Introduction
Cardiopulmonary arrest (CA) is a major health
concern that has a poor prognosis (1,2).
Although rigorous emergency procedures and cardiopulmonary
resuscitation (CPR) have decreased the mortality rates of CA,
long-term (≥12 month) survival following the restoration of
spontaneous circulation (ROSC) remains low (3,4).
Neurological damage is a common problem that adversely affects
patients following CA and ROSC, and results in limited long-term
survival. Although the rates of neurological disability among CA
survivors have decreased from 32.9 to 28.1% between the years 2002
and 2009 (5), there are no specific
therapies available to alleviate CA-associated brain injury.
The mechanisms underlying brain injury following CA
remain poorly understood. Mitogen-activated protein kinases (MAPKs)
are a family of signal transduction proteins, including
extracellular signal regulated kinase and p38-MAPK, that are
activated by cellular stresses (6).
Sustained expression of p38 MAPK is associated with neuronal death
and apoptosis (7), whereas the
inhibition of p38 MAPK is neuroprotective in cerebral focal
ischemia (8). As a result of these
neuroprotective effects, p38 MAPK inhibitors have been tested as
therapeutic agents for neural diseases (9).
Nuclear factor-κB (NF-κB) is a transcription factor
expressed throughout the nervous system (10). In response to ischemia, NF-κB
expression may promote cell death through apoptosis and necrosis
(11). The inhibition of NF-κB may
be able to reduce brain injury in rat models of middle cerebral
artery occlusion (12) and
hypoxic-ischemic brain damage (13).
These results suggest that therapies targeting NF-κB could reduce
brain injury following CA and subsequent CPR (14).
Pharmacological intervention, typically using
epinephrine, is essential in the management of CA (15). Previous studies have demonstrated
that epinephrine has a neuroprotective effect following CA;
however, the use of epinephrine for this purpose remains
controversial (16,17). Epinephrine has a number of
unfavorable side effects, such as long-term hypotension and
ventricular dysrhythmia; therefore, there is a requirement for
alternative therapies for CA-associated brain injury (18).
Vasopressin is a peptide hormone that functions as a
potent vasoconstrictor (19).
Previous laboratory studies have indicated that a combination
therapy of vasopressin and epinephrine improves histopathological
outcome and cerebral blood flow more successfully compared to
treatment with epinephrine alone (20–22).
However, these studies have not investigated whether vasopressin
alone or combined with epinephrine is able to reduce hippocampal
injury. In the present study, a rodent model of asphyxial cardiac
arrest is used to compare the effects of vasopressin and
epinephrine alone, or in combination, on hippocampal injury
following ROSC and the success rate of resuscitation.
Materials and methods
Animals and reagents
The Animal Care and Use Committee of Jilin
University (Changchun, China) approved the experimental procedures
performed in the present study. A total of 192 adult male
Sprague-Dawley rats (weight, 270±20 g; age, 8 weeks) were purchased
from the Experimental Animal Center at Jilin University and housed
with ad libitum access to food and water under conditions of
20±2°C, 55–60% humidity and a 12-h light/dark cycle. Polyclonal
anti-p38 MAPK and anti-NF-κB p65 antibodies were purchased from
Beijing Boosen Biological Technology Co., Ltd. (Beijing, China).
Vasopressin was purchased from Sigma-Aldrich (St. Louis, MO, USA)
and epinephrine was purchased from Shanghai Harvest Pharmaceutical
Co., Ltd. (Shanghai, China).
Surgical preparation of rats
The surgical procedure for asphyxial CA induction
was performed as described previously (23). Briefly, Sprague-Dawley rats were
anesthetized by intraperitoneal injection with 10% chloral hydrate
(0.03 ml/g; Shanghai Harvest Pharmaceutical Co., Ltd.).
Electrocardiographic monitoring was performed using limb leads (II)
to measure the heart rate. A tracheotomy was performed, followed by
intubation with an 18-gauge angiocatheter and mechanical
ventilation using an animal ventilator (DW-2000-type; Shanghai
Jiapeng Technology Co., Ltd., Shanghai, China). Catheterization of
the femoral vein was performed to administer sodium heparin (5
IU/ml; Benny Biochemical Pharmaceutical Co., Ltd., Changzhou,
China).
Induction of asphyxial cardiac arrest
in experimental rats
Following a 10-min equilibration period after the
operation, cardiac arrest was induced by clamping the tracheal tube
for 5 min. Cardiac arrest was confirmed by the loss of aortic
pulsations, defined as a mean arterial blood pressure <10 mmHg
(24). A total of 144 rats
undergoing asphyxial CA and subsequent CPR were randomly allocated
into three equally sized treatment groups: Rats treated with
vasopressin (0.8 U/kg); epinephrine (0.2 mg/kg); or vasopressin
(0.8 U/kg) plus epinephrine (0.2 mg/kg). An additional 48 rats
underwent a sham surgical procedure without CA induction or CPR.
After 10 min at room temperature, cardiac compression was performed
manually at a rate of 180 compressions/min over the chest, with
sufficient compression force to achieve 1/3 of the anteroposterior
chest diameter. The indicated drugs were administered once CPR
began. Ventilation was commenced using 100% oxygen at a breathing
rate of 70 breaths/min, with a tidal volume of 6 ml/kg and an
exhale to inhale ratio of 1:1.5.
Assessment of ROSC
ROSC was evaluated by two independent observers.
ROSC was indicated by the emergence of supraventricular rhythm
detected by the electrocardiogram monitor (78354C; Hewlett Packard
Enterprise, Palo Alto, CA, USA) and a mean arterial blood pressure
of ≥20 mmHg for 5 min (25).
