Introduction
Acute-on-chronic liver failure (ACLF) is
characterized by an acute deterioration of liver function in
patients with chronic liver disease, which is usually induced by a
precipitating factor such as sepsis, alcohol or the rupture and
bleeding of upper gastrointestinal varicose veins (1,2).
Occasionally, however, no specific precipitating event can be
identified. Although the exact pathophysiology of ACLF development
is unclear, unregulated inflammation is considered to be a major
contributing factor (2).
Furthermore, clinical manifestations are often characterized by
severe gastrointestinal tract symptoms, rapidly increased jaundice,
massive ascites, hepatic encephalopathy, hepatorenal syndrome
(HRS), coagulopathy with severe bleeding tendency and rapidly
progressing multi-organ failure, which may require liver
transplantation (3).
Occurring in addition to pre-existing chronic liver
diseases, ACLF is a deterioration syndrome characterized by the
emergence of massive liver cell necrosis, accompanied by severe
hepatic dysfunction with an associated mortality rate of up to
60–80% (4).
Due to severe functional liver impairment, patients
with ACLF lack bioactive substances for life-sustaining activities
and are affected by an accumulation of toxins (5), which causes multiple organ failure.
Therefore, defining early and accurate prognostic factors for
patients with ACLF is critically important when selecting an
optimal treatment schedule. Scoring systems addressing the severity
of liver disease, such as the Child-Pugh score (6) or the model for end-stage liver disease
(MELD) (7), have been investigated.
Indeed, Xun et al (8)
reported that the integrated MELD (iMELD) and MELD with
incorporation of sodium (MELD-Na) models predicted 3-month
mortality rates more accurately than traditional MELD. Furthermore,
Shi et al (9) demonstrated
that iMELD predicted hepatic-ACLF more accurately when compared
with various other scoring systems (9). However, the prognostic assessment of
ACLF in patients with multiple organ failure remains to be
examined. The present study aimed to characterize patients with
ACLF to facilitate the early recognition of the syndrome and to
refine the prognostic assessment of ACLF.
Patients and methods
Patients and primary endpoint
Patients with ACLF admitted to the First Hospital of
Jilin University (Changchun, China) between June 2010 and June 2014
were retrospectively recruited. The definition of ACLF was based on
the following Asia Pacific Association for Study of Liver criteria
(10): Acute hepatic insult
manifesting as jaundice and coagulopathy, complicated within 4
weeks by ascites and/or encephalopathy in a patient with previously
diagnosed or undiagnosed chronic liver disease. Jaundice [≥5 mg/dl
serum bilirubin (85 µmol/l)] and coagulopathy [international
normalized ratio (INR) >1.5 or prothrombin activity (PTA)
<40%] are mandatory for defining ACLF. Patients with
hepatocellular carcinoma, arterial hypertension, coronary heart
disease, diabetes and infectious diseases (with the exception of
viral hepatitis) were excluded from the study.
In addition, the diagnosis of hepatic encephalopathy
was based on the West Haven criteria (11). All medical treatments were recorded,
including absolute bed rest, etiological (especially antiviral,
lamivudine or entecavir) treatment, symptomatic treatment of
complications, liver cell membrane protective agents, biliary
stimulators, artificial liver support system, corticosteroid
treatment and general supportive measures such as intravenous
albumin and plasma.
Patients were divided into two groups according to
their prognosis, namely favorable and unfavorable groups. These two
subgroups were defined with reference to the primary endpoint of
this study, which was hospital discharge or mortality. This
investigation was approved by the Regional Ethics Committee of the
First Hospital of Jilin University and all patients signed written
informed consent forms.
Laboratory and clinical analysis
All patients with ACLF had fasting blood samples
drawn within 24 h of admission for assessment of liver function.
Blood samples were analyzed for levels of the following: Total bile
acid; γ-globulins; prealbumin; thrombin time (TT); hemoglobin;
serum sodium; alanine aminotransferase; aspartate aminotransferase;
alkaline phosphatase; γ-glutamyltranspeptidase; albumin (ALB);
total bilirubin (TBIL); cholinesterase; blood urea nitrogen; serum
creatinine (SCr); prothrombin time (PT); international normalized
ratio (INR); plasma prothrombin activity (PTA); white blood cell
count; platelet count (PLT); fasting blood glucose (FBS); and
α-fetoprotein. Incidence of hepatic encephalopathy, bacterial or
fungal infection, gastrointestinal bleeding, hepatorenal syndrome
and electrolyte disturbance were also recorded. Hepatic
encephalopathy is a clinical condition, characterized by the
presence of cerebral dysfunction in patients with liver disease
(11). To determine the presence of
bacterial or fungal infections, laboratory examination of swab
cultures was conducted. Infections included spontaneous
peritonitis, pneumonia and intestinal, oral cavity and urinary
tract infections. Gastrointestinal bleeding was determined by the
presence of the symptoms of hematemesis or hematochezia.
