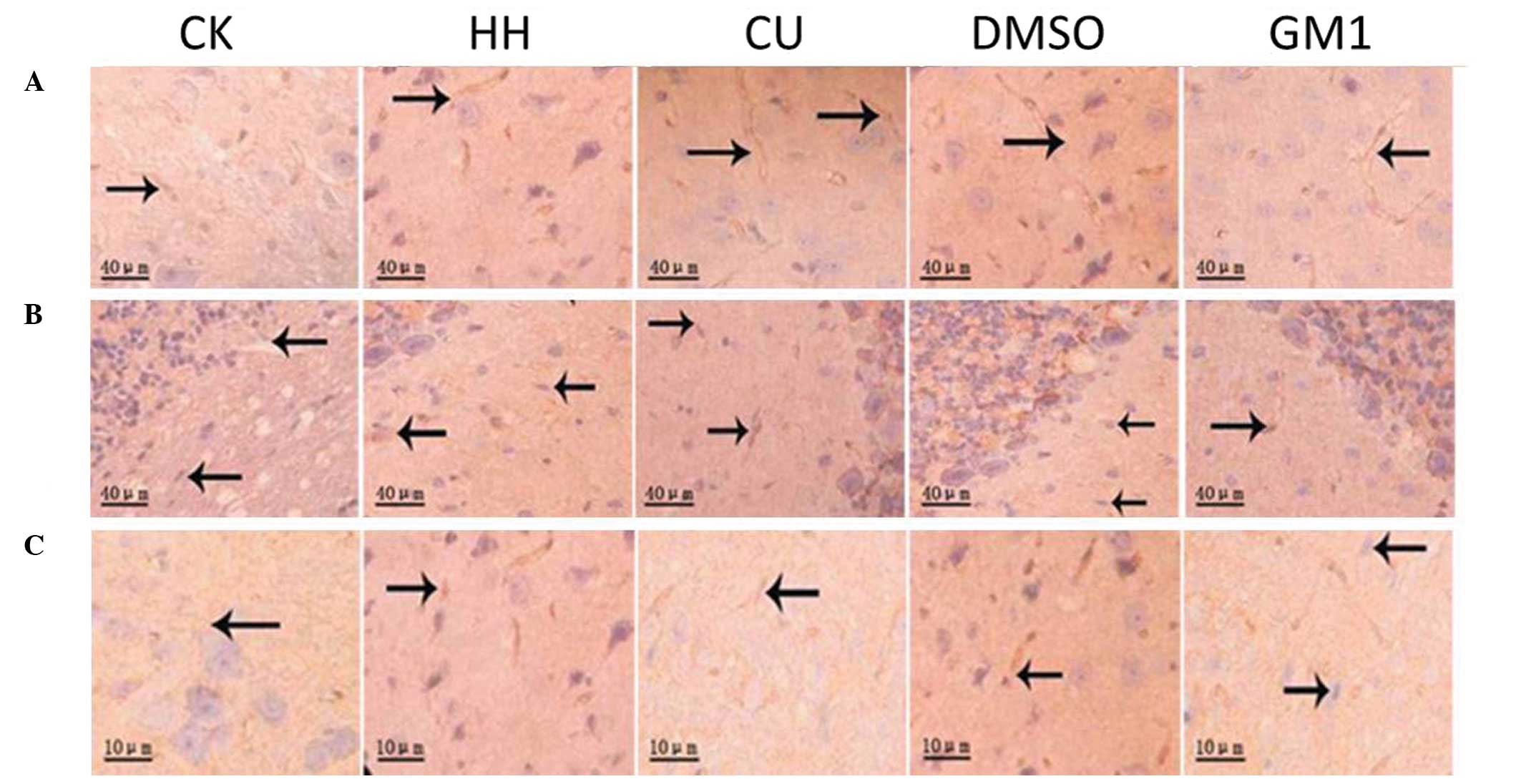
SABC immunohistochemical analysis of
AQP4 expression levels
In order to investigate the underlying mechanisms of
HHBD, the present study investigated the protein expression levels
of AQP4 in the brain cortex, cerebellum and brainstem tissue
samples from the various groups using the SABC immunohistochemical
method (Fig. 4). In addition,
optical density (OD) values of the AQP4 staining were quantified
and compared (Table I). AQP4 protein
expression levels were negligible in the brain cortex of the CK
group, as demonstrated by the minor AQP4 staining observed in the
cell membranes and axons. Conversely, elevated AQP4 protein
expression levels were detected in the HH group, as demonstrated by
dark brown staining of the cell membranes and axons along the
capillaries of the HH group tissue samples. Furthermore, the OD
value of AQP4 staining in the HH group was significantly increased,