Following the administration of each drug, the number of successful
ROSC cases and the length of time between CPR and ROSC was
recorded. If animals did not achieve ROSC after 10 min of CPR,
resuscitation was discontinued.
Microscopic analysis in the
hippocampus
Following anesthetization by intraperitoneal
injection with 30 mg/kg pentobarbital sodium (H. Lundbeck A/S,
Valby Denmark), the rats were sacrificed by decapitation. Tissue
from the hippocampal CA1 region was harvested at 1, 3, 6 and 12 h
after ROSC. At each time point, 12 rats were sacrificed.
Hematoxylin-eosin (HE) staining (Beyotime Institute of
Biotechnology, Shanghai, China) was performed according to standard
protocols and the tissue was evaluated by two independent
investigators using a light microscope (JEM-1200EX; Sweden).
Ultrastructural analysis of
hippocampal cells
For ultrastructural analysis, hippocampal CA1 tissue
samples (size, ~2×1×1 mm) were fixed with 2.5% glutaraldehyde
(Sigma-Aldrich) and embedded in EPON resin (Hexion Inc., Columbus,
OH, USA). Ultra-thin (50 nm) sections were cut using an ultra
microtome (LKB8800III; LKB Vertriebs GmbH, Vienna, Austria) and
stained with uranyl acetate (Shanghai Yanjing Biological Technology
Co., Ltd., Shanghai, China). An independent observer analyzed each
sample by transmission electron microscopy (JEM-1200EX; Japan
Electron Optics Laboratory Co., Ltd., Tokyo, Japan).
P38 MAPK and NF-κB p65 expression
levels
The expression levels of p38 MAPK and NF-κB p65 were
detected using immunohistochemistry. Briefly, 4-µm sections were
fixed in 5% formaldehyde for 7 days and dehydrated with decreasing
concentrations of ethanol. Paraffin sections were autoclaved
(C16S01; Supor Co., Ltd., Hangzhou, China) at 98°C in citrate
buffer (pH 6.0; Shanghai Meilian Biological Institute, Shanghai,
China) for 10 min. Sections were transferred to glass slides and
treated with 3% hydrogen peroxide for 15 min in order to inactivate
endogenous peroxides. The sections were then blocked using 1% goat
serum (Beyotime Institute of Biotechnology) in phosphate-buffered
saline (PBS) for 15 min at room temperature, then incubated with
rabbit anti-human p38 MAPK (1:200; bs-0637R; Beijing Boosen
Biological Technology Co., Ltd.) or NF-kB p65 (1:200; bs-3543R;
Beijing Boosen Biological Technology Co., Ltd.) polyclonal
antibodies in 1% goat serum for 12 h at 4°C. Following antibody
incubation, slides were incubated with biotin-conjugated mouse
anti-rabbit IgG (1:500; bs-0296P-Bio; Beijing Boosen Biological
Technology Co., Ltd.) for 10 min at 37°C. Slides were then washed
with PBS three times, incubated with horseradish peroxidase
(labeled with streptavidin; Beyotime Institute of Biotechnology)
for 10 min at 37°C, and incubated with 3,3′-diaminobenzidine
(Maixin Biotechnology Co., Ltd., Fuzhou, China) for 1–2 min. The
slides were stained with hematoxylin, and stained tissues were
analyzed by light microscopy (JEM-1200EX). Staining was assessed in
100 randomly selected cells under 10 fields in order to determine
the staining intensity and the percentage of positive cells.
Overall staining was measured using the immunoreactive score (IRS)
that is calculated as a product of the intensity and percentage
scores (26). Based on IRS, the
staining was categorized as negative (IRS, 0), weak (IRS, 2–3),
moderate (IRS, 4–5), and strong (IRS, 6–7). The staining score
criteria are detailed in Table
I.
 | Table I.Staining score criteria for
immunohistochemistry. |
Table I.
Staining score criteria for
immunohistochemistry.
| Positive cell number
(score: %) | Intensity of staining
score | Total score (degree
of positive expression) |
|---|
| 0 | 0:
No color |
0 |
| 1: ≤25 |
1:
Faint yellow |
2–3
(+) |
| 2: 26–50 |
2:
Pale brown |
4–5
(++) |
| 3: 50–75 | 3: Brown |
6–7
(+++) |
| 4: >75 |
|
|
Statistical analysis
Continuous variables were presented as the mean ±
standard deviation and categorical variables were expressed as
observed frequencies. Continuous variables were analyzed using
one-way analysis of variance and the Student-Newman-Keuls multiple
comparisons test, and categorical variables were compared using the
Fisher's exact test. Statistical analysis was performed using SPSS
version 16.0 software (SPSS, Inc., Chicago, IL, USA), and P<0.05
was considered to indicate a statistically significant
difference.
Results
Comparison of ROSC success rate
Prior to the induction of asphyxial CA, no
significant differences in the baseline characteristics were
observed among the four groups (Table
II). As presented in Table
III, the success rate of ROSC in rats treated with vasopressin
(39/48 rats), or with vasopressin plus epinephrine (42/48 rats),
was significantly higher compared with rats treated with
epinephrine alone (24/48 rats; P<0.05). In addition, the time
required to achieve ROSC following treatment with vasopressin, or
with vasopressin plus epinephrine, was significantly reduced
compared to the rats treated with epinephrine alone (P<0.05). In
addition, the administration number during ROSC following treatment
with vasopressin plus epinephrine was significantly reduced
compared with epinephrine alone (P<0.05).
 | Table II.Baseline characteristics of the
rats. |
Table II.
