Introduction
Sweet disease, which is also known as acute febrile
neutrophilic dermatosis, is a multisystemic inflammatory disease
that was first described in 1964 (1). It is characterized by fever, peripheral
neutrophil leukocytosis and tender erythematous skin lesions that
heal without scarring. The skin lesions of Sweet disease are
commonly asymmetrical and are predominantly located on the face,
neck and upper extremities (1).
Cutaneous manifestations of the disease may be preceded by a fever
lasting from several days to weeks (2). Skin biopsies of cutaneous edematous
erythematous plaques reveal deep dermal infiltration of mature
neutrophils and the absence of vasculitis (3). Several cases of Sweet disease with CNS
involvement have been reported (4–7) and
neuro-Sweet disease (NSD) has been proposed as a novel concept
(8). Although the aetiology of NSD
remains elusive, it has previously been suggested that NSD is a
distinct clinical entity that may account for various cases of
idiopathic encephalitis or meningitis (8,9).
Systemic corticosteroid therapy is highly effective for the
treatment of the majority of the neurologic symptoms detected in
patients with NSD (7,10,11). It
may be useful to classify NSD as benign, however, there is a high
frequency of symptom relapse (8,9). In the
present case report, a rare case of NSD with normal brain magnetic
resonance imaging (MRI) and positive modified acid-fast staining of
the cerebrospinal fluid (CSF). The patient's meningitis and skin
lesions were successfully treated with systemic corticosteroids,
and antiviral and anti-tuberculotic combination therapy.
Case report
In July 2013, a previously healthy 30-year-old
Chinese man presented with headache, neck stiffness and fever at
the First Affiliated Hospital of Xi'an Jiaotong University (Xi'an,
China). Although these symptoms were alleviated following treatment
with antibiotics, including 1 g compound polymyxin B ointment
(Zhejiang Reachall Pharmaceutical Co., Ltd., Zhejiang, China)
administered thrice daily and 3.75 g intravenous mezlocillin sodium