as compared with the CK group (P<0.05; Table I). Increased AQP4 staining and higher
OD values were similarly detected in brain tissue samples from the
DMSO-treated rats. However, AQP4 staining was significantly
decreased in the brain cortex of the CU and GM1 groups, as compared
with the HH group (P<0.05; Table
I). A similar phenomenon was detected in the cerebellum and
brainstem tissues. Increased AQP4 staining and higher OD values
were detected for the HH and DMSO groups, as compared with the CK,
CU and GM1 groups; thus suggesting that CU treatment is able to
significantly decrease HHBD-induced AQP-4 expression in various rat
brain tissues.
 | Table I.Detection of AQP4 protein expression
levels in brain tissues of the various treatment groups by SABC
immunohistochemistry (OD values; n=6). |
Table I.
Detection of AQP4 protein expression
levels in brain tissues of the various treatment groups by SABC
immunohistochemistry (OD values; n=6).
| Region | CK group | HH group | CU group | DMSO group | GM1 group |
|---|
| Cerebrum | 0.23±0.02 |
0.47±0.01a,b | 0.36±0.02 |
0.48±0.02a,b | 0.36±0.02 |
| Cerebellum | 0.24±0.03 |
0.48±0.02a,b | 0.38±0.02 |
0.49±0.02a,b | 0.38±0.03 |
| Brainstem | 0.22±0.03 |
0.40±0.03a,b | 0.30±0.03 |
0.40±0.02a,b | 0.29±0.03 |
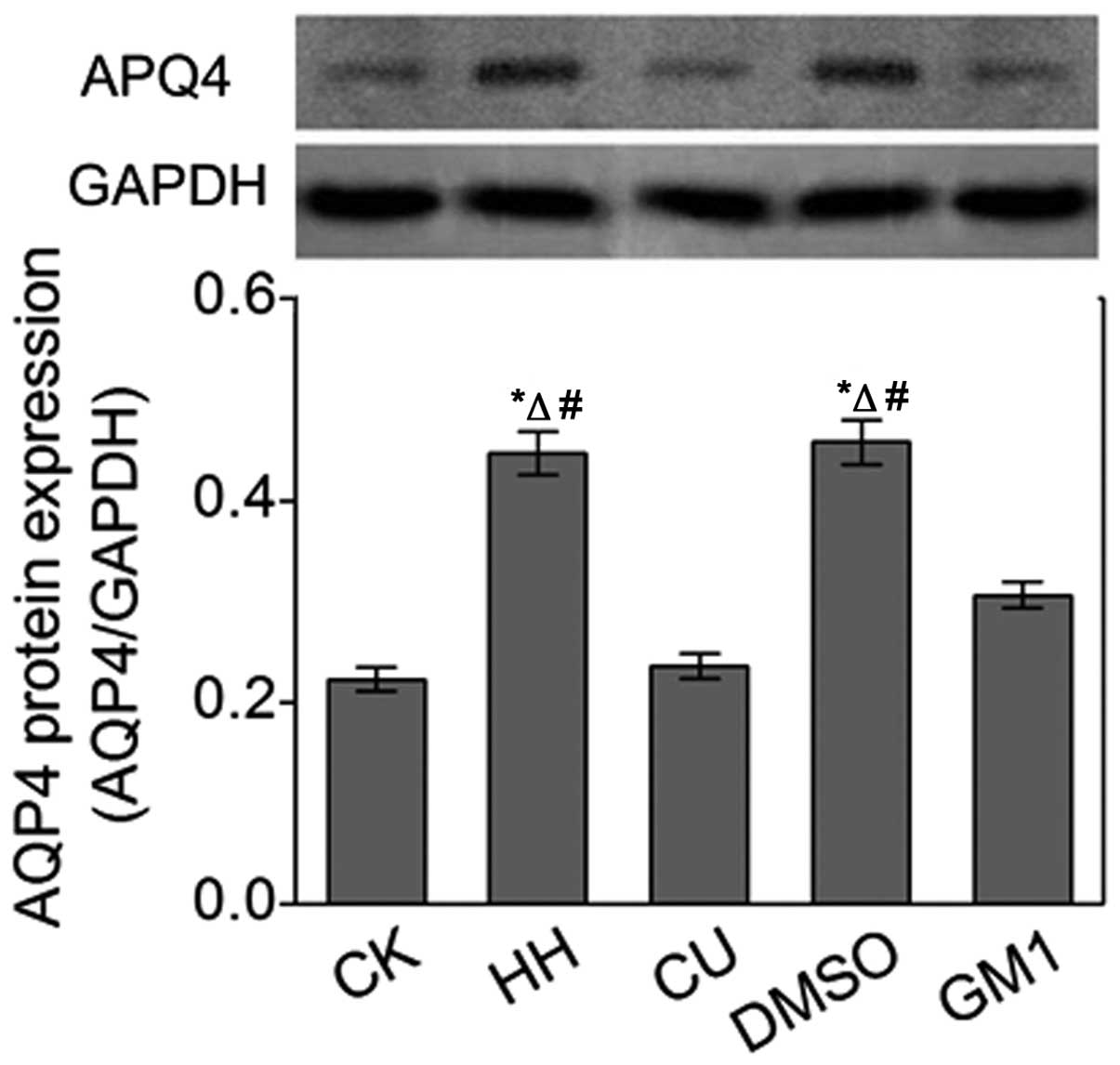
Western blot analysis of the AQP4
protein expression
Western blot analysis was conducted in order to
further investigate alterations in the protein expression levels of
AQP4 in the brain tissues derived from the various treatment
groups. The protein expression levels of AQP4 in the HH and DMSO
groups were significantly increased (P<0.05; Fig. 5), as compared with the CK group.
However, CU treatment inhibited the HHBD-induced increase in AQP4
protein expression levels in the CU group, as compared with the HH
group. In addition, GM1 treatment similarly inhibited AQP4
expression.