Baseline characteristics of the
rats.
| Group | Body weight (g) | Heart rate
(beats/min) | SBP (mmHg) | DBP (mmHg) |
|---|
| Sham control | 267.13±21.68 | 298±25.82 | 125.13±8.95 | 94.40±4.82 |
| Epinephrine | 270.00±17.57 | 301±17.57 |
131.00±10.00 | 94.13±5.19 |
| Vasopressin | 268.53±19.37 | 289±15.89 | 129.00±8.98 | 90.00±7.31 |
| Vasopressin +
epinephrine | 270.40±17.23 | 303±19.23 | 127.00±9.69 | 97.43±5.54 |
 | Table III.Comparison of ROSC. |
Table III.
Comparison of ROSC.
| Group | ROSC success
rate | ROSC time (sec) | Number of times
administered during ROSC |
|---|
| Sham control | 48/48 | N/A | N/A |
| Epinephrine | 24/48 | 262.00±17.89 | 2.40±0.89 |
| Vasopressin |
39/48a |
162.00±11.49a | 1.56±0.73 |
| Vasopressin +
epinephrine |
42/48a,b |
141.27±6.59a,b |
1.36±0.50a |
Histological analysis of the
hippocampus
In the hippocampus of sham control rats, normal
neurons free of edema were observed (Fig. 1A and B). In contrast, all rats
subjected to asphyxial CA displayed hippocampal neurons with
ambiguous or invisible nuclei, cytoplasmic cavities and neural
edema (Fig. 1C–J). At 1 h post-RPSC,
edema was not detected in the hippocampal neurons of rats treated
with a combination of vasopressin and epinephrine (Fig. 1K–N).
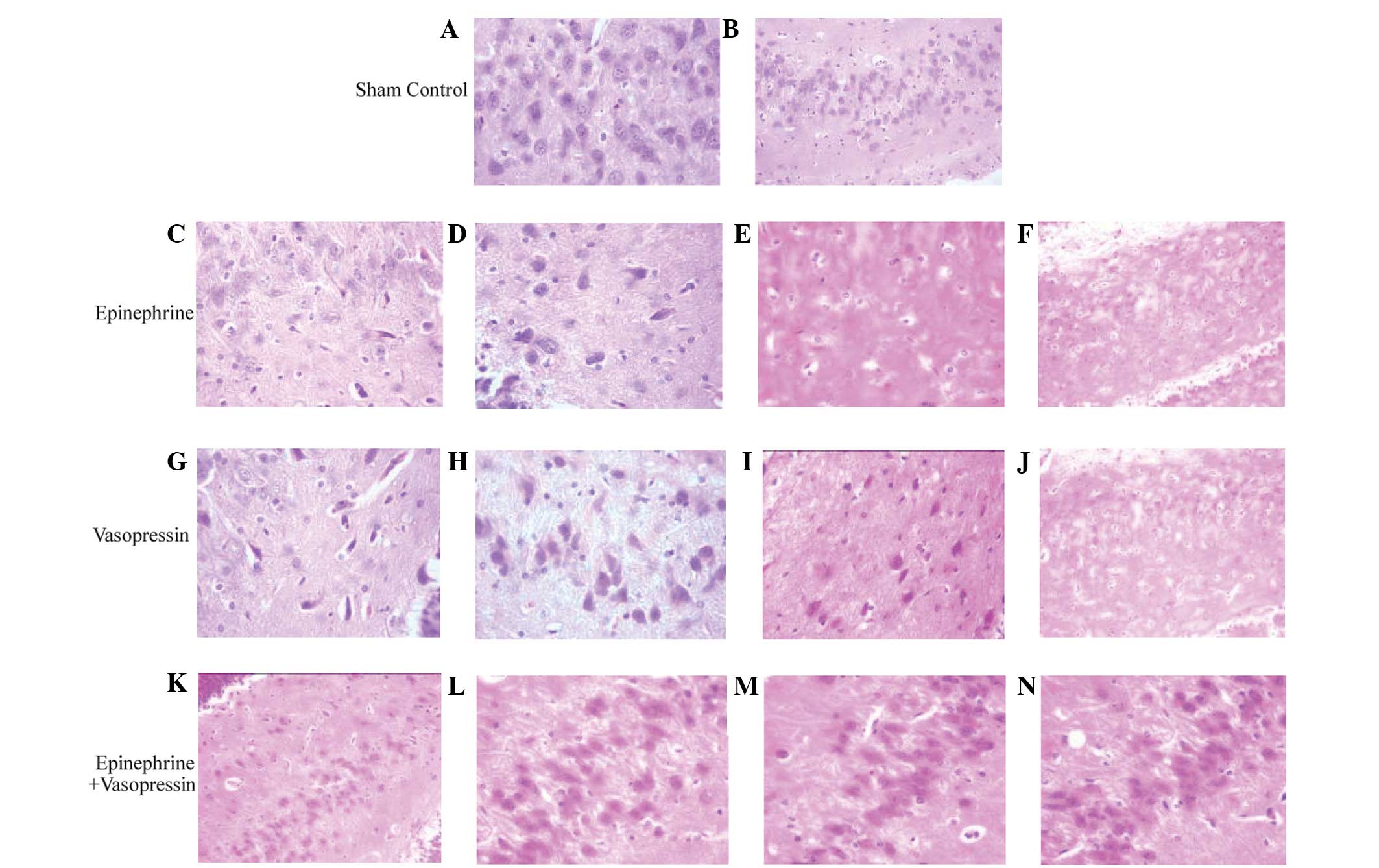 | Figure 1.Histological changes of hippocampal
CA1 region following asphyxial cardiac arrest. (A and B)
Hematoxylin and eosin (HE) stained pathological sections of
hippocampus in the sham control group presented with normal neurons
without edema. (C-F) HE stained hippocampal tissue from rats
treated with epinephrine presented with ambiguous or invisible
nuclei, cytoplasmic cavity and aggravated evident edema of
hippocampal tissue from rats sacrificed at (C) 1, (D) 3, (E) 6 and
(F) 12 h following restoration of spontaneous circulation (ROSC).