Hepatorenal syndrome was diagnosed as renal insufficiency (a plasma
creatinine level >1.5 mg/dl) that progressed over days or weeks
in the presence of severe liver disease, and in the absence of
recognized nephrotoxic agents. Finally, electrolyte disturbance was
assessed as two or more electrolyte disorders, such as hyperkalemia
and hyponatremia, occurring simultaneously.
Statistical analysis
All data are presented as mean values ± standard
deviation for continuous variables, and qualitative variables as
proportions with percentages. The association between ACLF
prognosis and biochemical indices or clinical complications was
determined by univariate analyses, and the forward Wald approach
was used for multivariate logistic regression models. Univariate
analyses were as follows: A t-test, for normal distributions; a
rank sum test, for non-normal distributions; and a chi-squared
test, for comparison of complications between the groups.
Furthermore, a comparative study of various liver function scoring
systems, including Child-Pugh, MELD, MELD-Na, serum sodium ratio
(MESO), and iMELD was performed (6–8,12). To compare the predictive values of
the various prognostic scoring systems, areas under the receiver
operating curve (ROC) were calculated. Statistical analyses were
performed using SPSS software version 18.0 (SPSS, Inc., Chicago,
IL, USA). A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Etiologies of ACLF
A total of 164 patients with ACLF were recruited
into the study. Hepatitis B virus (HBV) infection was the leading
etiology of ACLF, followed by alcoholism, as shown in Table I. Overlapping causes accounted for
the occurrence of ACLF in numerous patients, including 7 cases of
HBV infection associated with alcoholism, 6 cases of HBV associated
with drug-induced liver damage, 4 cases of alcoholic liver damage
associated with drug-induced liver damage, 2 cases of hepatitis C
(HCV) infection associated with alcohol liver damage, 1 case of
alcoholic liver damage associated with hepatitis E infection and 1
case of HCV infection associated with drug-induced liver
damage.
 | Table I.Etiological factors for
acute-on-chronic liver failure. |
Table I.
Etiological factors for
acute-on-chronic liver failure.
| Etiology | Cases (%) |
|---|
| Hepatitis B
infection | 88 (53.7) |
| Hepatitis C
infection | 3 (1.8) |
| Alcoholic liver
damage | 38 (23.2) |
| Drug-induced liver
damage | 5 (3.0) |
| Auto-immune liver
damage | 4 (2.4) |
| Cryptogenic liver
damage | 5 (3.0) |
| Overlapping
causes | 21 (12.8) |
Favorable and unfavorable group
inclusion
A total of 45 individuals were included in the
favorable group and 119 in the unfavorable group.
Associations of gender and age with
prognosis
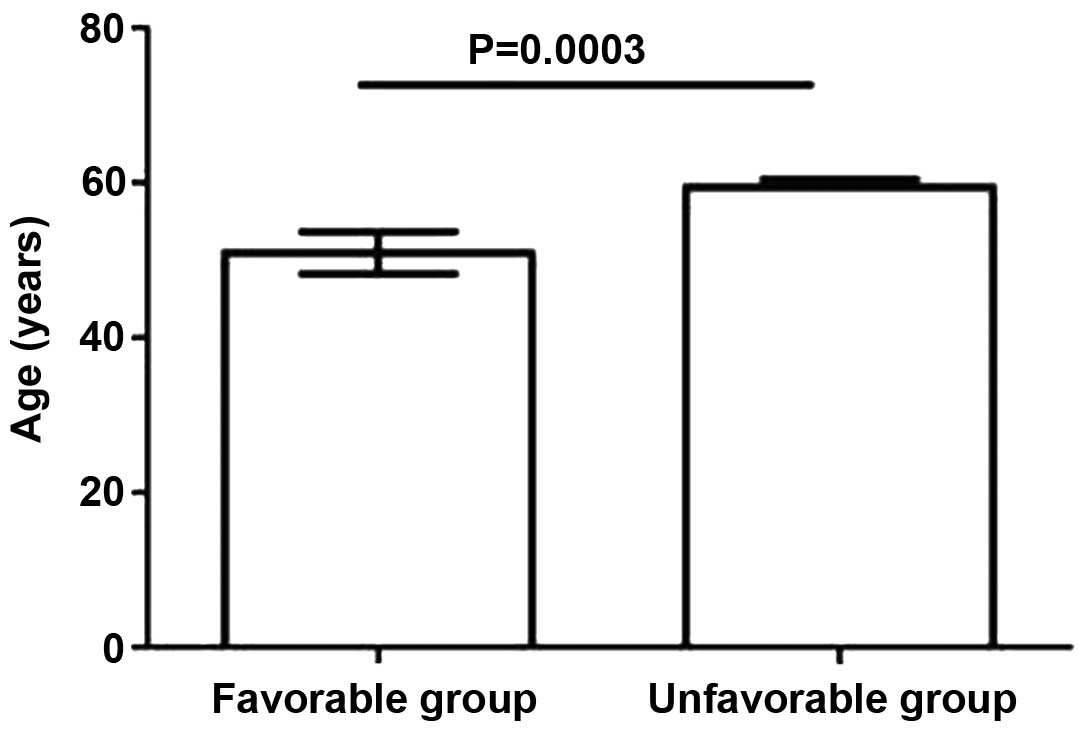
No statistically significant differences in
prognosis were revealed to be associated with gender. Conversely,
the results demonstrated that an older age was significantly
(P<0.05) associated with a poorer prognosis. Logistic regression
analysis demonstrated that age was an independent prognostic factor
for ACLF (P<0.05; Fig. 1).