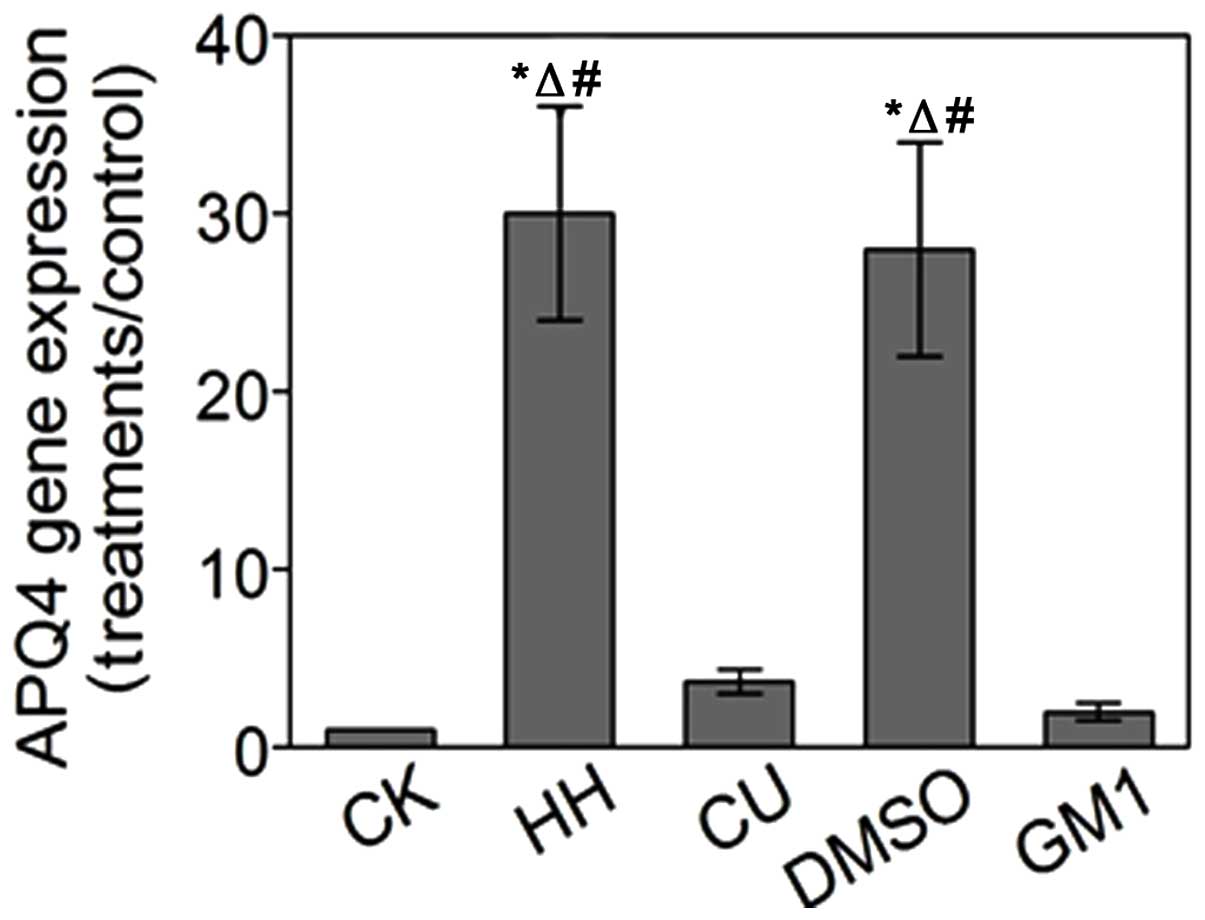
AQP4 gene expression analysis
The mRNA expression levels of AQP-4 in the various
treatment groups were detected by RT-qPCR. The mRNA expression
levels of AQP-4 were increased by 30-fold in the HH group and
28-fold in the DMSO group, as compared with the CK group.
Conversely, in the CU and GM1 groups, the mRNA expression levels of
AQP-4 were increased by 3.7-fold and 2.0-fold, as compared with the
CK group, respectively. These results were consistent with those of
the SABC immunohistochemical and western blot analyses (Fig. 6).
Discussion
HHBD, which is a common result of asphyxiation and
COPD, is detrimental to the nervous system and has previously been
associated with cognitive dysfunction, nervous system disorders and
mental retardation (27). In
addition, hypoxic brain damage accounts for the majority of
injuries observed in forensic cases (28). Therefore, analyzing the mechanisms
underlying HHBD, and identifying novel therapeutic drugs for the
treatment of hypoxic brain injury, are of great value in clinical
treatment and forensic pathology.