(G-J) HE stained hippocampal tissue from rats treated with
vasopressin presented with ambiguous or invisible nuclei, and
aggravated cytoplasmic cavity was detected [G-I, ×400 magnification
of hippocampal tissue from rats sacrificed at (G) 1, (H) 3, (I) 6
and (J) 12 h after ROSC. (K-N) HE stained hippocampal tissue from
rats treated with vasopressin plus epinephrine. Edema was not
evident and the nucleus was ambiguous. Hippocampal tissue at (K) 1,
(L) 3, (M) 6 and (N) 12 h after ROSC (magnification: A, C-E, G-I
and L-N, ×400; B, F, J and K, ×200). |
Ultrastructural changes of hippocampal
neurons
As presented in Fig.
2A, normal structures of the hippocampal neurons were observed
in the sham control group, while ultrastructural abnormalities
within the neurons of the hippocampus were observed following
asphyxial CA in rats treated with epinephrine (Fig. 2B and C). Asphyxial CA induced
prominent mitochondrial defects, including swollen mitochondria and
loss of the typical mitochondrial morphology (Fig. 2B). A loss of rough endoplasmic
reticulum and mitochondrial fragmentation was also observed in
neurons following asphyxial CA (Fig.
2C). These ultrastructural defects were attenuated by treatment
with vasopressin or vasopressin plus epinephrine (Fig. 2D–G).
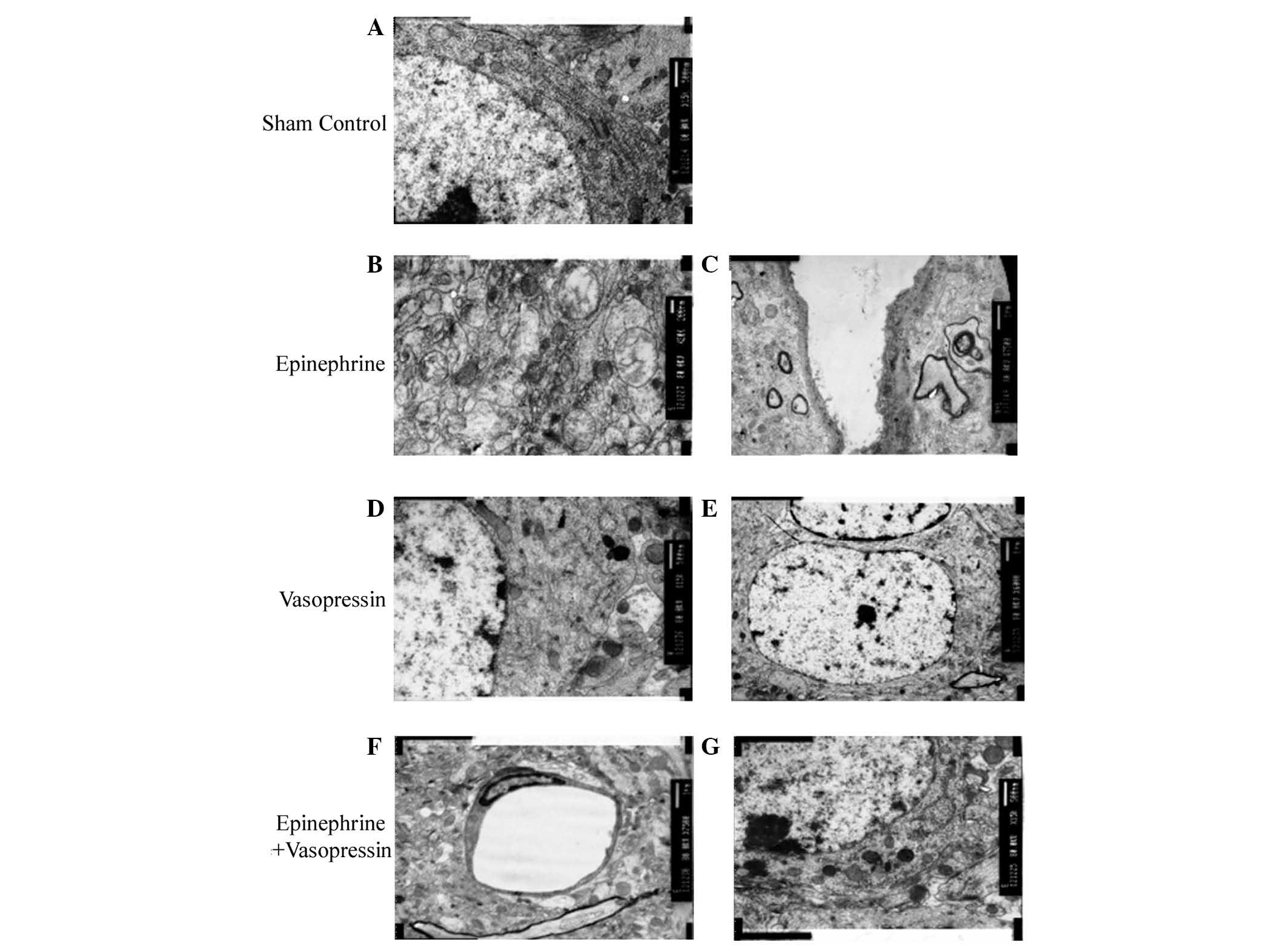 | Figure 2.Ultrastructural analysis of the
hippocampus CA1 region following asphyxial cardiac arrest
(magnification: ×8,000). (A) In the sham control group, large
quantities of rough endoplasmic reticulum, round mitochondria and
clear intramitochondrial ridges are observed. (B and C) In the
epinephrine treated group, (B) swollen mitochondria with focal