Comparison of biochemical parameters
between the two subgroups
Statistically significant differences between the
favorable and unfavorable subgroups were observed in a number of
biochemical parameters. Higher TT, TBIL, SCr, PT and INR values and
lower serum sodium, ALB, PTA, PLT and FBS values were associated
with a poor prognosis (P<0.05; Table
II). The other measured parameters revealed no significant
change.
 | Table II.Comparison of serum biochemical
parameters between the favorable and unfavorable subgroups. |
Table II.
Comparison of serum biochemical
parameters between the favorable and unfavorable subgroups.
| Parameters | Favorable group
(n=45) | Unfavorable group
(n=119) | P-value |
|---|
| GLO, g/l | 29.9±5.8 | 33.7±7.9 |
0.132 |
| TBA, µmol/l | 200.8±115.3 | 253.2±82.5 |
0.063 |
| PA, g/l | 0.12±0.04 | 0.14±0.11 |
0.564 |
| TT, sec | 21.8±2.6a | 26.6±4.4 | <0.001 |
| HB, g/l | 115.6±26.8 | 111.2±29.5 |
0.490 |
| Na+,
mmol/l |
134.3±4.5a | 125.5±5.5 | <0.001 |
| ALT, IU/l | 143.0
(31.0–325.0) | 103.0
(44.3–195.3) |
0.603 |
| AST, IU/l | 139.0
(63.5–217.5) | 136.5
(66.5–242.0) |
0.734 |
| ALP, IU/l | 117.0
(93.0–168.5) | 122.5
(94.5–169.7) |
0.938 |
| GGT, IU/l | 79.3
(28.0–148.0) | 66.0
(36.5–133.8) |
0.820 |
| ALB, g/l | 28.1
(27.0–32.1)a | 25.3
(21.2–26.9) | <0.001 |
| TBIL, µmol/l | 416.1
(230.1–512.3a | 501.3
(352.8–656.1) | <0.001 |
| CHE, IU/l | 2463.3
(1835.5–3933.5) | 2202.5
(1635.3–2660.5) |
0.080 |
| BUN, mmol/l | 4.8 (3.7–5.6) | 6.5 (3.6–11.8) |
0.071 |
| SCr, µmol/l | 60.2
(48.0–76.2)a | 98.0
(78.3–151.6) | <0.001 |
| PT, sec | 23.1
(21.6–24.9)a | 28.8
(23.1–35.6) | <0.001 |
| INR | 1.7
(1.6–2.0)a | 2.6 (2.1–3.1) | <0.001 |
| PTA, % | 37.0
(32.4–38.3)a | 31.0
(22.9–37.0) |
0.026 |
| WBC,
×109/l | 7.4 (4.7–9.9) | 6.7 (4.8–11.9) |
0.605 |
| PLT,
×109/l | 90.5
(59.0–127.5)a | 68.5
(52.3–79.8) |
0.025 |
| FBS, mmol/l | 4.9
(4.2–5.6)a | 4.3 (3.4–4.9) |
0.019 |
| AFP, ng/ml | 15.1
(3.5–152.2) | 16.7
(3.87–59.3) |
0.922 |
Comparison of complications between
the two subgroups
The incidence of bacterial or fungal infection
(including spontaneous peritonitis, pneumonia, intestinal
infection, oral cavity fungal infection, and urinary tract
infections), hepatic encephalopathy, HRS and electrolyte
disturbance were significantly associated with the prognosis of
patients with ACLF (P<0.05; Table
III).
 | Table III.Comparison of complications between
the favorable and unfavorable subgroups [n (%)]. |
Table III.