and sulbactam sodium (Shanxi C&Y Pharmaceutical Group Co.,
Ltd., Datong, China) administered twice daily, several painful
nodules subsequently developed on the patient's forehead. Two days
later, the patient's headache and neck stiffness symptoms had
worsened and intermittent fever (38–39°C) persisted. Nodules
subsequently developed on the patient's face, neck and chest;
therefore, antiviral drugs, including 0.3 g ribavirin granules
(Sichuan Baili Pharmaceutical Co., Ltd., Chengdu, China) twice
daily and acyclovir ointment (q.s.; Fujian Pacific Pharmaceutical
Co., Ltd., Jinjiang, China) every 4 h,. were administered for a
presumed herpes zoster infection; however, the symptoms did not
improve.
The patient was then admitted to Xijing Hospital
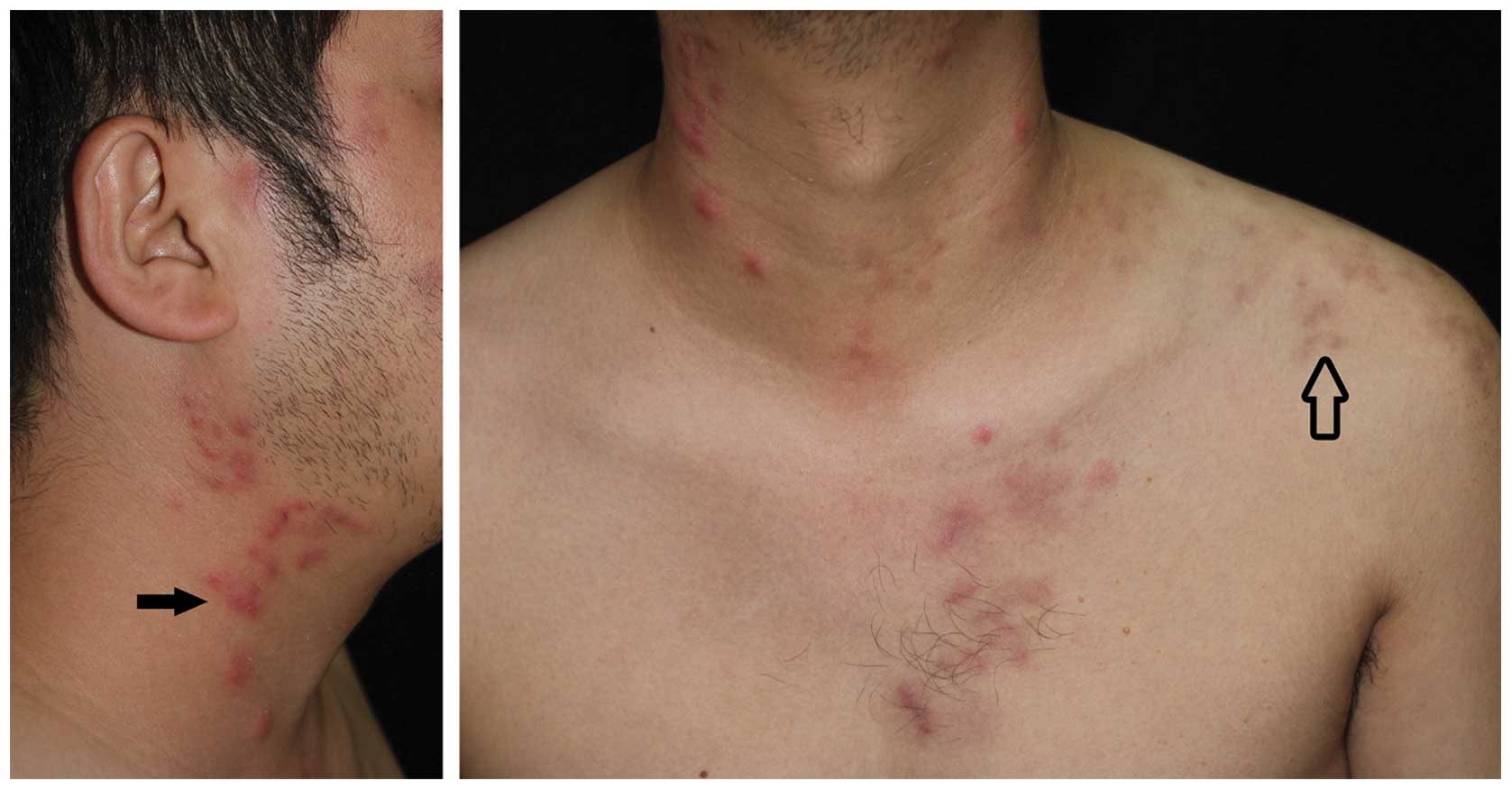
(Xi'an, China) on 22 July, 2013. Upon physical examination, raised
tender erythematous plaques were detected on the face, neck and
upper trunk of the patient. Pigmentation on the left shoulder was
also observed (Fig. 1).
Routine laboratory tests revealed the patient's
white blood cell (WBC) count was 10.36×109/l (normal
range, 3.5–9.5×109/l) with 82.4% neutrophils, the
erythrocyte sedimentation rate (ESR) was 99 mm/h (normal range,
0–20 mm/h), and the serum C-reactive protein (CRP) level was 132
mg/l (normal range, 0–5 mg/l). Serological tests demonstrated that
the patient was positive for antibodies to Epstein-Barr virus;
whereas tests for antibodies to hepatitis B and C viruses, human
immunodeficiency virus and Treponema pallidum were negative.
The lipopolysaccharide (LPS) level was 289 pg/ml (normal range,
0–10 pg/ml) and the 1,3-β-D-glucan level was within the normal
range. Blood culture for bacteria and T-spot tuberculosis tests
were negative. Extensive autoimmune screening revealed that the
patient was positive for antinuclear antibodies, however he did not
present with any of the typical symptoms of either systemic lupus
erythematosus, mixed connective tissue disease, rheumatoid
arthritis or dry syndrome; therefore this result was considered
insignificant. Tumor marker tests revealed no evidence of
malignancy.