breakdown of the intramitochondrial ridge, and membrane intrusion
into the mitochondrial cavity is observed; (C) slightly thickened
capillary vessels, incomplete or partially disappeared membrane
structures, and disarrangement of the myelin sheath is also
observed. (D and E) In the vasopressin treatment group, rough
endoplasmic reticulum, free ribosomes, round or oval-shaped
mitochondria with diminished intramitochondrial ridges and
lysosomes are observed. (F and G) Ultrastructure of the hippocampus
in the vasopressin plus epinephrine treated group presents with (F)
rough endoplasmic reticulum, free ribosomes, small rounded-shaped
mitochondria, compact ridges, increased lysosomes and a small
quantity of lipofuscin granules. (G) Flat capillary endothelial
cells, thin and intact membrane structures, and minimal protrusions
into the mitochondrial cavity are also observed. |
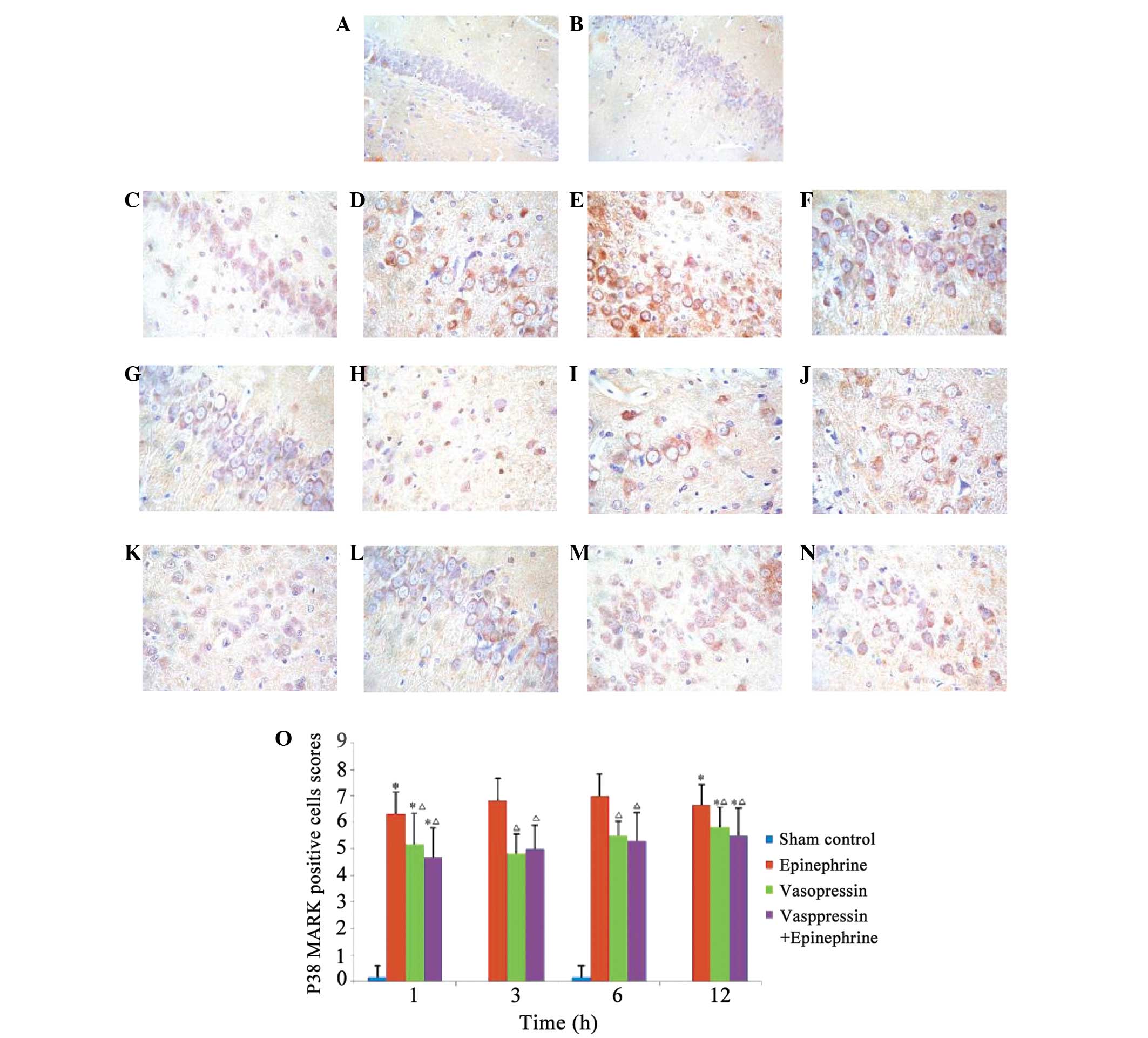
Induction of p38 MAPK expression by
asphyxial CA
A small quantity of p38 MAPK was detectable in the
hippocampal neurons of the sham control group; however, p38 MAPK
was abundant in all of the rats following asphyxial CA (Fig. 3A–D). Quantification of the staining
indicated that the expression level of p38 MAPK was significantly
higher following asphyxial CA (Fig.
3E; P<0.05). However, the expression level of p38 MAPK was
significantly reduced in rats treated with vasopressin, or
vasopressin plus epinephrine, in comparison with rats treated with
epinephrine alone (P<0.05). No significant difference was
observed between the expression levels of p38 MAPK in the rats
treated with vasopressin plus epinephrine compared with vasopressin
alone (P>0.05).