Previous studies have demonstrated that various
traditional Chinese medicines exert neuroprotective effects that
may be used to treat patients with nerve injuries (11,29). Our
previous study suggested that CU was able to alleviate HIBD-induced
brain edema in a rat model of HIBD (18), and the present study investigated the
effects of CU in a rat model of HHBD. In the HH group, neurons
appeared markedly swollen, organelles were disintegrated, and cells
exhibited signs of apoptosis. In addition, the water content in the
HH group cells was significantly higher, as compared with the CK
group cells. These results suggested that HH conditions induced
brain edema and destroyed brain tissue and cell structures in the
rats, which is consistent with a previous report (30).
In the present study, CU treatment alleviated the
HH-induced alterations observed in the HH group; thus suggesting
that CU was able to attenuate HHBD-induced brain edema. Dohare
et al (31) reported that CU
treatment decreased edema formation in a rat model of cerebral
thromboembolism in a dose-dependent manner. Similarly, Ghoneim
et al (32) demonstrated that
CU treatment attenuated ischemic-induced brain edema in the rat
forebrain, and King et al (33) reported that CU treatment
significantly decreased the water content and relieved brain edema
in a rat model of intracerebral hemorrhage. Despite differences in
these rat models and the brain tissues used, CU treatment was able
to attenuate brain edema in all cases (31–33);
however, the mechanisms underlying CU-mediated attenuation of brain
edema are yet to be defined thoroughly, and the precise mechanisms
may have differed among the various models.
AQPs are selective water channels that reside on the
cell membrane. A total of six AQPs have previously been identified
in mammalian brain tissues, of which AQP1 and AQP4 are the mostly
widely distributed in the central nervous system. It has previously
been demonstrated that AQP4, which is located on both sides of the
BBB, is primarily expressed in the end-feet of astrocytes and in
capillary endothelial cells. AQP4 may have an important role in
aiding the passage of water through the BBB, and therefore
upregulation of AQP4 may be a molecular mechanism underlying brain
edema (34,35).
The results of the present study suggested that CU
was able to attenuate chronic hypoxia-induced cerebral damage by
regulating AQP4 expression levels. SABC immunohistochemical
analysis detected significantly increased AQP4 protein expression
levels in the brain cortex, cerebellum and brainstem of rats in the
HH group, as compared with the CK group. However, the CU-treated
rats exhibited decreased AQP4 protein expression levels, as
compared with the HH group. Our previous study demonstrated that CU
was able to decrease the expression levels of AQP4 in the
hippocampus of a rat model of HIBD (18). In the present study, CU treatment was
able to reduce the AQP4 expression levels in various other brain
tissues following chronic HH. Furthermore, western blotting and
RT-qPCR were conducted in order to thoroughly investigate the AQP4
protein and mRNA expression levels, respectively. As expected, the
mRNA and protein expression levels of AQP4 were increased in the HH
group brain tissues, whereas CU treatment was able to inhibit this
elevation, and this was consistent with the SABC
imunohistochemistry results.
Laird et al (36) reported that pre- or post-treatment
with CU significantly alleviated head trauma-induced brain edema in
mice, and these protective effects were associated with
significantly decreased acute peri-contusional expression of
interleukin-1B and AQP4. In addition, Ji et al (37) demonstrated that chemically
synthesized CU was able to inhibit the expression of AQP4 and the
phosphorylation of c-Jun N-terminal kinase, in order to protect the
structure of the BBB and alleviate cerebral edema following focal
cerebral ischemia-reperfusion damage in rats. Furthermore, CU was
shown to attenuate edema in other nerve tissues, including spinal
cord tissue, and this was associated with the inhibition of AQP4
overexpression and the Janus kinase/signal transducers and
activators of transcription signaling pathway (38).
In the present study, downregulation of HHBD-induced
AQP4 expression had an important role in the CU-mediated
attenuation of brain edema. However, the signaling pathway
underlying CU-regulated AQP4 expression is currently unclear.