A subsequent lumbar puncture demonstrated a pressure
of >300 mmH2O, and cytological analysis indicated
that the CSF contained 80 leukocytes/mm3 (83%
lymphocytes; 8.5% mononuclear cells; 6% neutrophilic cells; and
2.5% plasma cells). These results were outside of the normal range
and were outside of the normal range, suggesting inflammation in
the patient's central nervous system. Immunoglobulin (Ig)M levels
were raised at 2.69 mg/l (normal range, 0–1.0 mg/l), which
indicated possible acute viral meningitis. Protein, glucose and
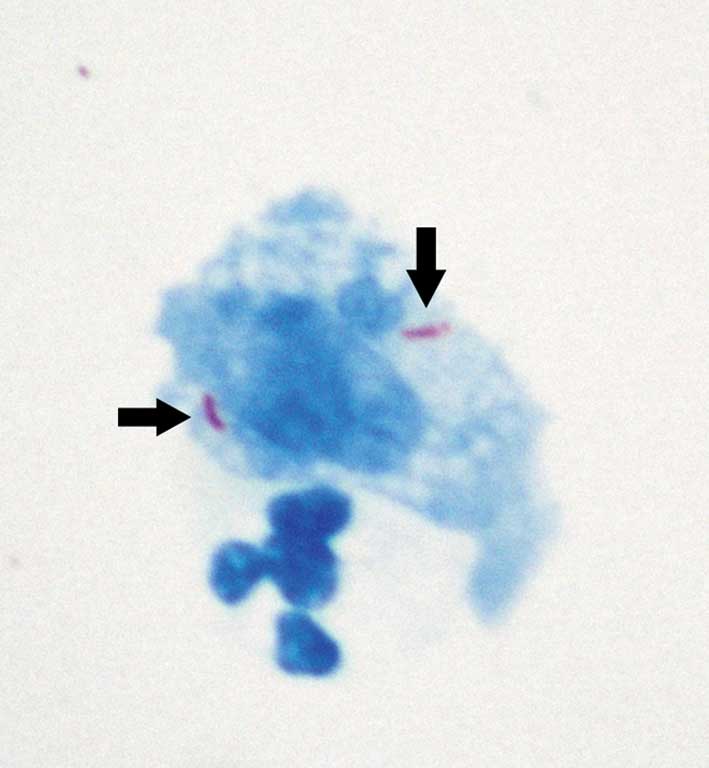
chloride levels were normal. A modified acid-fast stain test was
performed on the CSF sample according to the staining methods
described by Feng et al (12), which gave a positive result (Fig. 2). Alcian blue staining demonstrated
that the CSF was negative for Cryptococcus spp. Tests for
antibodies against common viruses were negative, and subsequent
brain MRI, electroencephalogram and X-ray examination of the chest
did not detect any abnormalities.
Since these results initially suggested the patient
was suffering from viral meningitis, intravenous ganciclovir (750
mg/day for 15 days) and dexamethasone (20 mg/day for 5 days, then
10 mg/day for 5 days) was administered, followed by oral prednisone
acetate tablet (40 mg/day, gradually tapered by 10 mg weekly). On
25 July 2013, a biopsy was taken from one of the erythematous
plaques on the left side of the patient's neck. The tissue sample
was paraffin-embedded and serial 3 µm-thick sections were cut and
subsequently stained with hematoxylin and eosin. Furthermore, the
positive CSF modified acid-fast stain result indicated infection
with Mycobacterium tuberculosis; therefore the patient
received anti-tuberculotic therapy whilst the results of the skin
biopsy were awaited. Anti-tuberculotic therapy included 0.45 g
rifampicin capsules (q.d.), 0.5 g pyrazinamide tablets (both
Chengdu Jinhua Pharmaceutical Co., Ltd., Chengdu, China) thrice
daily, 0.6 g isoniazid (q.d.; Tianjin Kingyork Group Co., Ltd.,
Tianjin, China) via an intravenous drip, ganciclovir (Hidragon
Pharmaceutical Co., Ltd., Shanghai, China), dexamethasone (Cisen
Pharmaceutical Co., Ltd., Jining, China) and prednisone acetate
(Tianyao Pharmaceutical Co., Ltd., Tianjin, China).
Following 3 days of treatment, the fever was
alleviated, and a second lumbar puncture indicated a pressure of
225 mmH2O, and CSF analysis detected 27
leukocytes/mm3 (96.5% lymphocytes; 3.5% mononuclear
cells; and 0% neutrophilic cells and plasma cells). The IgM level
was low at 1.22 mg/l, and the CSF modified acid-fast stain was
negative. The WBC count was 8.90×109/l (70.2%
neutrophils; 26.5% lymphocytes; 3.1% monocytes; and 0.2%
eosinophils), LPS was <5.00 pg/ml, and the ESR and CRP levels
had decreased to 40 mm/h and 0.92 mg/l, respectively. These
clinical findings indicated that treatment was effective and the
patient was recovering well.
