Introduction
The sinoatrial node (SAN) is located in the right
dorsal wall of the right atrium, and serves as the primary
pacemaker for initiating the heartbeat and controlling the rate and
rhythm of contraction (1).
Deficiencies in such function due to congenital defects, acquired
diseases, gene mutation and aging may lead to sinus node
dysfunction (sick sinus syndrome), necessitating pacemaking therapy
(2). The present primary therapy is
electronic pacemaker implantation. However, such devices are not
optimal because of the limited battery life, biological
responsiveness deficiency and other shortages (2). With respect to these concerns, on-going
research is focused on developing a biological pacemaker that is
able to integrate into the myocardium and provide a permanent cure
(2–5).
The ‘funny’ current, also called the If
current, is crucially involved in the spontaneous diastolic
depolarization of SAN cells. The If current is generated
by the hyperpolarization-activated cyclic nucleotide-gated channel
(HCN) family. Among the four known HCN isoforms, isoform 4 (encoded
by HCN4) strongly contributes to pacemaker function
(3). In previous studies, the
present authors showed that mouse HCN4
(mHCN4)-transfected mesenchymal stem cells (MSCs) are able
to generate a functional If current (3–5).
The heart is the largest source of bioelectricity in
the human body (6,7). Electrical signals are correlated with
cardiac depolarization and contraction, and electrical currents
have been administered to cells in order to mimic native heart
conditions in a number of prior biomimetic studies (7–9).
Furthermore, an electrical stimulation system has been designed to
deliver electrical signals mimicking those in the native heart by
the present authors (10). This
electric pulse current stimulation (EPCS) had been shown to be safe
in canine MSCs (cMSCs), and may be able to promote cardiogenesis in
cMSCs in our previous study (10).
However, it is not clear whether EPCS is able to influence the
properties of If current reconstructed in
HCN4-transfected cMSCs.
In the present study, cMSCs with high-level
expression of mHCN4 were produced using exogenous
transfection. The kinetic characteristics of the resulting
If current were analyzed. In addition, the mRNA and
protein expression levels of HCN4, connexin 43 (Cx43) and
Cx45 were evaluated using reverse transcription-quantitative
polymerase chain reaction (RT-qpcr) and western blot assays, and
the ultrastructures of the cardiac connective proteins were
characterized using transmission electron microscopy (TEM).
Materials and methods
cMSC culture
Under 30 mg/kg sodium pentobarbital (i.v.) and 1–2%
isoflurane anesthesia (Abbott Laboratories Co., Ltd., Shanghai,
China), cMSCs were harvested from bone marrow of three adult
Chinese rural canines (two males and one female; Third Military
Medical University (Chongqing, Chian), weighing 10–14 kg, as
previously described (5). cMDCs were
cultured in α-minimum essential medium (α-MEM) with L-glutamine and
ribo- and deoxyribonucleosides (SH30265.01B; Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA). cMSC purity was evaluated using
flow cytometry, as previously described (5), in order to detect the markers CD29+,
CD44+, CD34- and CD45-, and by the ability to differentiate into
adipogenic, chondrogenic and osteogenic lineages, using Oil Red O,
Alcian Blue and Toluidine Blue, and Alizarin Red staining,
respectively (5). Third generation
cMSCs were transfected with pLentis-mHCN4-GFP in the
presence of 2 µg/ml polybrene (H9268; Sigma-Aldrich, St. Louis, MO,
USA) at a multiplicity of infection (MOI) of 20 for 24 h. Following
48 h of transfection, 90% of the cMSCs cells exhibited green
fluorescent protein (GFP) expression, which indicated successful
transfection. These cMSCs were expanded using standard techniques
(3–5,10). In
order to produce consistent comparisons between
mHCN4-transfected cMSCs with or without EPCS induction, the
cells were divided into two groups: mHCN4-transfected cMSCs
(group A), and mHCN4-transfected cMSCs induced with EPCS
(group B). Ethical approval for the present study was obtained from
the Committee on the Ethics of Animal Experiments of Third Military
Medical University (SYXK2013-0012).
mHCN4 transfection
The lentiviral vector expressing mHCN4
(pLentis-mHCN4-GFP) was constructed by inserting the
mHCN4 gene into a pLentis-GFP vector using BamHI
(FD0054) and EcoRI (N41890)
restriction sites, using materials obtained from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
transcription of mHCN4 was promoted by a Spleen
Focus-Forming Virus promoter (Invitrogen). The lentiviral particles
were prepared using a calcium phosphate method, as previously
described (3–5).