Comparison of complications between
the favorable and unfavorable subgroups [n (%)].
| Complications | Total cases
(n=164) | Favorable group
(n=45) | Unfavorable group
(n=119) | χ2 | P-value |
|---|
| Infection | 108 (65.9) | 16 (35.6) | 92 (77.3) | 10.221 | 0.001 |
| HE | 63 (38.4) | 8 (17.8) | 55 (46.2) | 6.023 | 0.012 |
| HRS | 42 (25.6) | 5 (11.1) | 37 (31.1) | 10.829 | 0.001 |
| UGIB | 26 (15.9) | 6 (13.3) | 20 (16.8) | 0.509 | 0.474 |
| Electrolyte
disturbance | 122 (74.3) | 20 (44.4) | 102 (85.7) | 14.969 | <0.001 |
Comparative data based on multivariate
analysis
Logistic regression analysis identified that age,
hyponatremia, INR HRS and bacterial or fungal infection were
independent prognostic factors for ACLF (Table IV).
 | Table IV.Logistic regression analysis of 164
patients with ACLF. |
Table IV.
Logistic regression analysis of 164
patients with ACLF.
|
|
|
|
|
|
| 95% CI of OR |
|---|
|
|
|
|
|
|
|
|
|---|
| Factors | B | SE | χ2
value | P-value | OR | Lower limit | Upper limit |
|---|
| INR |
4.794 | 1.804 | 7.074 | 0.008 | 120.591 | 3.525 | 4.125 |
| Age |
0.225 | 0.088 | 6.769 | 0.007 | 1.253 | 1.057 | 1.486 |
| Hyponatremia | −0.389 | 0.128 | 9.348 | 0.002 | 0.679 | 0.527 | 0.871 |
| HRS |
1.096 | 0.002 | 4.621 | 0.031 | 2.511 | 1.533 | 4.702 |
| Infection |
2.934 | 1.289 | 5.196 | 0.024 | 18.827 | 1.507 | 234.838 |
Comparison of liver function scoring
systems between the two subgroups
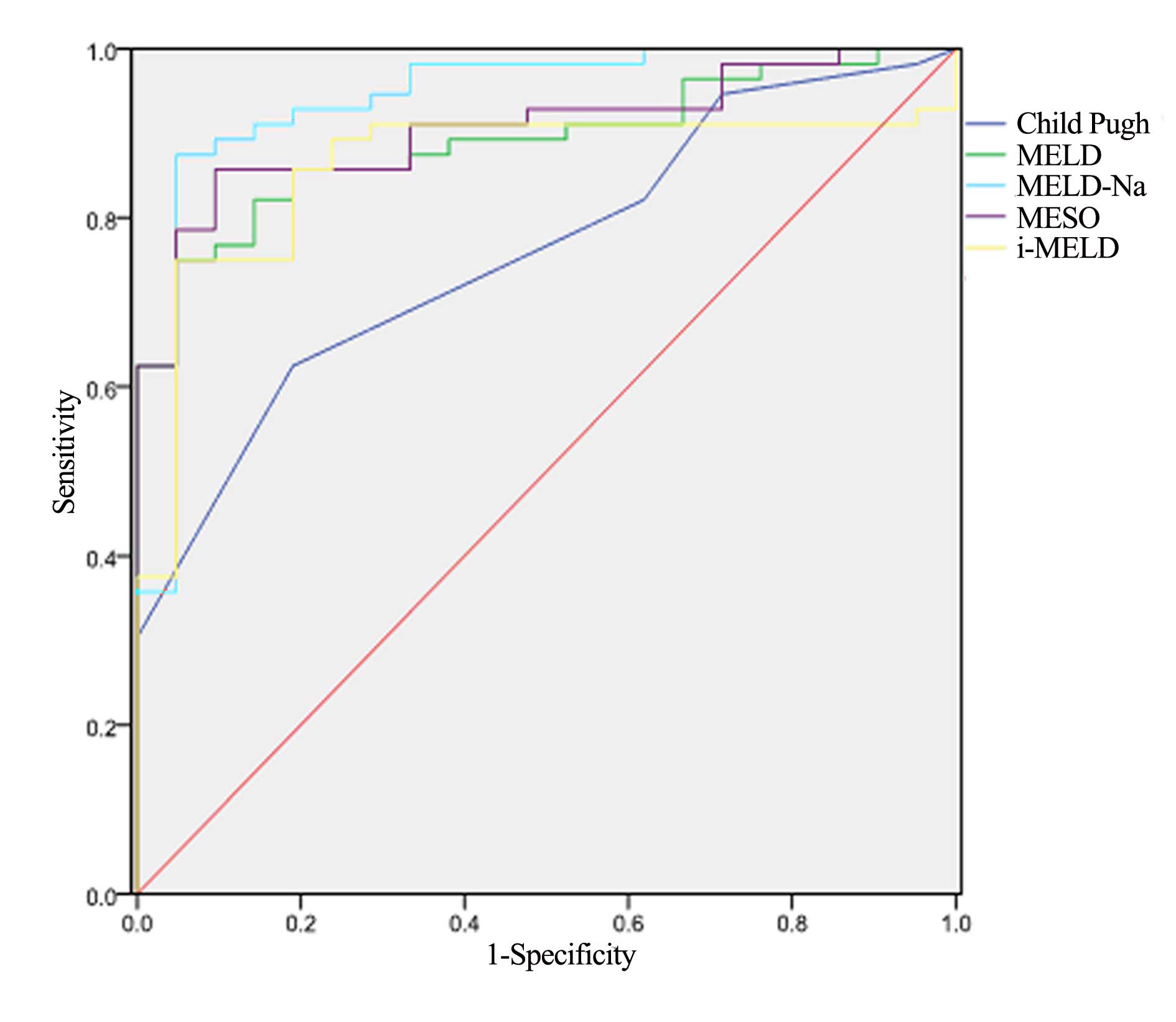
The five scoring systems (Child-Pugh, MELD, MELD-Na,
MESO and iMELD) produced results that were significantly different
between the favorable and unfavorable groups (P<0.001; Table V). ROC curves were created to
evaluate the predictive value of the five scoring systems. All
scores provided good predictive values, with areas under the curves
(AUCs) for the Child-Pugh, MELD, MELD-Na, MESO and iMELD scoring
systems of 0.760, 0.890, 0.940, 0.907 and 0.860, respectively.
However, the MELD-Na score had a significantly higher predictive