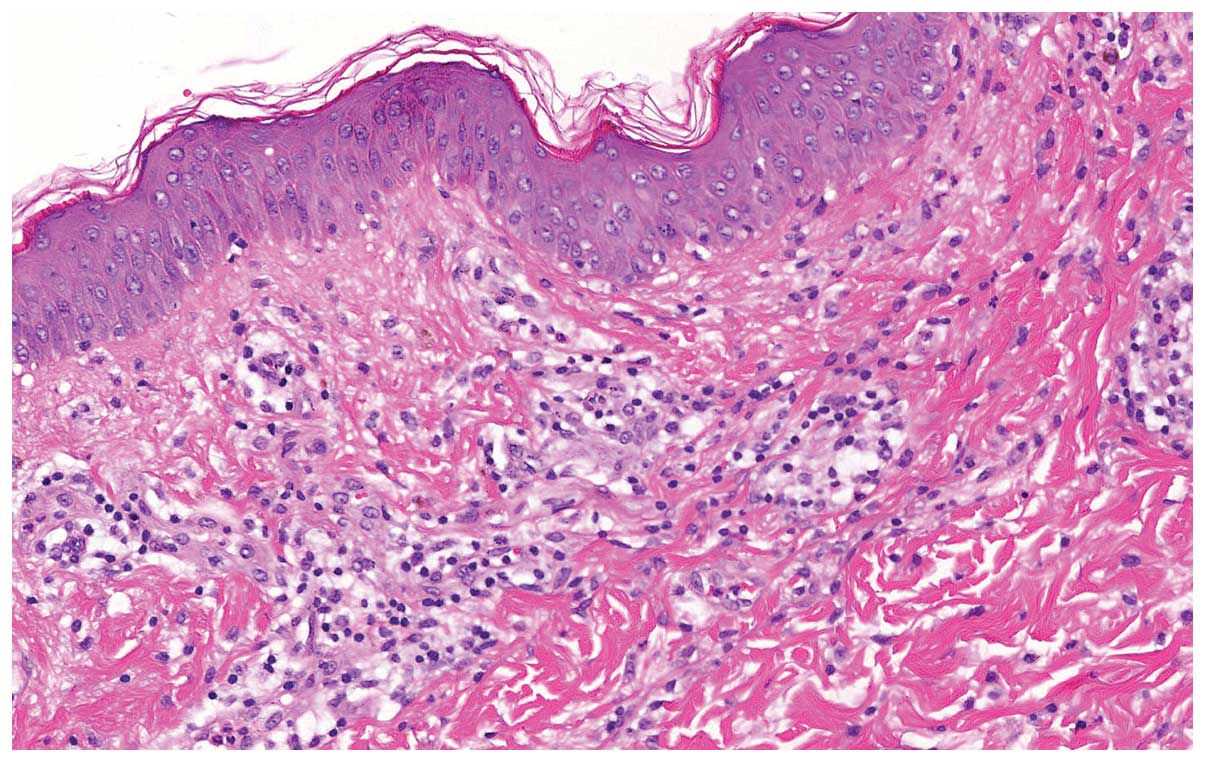
Subsequently, the skin biopsy demonstrated massive
infiltration of neutrophils into the dermis and dermal edema
without vasculitis (Fig. 3). These
findings were consistent with Sweet disease. Furthermore, human
leukocyte antigen (HLA) typing indicated the patient was positive
for Cw1 and negative for B51. Cw1 and B51 HLA typing is often used
for differential diagnosis of NSD (Positive Cw1 and negative B51)
from neuro-Behçet disease (NBD) (Positive B51) (9). The differences between NSD and NBD are
as follows: 1) Both sexes are evenly affected by NSD, whereas men
are affected ~3.4 times as frequently as women with NBD; 2)
individuals aged 30–70 are most commonly affected by NSD, whereas
NBD preferentially affects those aged 20–40; 3) in NSD, any region
of the CNS may be involved without site predilection, resulting in
various neurological symptoms, whereas the basal ganglia and
brainstem are preferentially affected in NBD; 4) some patients with
NSD demonstrate ocular signs, including episcleritis and
conjunctivitis, whereas uveitis is common in NBD; and 5) there is a
strong HLA-Cw1 and B54 association in NSD, whereas there is a high
frequency of HLA-B51 in patients with NBD (9). As a result of the clinical features,
CSF analysis and HLA typing, the patient was diagnosed with NSD,
not NBD.
The patient was discharged following 17 days of
hospitalization, at which point his headache and neck stiffness
were alleviated and the erythematous plaques had receded without
scarring. The patient was subsequently followed-up as an
outpatient, and he remains well on a tapering dose of prednisone
acetate tablets, oral aciclovir (300 mg/dose, 3 times/day for 6
days) and anti-tuberculotic drugs (for 3 months). At 15-month
follow-up, the patient demonstrated no recurrence of symptoms or
erythematous plaques. Written informed consent was obtained from
the patient prior to publication.