Previous studies have suggested that the brain edema-induced
upregulation of AQP4 expression may be associated with decreased
Na+-K+-adenosine triphosphatase (ATPase)
activity and dysfunctional Na+ influx/K+
efflux. Cerebral hypoxia and ischemia decreased the activity of
Na+-K+-ATPase, which was associated with an
imbalance in the osmotic pressure on both sides of the cell
membrane, activation of osmotic pressure sensors, upregulation of
AQP4 expression and signs of edema (39,40).
Therefore, Na+-K+-ATPase may be an
intermediate component of the CU-regulated AQP4 expression signal
transduction pathway; however further experiments are required to
investigate this.
The present study investigated the effects of GM1
against HHBD-induced brain edema. GM1 is a widely recognized
chemical that exerts neuroprotective effects (41). The results of the present study
suggested that GM1 and CU treatment were able to alleviate
HHBD-induced brain edema and AQP4 expression. GM1 has previously
been shown to maintain the activities of the
Na+-K+-ATP and
Ca2+-Mg2+-ATP enzymes in the membrane of
central nerve cells, which in turn maintained the intracellular ion
balance, attenuated edema of nerve cells and prevented the
accumulation of Ca2+ in the cells (42). Therefore, CU and GM1 may share a
common signaling pathway in their regulation of AQP4 expression;
however further research is required in order to confirm this.
In conclusion, the results of the present study
suggested that CU may alleviate chronic HH-induced brain edema in a
rat model of HHBD by inhibiting HHBD-induced upregulation of AQP4.
Therefore, CU may be considered a potential therapeutic drug for
the treatment of patients with chronic HHBD. Further experiments,
in particular clinical experiments, are required in order to
evaluate the potential clinical application of CU.
Acknowledgements
The present study was supported by the Shanghai Key
Lab of Forensic Medicine (grant no. KF1403); the Natural Science
Foundation of Zhejiang Province (grant no. LQ13H150002); and the
Wenzhou Municipal Science and Technology Bureau Science and
Technology Planning Project (grant no. Y20100185).
References
|
1
|
Bakay L and Lee JC: The effect of acute
hypoxia and hypercapnia on the ultrastructure of the central
nervous system. Brain. 91:697–706. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yu L, Yi J, Ye G, Zheng Y, Song Z, Yang Y,
Song Y, Wang Z and Bao Q: Effects of curcumin on levels of nitric
oxide synthase and AQP-4 in a rat model of hypoxia-ischemic brain
damage. Brain Res. 1475:88–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ainslie PN and Ogoh S: Regulation of
cerebral blood flow in mammals during chronic hypoxia: A matter of
balance. Exp Physiol. 95:251–262. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Donkin JJ and Vink R: Mechanisms of
cerebral edema in traumatic brain injury: Therapeutic developments.
Curr Opin Neurol. 23:293–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Von Sarnowski B, Guerra Kleist-Welch W,
Kohlmann T, Moock J, Khaw AV, Kessler C, Schminke U and Schroeder
HW: Long-term health-related quality of life after decompressive
hemicraniectomy in stroke patients with life-threatening
space-occupying brain edema. Clin Neurol Neurosurg. 114:627–633.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rite I, Machado A, Cano J and Venero JL:
Intracerebral VEGF injection highly upregulates AQP4 mRNA and
protein in the perivascular space and glia limitans externa.
Neurochem Int. 52:897–903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshida H, Yanai H, Namiki Y,
Fukatsu-Sasaki K, Furutani N and Tada N: Neuroprotective effects of
edaravone: A novel free radical scavenger in cerebrovascular
injury. CNS Drug Rev. 12:9–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mcconeghy KW, Hatton J, Hughes L and Cook
AM: A review of neuroprotection pharmacology and therapies in
patients with acute traumatic brain injury. CNS Drugs. 26:613–636.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gaire BP and Kim H: Neuroprotective
effects of Fructus Chebulae extracts on experimental models
of cerebral ischemia. J Tradit Chin Med. 34:69–75. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu QS, Chen XY, Zhuang SJ and Li KQ:
Research on effect of Baimai powder effective compounds group
promotes neurogenesis and maintains of neural stem cells after
cerebral infarction. Zhongguo Zhong Yao Za Zhi. 38:3776–3781.