value compared with the other scoring systems (Table VI; Fig.
2).
 | Table V.Comparison of liver function scoring
systems between favorable and unfavorable subgroups (mean ±
standard deviation). |
Table V.
Comparison of liver function scoring
systems between favorable and unfavorable subgroups (mean ±
standard deviation).
| Prediction
models | Favorable group
(n=45) | Unfavorable group
(n=119) | P-value |
|---|
| Child-Pugh | 11.3±1.2 | 12.9±1.4 | <0.001 |
| MELD | 19.8±4.4 | 29.5±6.9 | <0.001 |
| MELD-Na | 20.2±7.1 |
43.5±13.9 | <0.001 |
| MESO | 14.6±3.3 | 23.2±5.9 | <0.001 |
| iMELD | 40.4±6.3 |
55.7±12.8 | <0.001 |
 | Table VI.Optimal threshold, sensitivity,
specificity, areas under the curve, and 95% confidence interval of
the five scoring systems for prognostic evaluation of patients with
ACLF. |
Table VI.
Optimal threshold, sensitivity,
specificity, areas under the curve, and 95% confidence interval of
the five scoring systems for prognostic evaluation of patients with
ACLF.
| Prediction
models | Optimal
threshold | Sensitivity
(%) | Specificity(%) | Area under the
curve | 95% CI |
|---|
| Child-Pugh | 12.6 | 0.625 | 0.811 | 0.760 | (0.650, 0.871) |
| MELD | 26.1 | 0.750 | 0.952 | 0.890 | (0.819, 0.961) |
| MELD-Na | 27.3 | 0.875 | 0.952 | 0.940 | (0.868, 1.000) |
| MESO | 18.2 | 0.858 | 0.906 | 0.907 | (0.839, 0.973) |
| iMELD | 48.3 | 0.750 | 0.952 | 0.860 | (0.770, 0.949) |
Discussion
ACLF is a severe condition associated with various
etiological factors (13), including
viral infection, chronic alcohol abuse, use of illicit drugs and
autoimmune liver disease. In the investigated cohort, the
predominant causes of ACLF were HBV infection, chronic alcohol
abuse, and various combinations of other etiologies. The prevailing
role of HBV in the occurrence of ACLF was expected due to the high
prevalence of HBV in China (14).
Alcoholism and alternative combined etiologies are the two other
main types of etiological factors contributing to the disease
occurrence.