Discussion
The patient presented with clinical features of
viral meningitis and the cutaneous manifestations of classical
Sweet disease. Although the brain MRI was normal, which has rarely
been demonstrated in previous patients with NSD (4,9,13–16), the
present patient fulfilled all the essential points of the
diagnostic criteria for NSD, including: i) high fever of 38–40°C;
ii) painful and dull red erythematous plaques on the face, neck and
upper part of the trunk; iii) neutrophilic infiltration of the
dermis without uveitis; iv) positive HLA-Cw1 and negative HLA-B51;
and v) good response to corticosteroid therapy (8). Combination therapy with corticosteroids
and antiviral agents was initiated due to suspected viral
meningitis, prior to the final diagnosis of NSD. In addition to
corticosteroids and antiviral therapy, anti-tuberculotic agents
were also administered as the CSF modified acid-fast stain was
positive, which suggested that the patient was concurrently
suffering from an endogenous infection of M. tuberculosis
and, therefore, corticosteroid administration would have lead to
the spread of M. tuberculosis. The patient responded well to
these drugs. Given the rarity of NSD, it is understandable that the
correct diagnosis was not immediately achieved. The present case
reinforces the value of an early skin biopsy in order to confirm
the diagnosis, avoid unnecessary therapy, allow the prompt
initiation of the appropriate treatment and achieve successful
therapeutic management.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81070950).
References
|
1
|
Sweet RD: An acute febrile neutrophilic
dermatosis. Br J Dermatol. 76:349–356. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen PR: Sweet's syndrome - a
comprehensive review of an acute febrile neutrophilic dermatosis.
Orphanet J Rare Dis. 2:342007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Going JJ, Going SM, Myśkoẃ MW and
Beveridge GW: Sweet's syndrome: Histological and
immunohistochemical study of 15 cases. J Clin Pathol. 40:175–179.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hisanaga K, Hosokawa M, Sato N, Mochizuki
H, Itoyama Y and Iwasaki Y: Neuro-sweet disease: Benign recurrent
encephalitis with neutrophilic dermatosis. Arch Neurol.
56:1010–1013. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noda K, Okuma Y, Fukae J, Fujishima K,
Goto K, Sadamasa H, Yoshiike T and Mizuno Y: Sweet's syndrome
associated with encephalitis. J Neurol Sci. 188:95–97. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stenzel W, Frosch PJ and Schwarz M:
Sweet's syndrome associated with acute benign encephalitis. A drug
induced aetiology. J Neurol. 250:770–771. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nobeyama Y and Kamide R: Sweet's syndrome
with neurologic manifestation: Case report and literature review.
Int J Dermatol. 42:438–443. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hisanaga K, Iwasaki Y and Itoyama Y:
Neuro-Sweet Disease Study Group: Neuro-Sweet disease: Clinical
manifestations and criteria for diagnosis. Neurology. 64:1756–1761.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hisanaga K: Neuro-neutrophilic disease:
Neuro-Behcet disease and Neuro-Sweet disease. Intern Med.
46:153–154. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dunn TR, Saperstein HW, Biederman A and
Kaplan RP: Sweet syndrome in a neonate with aseptic meningitis.
Pediatr Dermatol. 9:288–292. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Druschky A, von den Driesch P, Anders M,
Claus D and Neundörfer B: Sweet's syndrome (acute febrile
neutrophilic dermatosis) affecting the central nervous system. J
Neurol. 243:556–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng GD, Shi M, Ma L, Chen P, Wang BJ,
Zhang M, Chang XL, Su XC, Yang YN, Fan XH, et al: Diagnostic
accuracy of intracellular Mycobacterium tuberculosis
detection for tuberculous meningitis. Am J Respir Crit Care Med.
189:475–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kokubo Y, Kuzuhara S, Isoda K, Sato K,
Kawada N and Narita Y: Neuro-Sweet disease: Report of the first
autopsy case. J Neurol Neurosurg Psychiatry. 78:997–1000. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Niwa F, Tokuda T, Kimura M, Azuma Y,
Mizuno T and Nakagawa M: Self-remitting and reversible parkinsonism
associated with neuro-Sweet disease. Intern Med. 49:1201–1204.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fukae J, Noda K, Fujishima K, Wada R,
Yoshiike T, Hattori N and Okuma Y: Successful treatment of
relapsing neuro-Sweet's disease with corticosteroid and dapsone
combination therapy. Clin Neurol Neurosurg. 109:910–913. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kimura A, Sakurai T, Koumura A, Suzuki Y,
Tanaka Y, Hozumi I, Nakajima H, Ichiyama T and Inuzuka T:
Longitudinal analysis of cytokines and chemokines in the
cerebrospinal fluid of a patient with Neuro-Sweet disease
presenting with recurrent encephalomeningitis. Intern Med.
47:135–141. 2008. View Article : Google Scholar : PubMed/NCBI
|