EPCS induction
An electric stimulation device designed in our lab
provided a constant electric signal to the cMSCs, which had been
shown to be safe to cMSCs and to promote cardiogenesis in cMSCs.
The property of the electrical signal was pulse polarity-altered
positive and negative variations (rectangular, 2 msec; current
intensity, 40 µA; current frequency, 2 Hz) (10). For EPCS, the transfected GFP-positive
cells in group B were reseeded in α-MEM at a density of 2×105 cells
into 60-mm culture dishes (430166; Corning Incorporated, Corning,
NY, USA) with platinum electrode (Chow Sang Sang Jewellery
Corporation, Hong Kong, China). The EPCS treatment was initiated on
the next day after seeding, lasting 3–6 h per day for 5 days until
the cells reached ~95% confluence. α-MEM was changed once during
this treatment period. Each experiment was repeated three
times.
Patch clamp studies for If
current detection in transfected cMSCs
Following EPCS induction for 5 days, the cMSCs in
group B and parallel cMSCs in group A were subjected to whole-cell
patch clamp detection. The If current was recorded using
a voltage clamp (Axon Instruments. Foster City, CA, USA) with an
Axopatch 200B amplifier (Molecular Devices, LLC, Sunnyvale, CA,
USA) (3–5). The pipette solution contained the
following (in mmol/l): 5.0 Na2-phosphocreatine, 5.0 Mg2-adenosine
triphosphate, 110 K aspartate, 0.1 GTP (Sigma-Aldrich), 1.0 MgCl2,
20 KCl, 0.05 EGTA and 10 HEPES (Sangon Biotech Co., Ltd., Shanghai,
China); pH was adjusted to 7.2 with KOH (Zhengzhongyi Biotech Co.,
Ltd., Beijing, China). The extracellular solution contained the
following (in mmol/l): 0.33 NaH2PO4, 1.0 MgCl2, 1.8
CaCl2, 5.4 KCl, 136 NaCl, 10 glucose and 10 HEPES; pH
was adjusted to 7.4 with NaOH (Sangon Biotech Co., Ltd.). When
required, 4 mmol/l CsCl was added into the bath solution. Cs+ is a
specific pacemaker current blocker to block If current
and has been used to confirm the If current. The sealing
resistance was >4 mΩ and the temperature was maintained at
25±1°C.
In preparation for patch clamping, cells were
trypsinized (SH30042.01; Hyclone; GE Healthcare Life Sciences) for
10–20 sec, while remaining attached to the coverslips. The membrane
capacity was measured by the application of a voltage clamp step,
and current density was shown as the value of the peak current per
capacity. If current was detected using custom voltage
clamp methods, as previously described (3–5).
Normalized tail currents were recorded to construct the fully
activated current-voltage relation. The slope factor (k) and the
half-maximal activation voltage (V1/2) were obtained by fitting the
activation curves to the Boltzmann equation, as follows:
Itail/Imax = A/{1.0 + exp
[(V-V1/2)/k]}. The time constants of activation were
determined by fitting current traces to a single exponential
function.
Immunofluorescence analysis
Immunofluorescence was performed according to
previously reported methods (10).
Transfected cMSCs (1.5×104 cells/cm2) were fixed with 4%
paraformaldehyde (Wuhan Boster Biological Technology, Ltd., Wuhan,
China) for 15 min at room temperature, washed in phosphate-buffered
saline (PBS; AR0030; Wuhan Boster Biological Technology, Ltd.),
then treated with 0.2% Triton X-100 (T8787; Sigma-Aldrich) for 15
min. Cells were incubated with rabbit polyclonal anti-HCN4
antibody (1:100; ab69054; Abcam, Cambridge, UK) overnight at 4°C.