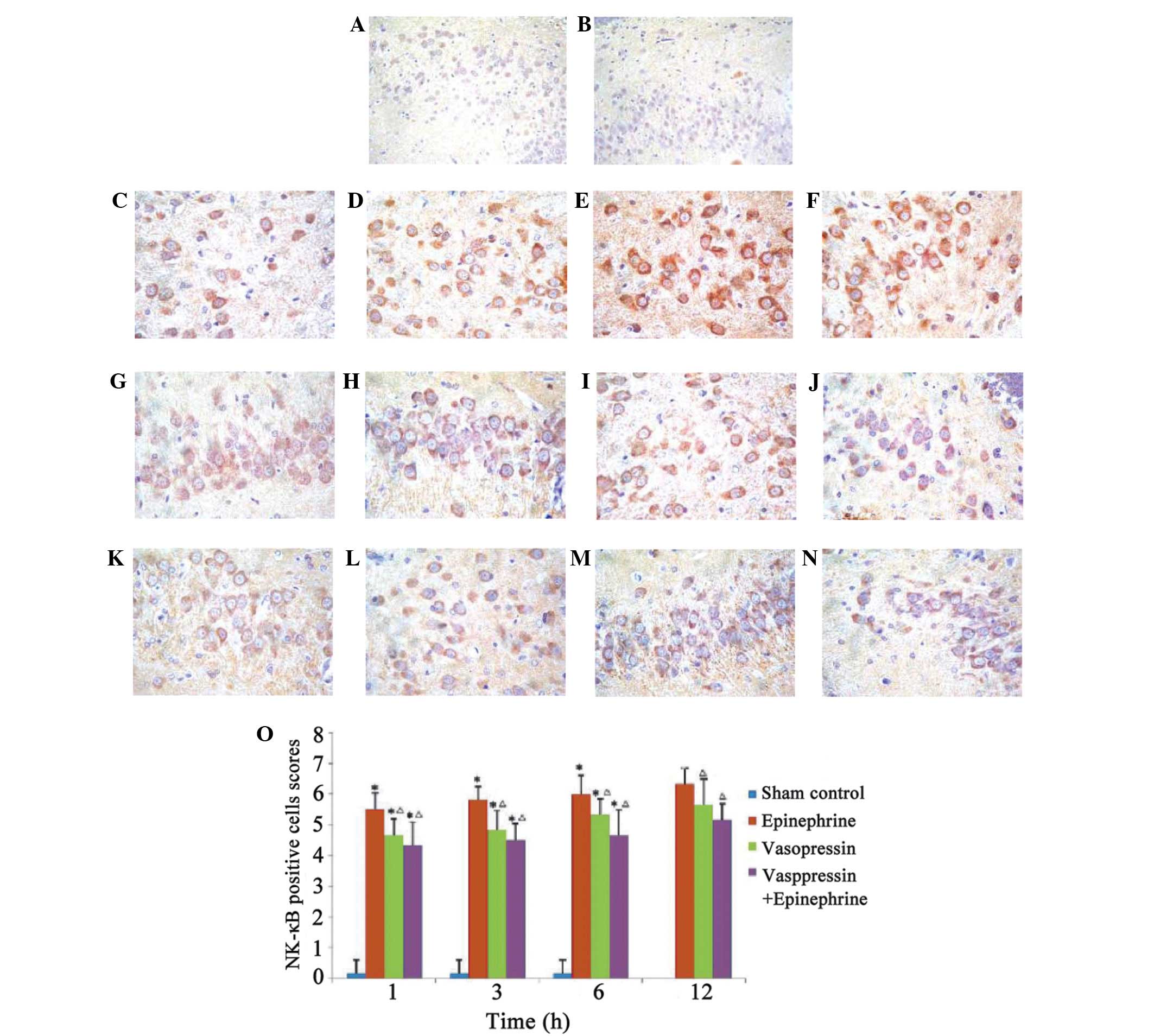
NF-κB p65 expression following
asphyxial CA and treatment with vasopressin
Very low expression levels of NF-κB p65 were
detected in the sham control group, while abundant NF-κB p65
staining was detected following asphyxial CA (Fig. 4A–D). Positive staining scores of
NF-κB p65 were significantly higher in each asphyxial CA group in
comparison with the sham control group (Fig. 4E; P<0.05). As observed in the MAPK
analyses, NF-κB p65 staining was significantly reduced in rats
treated with vasopressin, or vasopressin plus epinephrine, in
comparison with those treated with epinephrine alone (P<0.05).
There were no significant differences between the synergistic
effects from combining vasopressin and epinephrine.
Discussion
The present study demonstrated that treatment with
vasopressin following CA improved the chance of survival and
attenuated ultrastructural changes associated with hippocampal
injury. In addition, the expression of p38 MAPK and NF-κB p65 was
significantly reduced in the hippocampus of rats treated with
vasopressin, as compared with those treated with epinephrine.
Furthermore, combination therapy of vasopressin and epinephrine
appeared to have a synergistic effect in attenuating hippocampal
injury; however, they did not induce these effects using the
presently investigated mechanisms.
Animal studies have demonstrated that CA and CPR are
able to promote injury in selectively vulnerable zones of the
brain, including the hippocampus (27). The present study demonstrated that
simultaneous administration of vasopressin and epinephrine during
CPR improved the histopathological outcome following ROSC. These
results are consistent with a previous study, which demonstrated
that combination therapy with epinephrine and vasopressin improved
the histopathological outcome, as compared with epinephrine alone
(22).