In the present study, patients with ACLF of older
age groups, with higher PT, TT, INR, TBIL and SCr and lower serum
sodium, PTA, ALB, PLT and FBS were more likely to have a poor
prognosis. Complications such as bacterial or fungal infection,
hepatic encephalopathy, HRS and electrolyte disturbance were also
associated with poor prognosis. The results of the present
investigation are concordant with those of Lal et al
(15), who reported that high INR
was an independent ACLF prognostic factor.
Cellular immunity is impaired in patients with ACLF,
increasing the risk of infection and the infection-associated
mortality rate (16). Accordingly,
the results of this study illustrate a high frequency of infections
(observed in 108 cases) and demonstrate that bacterial or fungal
infections are an independent prognostic factor for ACLF.
Encephalopathy is another severe ACLF complication
(17–19), and was observed in 63 of the cases
analyzed (38.4%). A number of the identified complications,
including infection and electrolyte abnormalities, arise relatively
suddenly, and may exacerbate the disturbances attributable to liver
failure or exert a direct effect on the brain (20).
Upper gastrointestinal hemorrhage is a critical
complication associated with ACLF; it is predominantly associated
with high intravascular pressure, blood coagulation dysfunction and
esophageal gastric varices, which exist prior to the occurrence of
the bleeding (21). Through blood
volume reduction, organ ischemia and hypoxia, multiple organ
function failure may occur (22).
Upper gastrointestinal bleeding was observed in 15.9% of the
patients in the present study, and was not a prognostic factor.
HRS was identified as a strong prognostic risk
factor within the present cohort. HRS corresponds to functional
renal failure. In ACLF, renal vascular resistance increases
progressively causing renal hypoperfusion. Furthermore,
self-regulation of the renal perfusion function is also affected,
and a small decrease in blood volume may lead to marked reduced
renal perfusion, a further factor associated with kidney damage
(23).
A total of 122 cases (74.3%) of electrolyte
disturbances, such as hyperkalemia and/or hyponatremia, were
observed. These were predominantly associated with insufficient
intake of food nutrients, vomiting, diarrhea, digestive disorders,
long-term use of diuretics or a large amount of ascites drainage,
long-term application of hypertonic glucose liquid and secondary
aldosteronism. The present study demonstrated that the incidence of
electrolyte disorder was significantly higher in the unfavorable
group, as compared with the favorable group.
The most common type of electrolyte disturbances
associated with patients with ACLF in the present study was
hyponatremia, which was found to be an independent prognostic
factor. Hyponatremia is primarily the result of solute-free water
retention in liver cirrhosis. The proposed mechanism underlying
this process is an association between the release of antidiuretic
hormones and splanchnic arterial vasodilatation leading to reduced
systemic vascular resistance (24–26).
The Child-Pugh score is the most commonly used
evaluation system to assess hepatic reserve function in cirrhotic
patients, facilitating evaluation of their prognosis. However, the
Child-Pugh score, initially designed for patients with
portosystemic shunt surgery, is associated with certain
difficulties and inaccuracies (27,28). The
Child-Pugh classification uses TBIL, ALB, PT, presence of ascites
and encephalopathy to reach a score. However, ALB, ascites and
encephalopathy are subject to medical intervention. Furthermore,
the Child-Pugh classification does not recognize a TBIL level
>51 µmol/l. Additionally, ALB levels tend to be low immediately
subsequent to bleeding or transfusions (29). The MELD scoring system was developed
by the Mayo Clinic team, and was originally used to predict the
prognosis of portal hypertension patients following transjugular
intrahepatic portosystemic shunt (27). Kumar et al (30) reported that a MELD score that did not
decrease by week 2 generated a 93.8% predictive chance of survival
for the following 60 days. Ruf et al (31) demonstrated that, in ACLF,
hyponatremia and the MELD score were risk factors that may affect
the mortality of patients with liver failure, and supported the
hypothesis that comprehensive serum sodium and a MELD score may
provide a more accurate predictive approach. The MELD-Na model,
established by Biggins et al (32), exhibited a more optimal predictive
capacity than the MELD model. Using the MELD-Na model, Huo et
al (12) established the MESO
model, a non-invasive predictor of increased portal pressure in
cirrhosis, which was superior to the MELD score in predicting
patient mortality (12). Luca et
al (33) proposed the addition
of age and serum sodium concentrations to the MELD score system,
resulting in the iMELD system, which allowed for enhancement of its
predictive capacity. In the present study, all five scoring systems
were validated as prognostic indicators for ACLF patients.