2013.(In Chinese). PubMed/NCBI
|
|
11
|
Gupta YK, Briyal S and Gulati A:
Therapeutic potential of herbal drugs in cerebral ischemia. Indian
J Physiol Pharmacol. 54:99–122. 2010.PubMed/NCBI
|
|
12
|
Bu Y, Lee K, Jung HS and Moon SK:
Therapeutic effects of traditional herbal medicine on cerebral
ischemia: A perspective of vascular protection. Chin J Integr Med.
19:804–814. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou H, Beevers CS and Huang S: Targets of
curcumin. Current drug targets. 12:332–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wilken R, Veena MS, Wang MB and Srivatsan
ES: Curcumin: A review of anti-cancer properties and therapeutic
activity in head and neck squamous cell carcinoma. Mol Cancer.
10:122011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SY, Chen Y, Li YP, Chen SH, Tan JH,
Ou TM, Gu LQ and Huang ZS: Design, synthesis and biological
evaluation of curcumin analogues as multifunctional agents for the
treatment of Alzheimer's disease. Bioorg Med Chem. 19:5596–5604.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tu XK, Yang WZ, Chen JP, Chen Y, Ouyang
LQ, Xu YC and Shi SS: Curcumin inhibits TLR2/4-NF-κB signaling
pathway and attenuates brain damage in permanent focal cerebral
ischemia in rats. Inflammation. 37:1544–1551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuo CP, Lu CH, Wen LL, Cherng CH, Wong CS,
Borel CO, Ju DT, Chen CM and Wu CT: Neuroprotective effect of
curcumin in an experimental rat model of subarachnoid hemorrhage.
Anesthesiology. 115:1229–1238. 2011.PubMed/NCBI
|
|
18
|
Yu L, Yi J, Ye G, Zheng Y, Song Z, Yang Y,
Song Y, Wang Z and Bao Q: Effects of curcumin on levels of nitric
oxide synthase and AQP-4 in a rat model of hypoxia-ischemic brain
damage. Brain Res. 1475:88–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu L, Yi J, Ye G, Zheng Y, Song Z, Yang Y,
Song Y, Wang Z and Bao Q: Effects of curcumin on levels of nitric
oxide synthase and AQP-4 in a rat model of hypoxia-ischemic brain
damage. Brain Res. 1475:88–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Hua LH: Preservation of animal tissue
within the RNA method to improve. Sheng Wu Ji Shu Tong Bao.
4:2010.(In Chinese).
|
|
21
|
Chen FH, Wang L and Hu LH: Real time
fluorescent quantitative RT-PCR reference genes selection. Zhong
Guo Lin Chuang Jian Yan Za Zhi. 23:393–395. 2005.(In Chinese).