Ultrastructural analyses in the present study
demonstrated that vasopressin alone, or in combination with
epinephrine, reduced edema and mitochondrial damage in hippocampal
neurons. These beneficial results may have been due to the high
cerebral blood flow induced by vasopressin (28). In addition to reducing hippocampal
injury, combination therapy with vasopressin and epinephrine may
permit lower doses of epinephrine, thereby minimizing adverse side
effects.
p38 MAPK is activated following cerebral ischemia
and contributes to ischemic/hypoxic neuronal cell death (29,30). In
the present study, immunohistochemistry demonstrated that p38 MAPK
expression levels were significantly elevated for up to 12 h
post-ROSC, thus suggesting that p38 MAPK may be activated by
hypoxia and ischemia/reperfusion injury. Treatment with epinephrine
alone did not significantly affect the expression levels of p38
MAPK following ROSC; however, vasopressin alone or in combination
with epinephrine significantly reduced p38 MAPK expression levels
in the hippocampus, as assessed by immunohistochemistry.
The fate of cerebral cells under anoxic conditions
or in ischemia/reperfusion injury is partly determined by proteins
of the apoptotic cascade, including NF-κB (10). Inhibition of the apoptotic pathway
activated by NF-κB may attenuate cerebral injury (10). In addition, NF-κB is a critical
transcription factor involved in inflammatory mediator induction;
therefore, inhibition of NF-κB signaling may inhibit the expression
of inflammatory mediators and attenuate subsequent inflammatory
injury (10).
The results from the present study indicated that
NF-κB p65 expression was elevated following asphyxial CA and ROSC,
suggesting that these pathways are involved in hippocampal injury.
It was also observed that combination therapy with vasopressin and
epinephrine reduced NF-κB p65 expression levels to a greater extent
than treatment with epinephrine alone. In addition, the results of
the present study suggested that vasopressin was able to improve
the post-ROSC outcome by suppressing apoptosis.
One limitation of the present study was the 12-h
observation period following resuscitation. Necrosis is difficult
to detect within 12 h following ROSC; an observation time of ≥96 h
would more accurately indicate hippocampal changes. In addition,
the present study did not record neurological deficit scale scores,
which would permit the analysis of the correlation between the
extent of hippocampal injury and neurological function.
Furthermore, there was no measurement of cerebral blood flow during
CPR and in the post-resuscitation period; cerebral blood flow would
indicate the mechanism by which vasopressin exerts its protective
effects. Finally, no rats were administered a vehicle substance;
vehicle controls would be required in order to accurately compare
the effects of epinephrine and vasopressin with the effects
observed following no pharmacological intervention. Future studies
are required to address these issues.
In conclusion, the present study demonstrated that
the administration of vasopressin efficiently attenuated
hippocampal injury during ROSC in a rat model of asphyxial CA, and
was superior compared to treatment with epinephrine. In comparison
to treatment with epinephrine, vasopressin alone and in combination
with epinephrine was associated with more frequent ROSC and a more
effective attenuation of hippocampal injury. The neuroprotective
effects observed in the present study may be attributed to the
inhibition of p38 MAPK and NF-κB expression. Additional studies
specifically addressing the effects of vasopressin on neurological
outcomes are required in order to determine the mechanisms by which
vasopressin reduces hippocampal injury.
Acknowledgements
The present study was supported by a research grant
from the National Natural Science Foundation of China (grant no.
81471830).
References
|
1
|
Gueugniaud PY, David JS, Chanzy E, Hubert
H, Dubien PY, Mauriaucourt P, Bragança C, Billères X,
Clotteau-Lambert MP, Fuster P, et al: Vasopressin and epinephrine
vs. epinephrine alone in cardiopulmonary resuscitation. N Engl J
Med. 359:21–30. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mentzelopoulos SD, Zakynthinos SG, Tzoufi
M, Katsios N, Papastylianou A, Gkisioti S, Stathopoulos A,
Kollintza A, Stamataki E and Roussos C: Vasopressin, epinephrine
and corticosteroids for in-hospital cardiac arrest. Arch Intern
Med. 169:15–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rudiger A, Tobler D and Estlinbaum W:
Frequency and outcome of in-hospital resuscitation outside the
ICU-setting. Swiss Med Wkly. 134:59–62. 2004.PubMed/NCBI
|
|
4
|
Stiell IG, Wells GA, Field B, Spaite DW,
Nesbitt LP, De Maio VJ, Nichol G, Cousineau D, Blackburn J, Munkley
D, et al: Advanced cardiac life support in out-of-hospital cardiac
arrest. N Engl J Med. 351:647–656. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Girotra S, Nallamothu BK, Spertus JA, Li
Y, Krumholz HM and Chan PS: American Heart Association Get with the
Guidelines-Resuscitation Investigators: Trends in survival after
in-hospital cardiac arrest. N Engl J Med. 367:1912–1920. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo CL, Li QQ, Chen XP, Zhang XM, Li LL,
Li BX, Zhao ZQ and Tao LY: Lipoxin A4 attenuates brain damage and
downregulates the production of pro-inflammatory cytokines and
phosphorylated mitogen-activated protein kinases in a mouse model
of traumatic brain injury. Brain Res. 1502:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maroney AC, Glicksman MA, Basma AN, Walton
KM, Knight E Jr, Murphy CA, Bartlett BA, Finn JP, Angeles T,
Matsuda Y, et al: Motoneuron apoptosis is blocked by CEP-1347 (KT
7515), a novel inhibitor of the JNK signaling pathway. J Neurosci.