When establishing the accuracy of survival rate
prediction at three months, the scoring systems with the better
diagnostic or predictive value were those with the greater AUC of
the ROC curve. In the present study, when considering that
AUC>0.7 is the accepted threshold for clinical application; the
five scoring systems demonstrated accurate predictive values for
ACLF prognosis. Notably, the MELD-Na score was the most effective
scoring system. Therefore, incorporating the serum sodium level
into the MELD score would significantly improve the prediction
accuracy of the prognosis of patients with ACLD.
In conclusion, age, hyponatremia, INR, HRS and
bacterial or fungal infection were identified to be independent
risk factors associated with ACLF prognosis. The MELD-Na score was
the most efficient liver function evaluation system. The results of
the present study may facilitate the prognostic assessment of
patients with ACLF, and lead to improved overall management of this
severe liver condition.
References
|
1
|
Fan HL, Yang PS, Chen HW, Chen TW, Chan
DC, Chu CH, Yu JC, Kuo SM and Hsieh CB: Predictors of the outcomes
of acute-on-chronic hepatitis B liver failure. World J
Gastroenterol. 18:5078–5083. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jalan R, Gines P, Olson JC, Mookerjee RP,
Moreau R, Garcia-Tsao G, Arroyo V and Kamath PS: Acute-on chronic
liver failure. J Hepatol. 57:1336–1348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wigg AJ, McCormick R, Wundke R and Woodman
RJ: Efficacy of a chronic disease management model for patients
with chronic liver failure. Clin Gastroenterol Hepatol.
11:850–858.e4. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finkenstedt A, Nachbaur K, Zoller H,
Joannidis M, Pratschke J, Graziadei IW and Vogel W:
Acute-on-chronic liver failure: Excellent outcomes after liver
transplantation but high mortality on the wait list. Liver Transpl.
19:879–886. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Katoonizadeh A, Laleman W, Verslype C,
Wilmer A, Maleux G, Roskams T and Nevens F: Early features of
acute-on-chronic alcoholic liver failure: A prospective cohort
study. Gut. 59:1561–1569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lucey MR, Brown KA, Everson GT, Fung JJ,
Gish R, Keeffe EB, Kneteman NM, Lake JR, Martin P, McDiarmid SV, et
al: Minimal criteria for placement of adults on the liver
transplant waiting list: A report of a national conference
organized by the American Society of Transplant Physicians and the
American Association for the Study of Liver Diseases. Liver Transpl
Surg. 3:628–637. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kamath PS, Wiesner RH, Malinchoc M,
Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER and Kim
WR: A model to predict survival in patients with end-stage liver
disease. Hepatology. 33:464–470. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xun YH, Shi JP, Li CQ, Li D, Shi WZ, Pan
QC, Guo JC and Zang GQ: Prognostic performance of a series of model
for end-stage liver disease and respective Δ scores in patients
with hepatitis B acute-on-chronic liver failure. Mol Med Rep.
9:1559–1568. 2014.PubMed/NCBI
|
|
9
|
Shi Y, Yang Y, Hu Y, Wu W, Yang Q, Zheng
M, Zhang S, Xu Z, Wu Y, Yan H and Chen Z: Acute-on-chronic liver
failure precipitated by hepatic injury is distinct from that
precipitated by extrahepatic insults. Hepatology. 62:232–242. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sarin SK, Kumar A, Almeida JA, Chawla YK,
Fan ST, Garg H, de Silva HJ, Hamid SS, Jalan R, Komolmit P, et al:
Acute-on-chronic liver failure: Consensus recommendations of the
Asian Pacific Association for the Study of the Liver (APASL).
Hepatol Int. 3:269–282. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferenci P, Lockwood A, Mullen K, Tarter R,
Weissenborn K and Blei AT: Hepatic encephalopathy - definition,
nomenclature, diagnosis and quantification: Final report of the
Working Party at the 11th World Congresses of Gastroenterology,
Vienna, 1998. Hepatology. 35:716–721. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huo TI, Wang YW, Yang YY, Lin HC, Lee PC,
Hou MC, Lee FY and Lee SD: Model for end-stage liver disease score
to serum sodium ratio index as a prognostic predictor and its
correlation with portal pressure in patients with liver cirrhosis.
Liver Int. 27:498–506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee W, Squires RH Jr, Nyberg SL, Doo E and
Hoofnagle JH: Acute liver failure: Summary of a workshop.