|
|
22
|
Shibata M, Yamawaki T, Sasaki T, Hattori
H, Hamada J, Fukuuchi Y, Okano H and Miura M: Upregulation of Akt
phosphorylation at the early stage of middle cerebral artery
occlusion in mice. Brain Res. 942:1–10. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Beziaud T, Chen XR, El Shafey N, Fréchou
M, Teng F, Palmier B, Beray-Berthat V, Soustrat M, Margaill I,
Plotkine M, et al: Simvastatin in traumatic brain injury: Effect on
brain edema mechanisms. Crit Care Med. 39:2300–2307. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun L, Yang L, Xu YW, Liang H, Han J, Zhao
RJ and Cheng Y: Neuroprotection of hydroxysafflor yellow A in the
transient focal ischemia: Inhibition of protein
oxidation/nitration, 12/15-lipoxygenase and blood-brain barrier
disruption. Brain Res. 1473:227–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang Y, Cai D and Chen Y: Thrombin
inhibits aquaporin 4 expression through protein kinase C-dependent
pathway in cultured astrocytes. J Mol Neurosci. 31:83–93. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng GQ, Wang Y and Wang XT: Chronic
hypoxia-hypercapnia influences cognitive function: A possible new
model of cognitive dysfunction in chronic obstructive pulmonary
disease. Med Hypotheses. 71:111–113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oechmichen M and Meissner C: Cerebral
hypoxia and ischemia: The forensic point of view: A review. J
Forensic Sci. 51:880–887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen YF: Traditional Chinese herbal
medicine and cerebral ischemia. Front Biosci (Elite Ed). 4:809–817.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeremitsky E, Omert L, Dunham CM, Protetch
J and Rodriguez A: Harbingers of poor outcome the day after severe
brain injury: Hypothermia, hypoxia and hypoperfusion. J Trauma.
54:312–319. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dohare P, Garg P, Jain V, Nath C and Ray
M: Dose dependence and therapeutic window for the neuroprotective
effects of curcumin in thromboembolic model of rat. Behav Brain
Res. 193:289–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghoneim AI, Abdel-Naim AB, Khalifa AE and
El-Denshary ES: Protective effects of curcumin against
ischaemia/reperfusion insult in rat forebrain. Pharmacol Res.
46:273–279. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
King MD, Mccracken DJ, Wade FM, Meiler SE,
Alleyne CH Jr and Dhandapani KM: Attenuation of hematoma size and
neurological injury with curcumin following intracerebral
hemorrhage in mice. J Neurosurg. 115:116–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Papadopoulos MC and Verkman AS:
Aquaporin-4 and brain edema. Pediatr Nephrol. 22:778–784. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Loreto C and Reggio E: Aquaporin and
vascular diseases. Curr Neuropharmacol. 8:105–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Laird MD, Sukumari-Ramesh S, Swift AE,
Meiler SE, Vender JR and Dhandapani KM: Curcumin attenuates
cerebral edema following traumatic brain injury in mice: A possible
role for aquaporin-4? J Neurochem. 113:637–648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ji FT, Cao MH, Liang JJ, Liu L, Li F and
Bu XZ: Effects of chemical synthesized curcumin preconditioning on
the expression of AQP 4 and cerebral edema after focal cerebral
ischemia/reperfusion damage in rats. Zhong Guo Yao Li Xue Tong Bao.
4:0192011.(In Chinese).
|
|
38
|
Zu J, Wang Y, Xu G, Zhuang J, Gong H and
Yan J: Curcumin improves the recovery of motor function and reduces
spinal cord edema in a rat acute spinal cord injury model by
inhibiting the JAK/STAT signaling pathway. Acta Histochem.
116:1331–1336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moftakhar P, Lynch MD, Pomakian JL and
Vinters HV: Aquaporin expression in the brains of patients with or
without cerebral amyloid angiopathy. J Neuropathol Exp Neurol.
69:1201–1209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang B, Zador Z and Verkman A: Glial cell
aquaporin-4 overexpression in transgenic mice accelerates cytotoxic
brain swelling. J Biol Chem. 283:15280–15286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vieira KP, De Almeida E, Silva Lima,
Zollner AR, Malaguti C, Vilella CA and De Lima Zollner R:
Ganglioside GM1 effects on the expression of nerve growth factor
(NGF), Trk-A receptor, proinflammatory cytokines and on autoimmune
diabetes onset in non-obese diabetic (NOD) mice. Cytokine.
42:92–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen ZG, Lu YC, Zhu C, Zhang GJ, Ding XH
and Jiang JY: Effects of ganglioside GM1 on reduction of brain
edema and amelioration of cerebral metabolism after traumatic brain
injury. Chin J Traumatol. 6:23–27. 2003.PubMed/NCBI
|