18:104–111. 1998.PubMed/NCBI
|
|
8
|
Barone FC, Irving EA, Ray AM, Lee JC,
Kassis S, Kumar S, Badger AM, Legos JJ, Erhardt JA, Ohlstein EH, et
al: Inhibition of p38 mitogen-activated protein kinase provides
neuroprotection in cerebral focal ischemia. Med Res Rev.
21:129–145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yasuda S, Sugiura H, Tanaka H, Takigami S
and Yamagata K: p38 MAP kinase inhibitors as potential therapeutic
drugs for neural diseases. Cent Nerv Syst Agents Med Chem.
11:45–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Loo G, De Lorenzi R, Schmidt H, Huth
M, Mildner A, Schmidt-Supprian M, Lassmann H, Prinz MR and
Pasparakis M: Inhibition of transcription factor NF-kappaB in the
central nervous system ameliorates autoimmune encephalomyelitis in
mice. Nat Immunol. 7:954–961. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Niu YL, Zhang WJ, Wu P, Liu B, Sun GT, Yu
DM and Deng JB: Expression of the apoptosis-related proteins
caspase-3 and NF-kappaB in the hippocampus of Tg2576 mice. Neurosci
Bull. 26:37–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu L, Zhan Y, Wang Y, Feuerstein GZ and
Wang X: Recombinant adenoviral expression of dominant negative
IkappaBalpha protects brain from cerebral ischemic injury. Biochem
Biophys Res Commun. 299:14–17. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
van der Kooij MA, Nijboer CH, Ohl F,
Groenendaal F, Heijnen CJ, van Bel F and Kavelaars A: NF-kappaB
inhibition after neonatal cerebral hypoxia-ischemia improves
long-term motor and cognitive outcome in rats. Neurobiol Dis.
38:266–272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang JY, Shen J, Gao Q, Ye ZG, Yang SY,
Liang HW, Bruce IC, Luo BY and Xia Q: Ischemic postconditioning
protects against global cerebral ischemia/reperfusion-induced
injury in rats. Stroke. 39:983–990. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Callaway CW: Epinephrine for cardiac
arrest. Curr Opin Cardiol. 28:36–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakahara S, Tomio J, Nishida M, Morimura
N, Ichikawa M and Sakamoto T: Association between timing of
epinephrine administration and intact neurologic survival following
out-of-hospital cardiac arrest in Japan: A population-based
prospective observational study. Acad Emerg Med. 19:782–792. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakahara S, Tomio J, Takahashi H, Ichikawa
M, Nishida M, Morimura N and Sakamoto T: Evaluation of pre-hospital
administration of adrenaline (epinephrine) by emergency medical
services for patients with out of hospital cardiac arrest in Japan:
Controlled propensity matched retrospective cohort study. BMJ.
347:f68292013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lurie KG, Voelckel WG, Iskos DN, McKnite
SH, Zielinski TM, Sugiyama A, Wenzel V, Benditt D and Lindner KH:
Combination drug therapy with vasopressin, adrenaline (epinephrine)
and nitroglycerin improves vital organ blood flow in a porcine
model of ventricular fibrillation. Resuscitation. 54:187–194. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aoyagi T, Koshimizu TA and Tanoue A:
Vasopressin regulation of blood pressure and volume: Findings from
V1a receptor-deficient mice. Kidney Int. 76:1035–1039. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mayr VD, Wenzel V, Voelckel WG, Krismer
AC, Mueller T, Lurie KG and Lindner KH: Developing a vasopressor
combination in a pig model of adult asphyxial cardiac arrest.
Circulation. 104:1651–1656. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stadlbauer KH, Wagner-Berger HG, Wenzel V,
Voelckel WG, Krismer AC, Klima G, Rheinberger K, Pechlaner S, Mayr
VD and Lindner KH: Survival with full neurologic recovery after
prolonged cardiopulmonary resuscitation with a combination of
vasopressin and epinephrine in pigs. Anesth Analg. 96:1743–1749.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Varvarousi G, Johnson EO, Goulas S,
Agrogiannis G, Valsamakis N, Perrea D, Stefanadis C, Papadimitriou
L and Xanthos T: Combination pharmacotherapy improves neurological
outcome after asphyxial cardiac arrest. Resuscitation. 83:527–532.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kono S, Bito H, Suzuki A, Obata Y,
Igarashi H and Sato S: Vasopressin and epinephrine are equally
effective for CPR in a rat asphyxia model. Resuscitation.
52:215–219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen MH, Xie L, Liu TW, Song FQ and He T:
Naloxone and epinephrine are equally effective for cardiopulmonary
resuscitation in a rat asphyxia model. Acta Anaesthesiol Scand.
50:1125–1130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen MH, Liu TW, Xie L, Song FQ and He T:
Does naloxone alone increase resuscitation rate during
cardiopulmonary resuscitation in a rat asphyxia model? Am J Emerg
Med. 24:567–572. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
27
|
Lim C, Alexander MP, LaFleche G, Schnyer
DM and Verfaellie M: The neurological and cognitive sequelae of
cardiac arrest. Neurology. 63:1774–1778. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wenzel V, Linder KH, Augenstein S, Prengel
AW and Strohmenger HU: Vasopressin combined with epinephrine
decreases cerebral perfusion compared with vasopressin alone during
cardiopulmonary resuscitation in pigs. Stroke. 29:1462–1467,
Discussion 1467–1468. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Philpott KL and Facci L: MAP kinase
pathways in neuronal cell death. CNS Neurol Disord Drug Targets.
7:83–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sugino T, Nozaki K, Takagi Y, Hattori I,
Hashimoto N, Moriguchi T and Nishida E: Activation of
mitogen-activated protein kinases after transient forebrain
ischemia in gerbil hippocampus. J Neurosci. 20:4506–4514.
2000.PubMed/NCBI
|