Hepatology. 47:1401–1415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ostapowicz G, Fontana RJ, Schiødt FV,
Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE,
Hynan L, et al: Results of a prospective study of acute liver
failure at 17 tertiary care centers in the United States. Ann
Intern Med. 137:947–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lal J, Thapa BR, Rawal P, Ratho RK and
Singh K: Predictors of outcome in acute-on-chronic liver failure in
children. Hepatol Int. 5:693–697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wasmuth HE, Kunz D, Yagmur E,
Timmer-Stranghöner A, Vidacek D, Siewert E, Bach J, Geier A,
Purucker EA, Gressner AM, et al: Patients with acute on chronic
liver failure display ‘sepsis-like’ immune paralysis. J Hepatol.
42:195–201. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Crippin JS, Gross JB Jr and Lindor KD:
Increased intracranial pressure and hepatic encephalopathy in
chronic liver disease. Am J Gastroenterol. 87:879–882.
1992.PubMed/NCBI
|
|
18
|
Jalan R, Dabos K, Redhead DN, Lee A and
Hayes PC: Elevation of intracranial pressure following transjugular
intrahepatic portosystemic stent-shunt for variceal haemorrhage. J
Hepatol. 27:928–933. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Donovan JP, Schafer DF, Shaw BW Jr and
Sorrell MF: Cerebral edema and increased intracranial pressure in
chronic liver disease. Lancet. 351:719–721. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Olson JC, Wendon JA, Kramer DJ, Arroyo V,
Jalan R, Garcia-Tsao G and Kamath PS: Intensive care of the patient
with cirrhosis. Hepatology. 54:1864–1872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cremers I and Ribeiro S: Management of
variceal and nonvariceal upper gastrointestinal bleeding in
patients with cirrhosis. Therap Adv Gastroenterol. 7:206–216. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bumaschny E, Doglio G, Pusajó J, Vetere L,
Parra C, Grosso RM and Schieppati E: Postoperative acute
gastrointestinal tract hemorrhage and multiple-organ failure. Arch
Surg. 123:722–726. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Solís-Muñoz P: Acute on chronic liver
failure and prognostic factors: Time for reevaluation. Rev Esp
Enferm Dig. 103:169–176. 2011.PubMed/NCBI
|
|
24
|
Wiesner R, Edwards E, Freeman R, Harper A,
Kim R, Kamath P, Kremers W, Lake J, Howard T, Merion RM, et al:
Model for end-stage liver disease (MELD) and allocation of donor
livers. Gastroenterology. 124:91–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Arroyo V and Colmenero J: Ascites and
hepatorenal syndrome in cirrhosis: Pathophysiological basis of
therapy and current management. J Hepatol. 38(Suppl 1): S69–S89.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schrier RW, Gurevich AK and
Cadnapaphornchai MA: Pathogenesis and management of sodium and
water retention in cardiac failure and cirrhosis. Semin Nephrol.
21:157–172. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Malinchoc M, Kamath PS, Gordon FD, Peine
CJ, Rank J and ter Borg PC: A model to predict poor survival in
patients undergoing transjugular intrahepatic portosystemic shunts.
Hepatology. 31:864–871. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Conn HO: A peek at the Child-Turcotte
classification. Hepatology. 1:673–676. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Al Sibae MR and Cappell MS: Accuracy of
MELD scores in predicting mortality in decompensated cirrhosis from
variceal bleeding, hepatorenal syndrome, alcoholic hepatitis, or
acute liver failure as well as mortality after non-transplant
surgery or TIPS. Dig Dis Sci. 56:977–987. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kumar R, Krishnamoorthy TL, Tan HK, Lui HF
and Chow WC: Change in model for end-stage liver disease score at
two weeks, as an indicator of mortality or liver transplantation at
60 days in acute-on-chronic liver failure. Gastroenterol Rep.
3:122–127. 2015. View Article : Google Scholar
|
|
31
|
Ruf AE, Kremers WK, Chavez LL, Descalzi
VI, Podesta LG and Villamil FG: Addition of serum sodium into the
MELD score predicts waiting list mortality better than MELD alone.
Liver Transpl. 11:336–343. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Biggins SW, Kim WR, Terrault NA, Saab S,
Balan V, Schiano T, Benson J, Therneau T, Kremers W, Wiesner R, et
al: Evidence-based incorporation of serum sodium concentration into
MELD. Gastroenterology. 130:1652–1660. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luca A, Angermayr B, Bertolini G, Koenig
F, Vizzini G, Ploner M, Peck-Radosavljevic M, Gridelli B and Bosch
J: An integrated MELD model including serum sodium and age improves
the prediction of early mortality in patients with cirrhosis. Liver
Transpl. 13:1174–1180. 2007. View
Article : Google Scholar : PubMed/NCBI
|