Following washing three times with PBS for 5 min, the cells were
incubated with Alexa FluorTM 647-conjugated donkey anti-rabbit IgG
(1:200; A-31573; Invitrogen) for 60 min at 25±1°C. After further
washing with PBS, the cells were mounted with Antifade Mounting
Medium (Beyotime Institute of Biotechnology, Shanghai, China).
Nuclei were stained with 4′,6-diamidino-2-phenylindole (D9542;
Sigma-Aldrich) as a location control. Fluorescent images were
obtained using an inverted laser confocal microscope (LSM 710; Carl
Zeiss Microscopy GmbH, Cologne, Germany). The results were analyzed
using ZEN lite software, 2011 edition (Carl Zeiss Microscopy
GmbH).
TEM
Cells were fixed in caco-dylate-buffered 1% osmium
tetroxide, then dehydrated and embedded in Epon 812 (both Sangon
Biotech Co., Ltd.) for ultra-thin sectioning. The ultrastructures
of connection protein were examined by TEM using a TecnaiTM-10
electron microscope (FEI Company, Hillsboro, OR, USA) and operated
at a high-tension setting of 80 kV with a magnification of
x135,000.
RT-qpcr analysis
RT-qPCR was conducted according to previously
described methods (11). Briefly,
total mRNA was extracted using TRIzol (Invitrogen) and purified.
For RNA purification, the RNA was treated with chloroform,
centrifuged at 12,000 × g for 15 min at 4°C to collect the
supernatant prior to treatment with isoamylalcohol and vortexing
for 10 min at room temperature. Following centrifugation at 12,000
× g for 15 min at 4°C, the supernatant was discarded and the
subsequent sediment RNA was reverse transcribed into cDNA at 37°C
for 15 min and 98°C for 5 min using a ReverTra Ace qPCR RT kit
(FSQ-101; Toyobo Co., Ltd., Osaka, Japan). RT-qPCR amplification
was performed using a 12.5 µl SYBR® Green (2X) Realtime PCR Master
Mix kit (QPK-201; Toyobo Co., Ltd.), 1 µl forward primer, 1 µl
reverse primer, 5 µl cDNA and 5.5 µl water on a Stratagene Mx3000P
qPCR system (Agilent Technologies, Inc., Santa Clara, CA, USA),
according to the manufacturer's instructions. Thermal cycling was
performed as follows: 95°C for 30 sec, followed by 40 cycles of
95°C for 30 sec, 60°C for 30 sec and 72°C for 20 sec, and 72°C for
10 min prior to holding at 4°C. The primer sequences used were
designed as follows: HCN4 forward, 5′-AGTTGCGTTTCGAGGTCTT-3′
and reverse, 5′-CTTTGTTGCCCTTAGTGAGC-3′; Cx43 forward,
5′-TGCTATGACAAATCCTTCCCAATC-3′ and reverse,
5′-GCCGTGCTCTTCAATTCCATACTT-3′; Cx45 forward,
5′-CAGCAGACTTCCTTGCCCTCATA-3′ and reverse,
5′-CTTAGCATTGGACAGTTCGGTGT-3′; GAPDH forward,
5′-GAGATCCCGCCAACATCAAA-3′ and reverse, 5′GGCATCAGCAGAAGGAGCAG3′
(Invitrogen). Quantitative measurements were determined using the
comparative Ct (2-ΔΔCq) method (12). All samples were normalized against
the endogenous level of GAPDH. All results were repeated in
triplicate and were analyzed using MxPro-Mx3000P software (Agilent
Technologies, Inc.).
Western blot analysis
Western blot assays were performed according to
previously described methods (10,11,13).
Briefly, cMSCs were lysed with RIPA buffer containing
phenylmethylsulfonyl fluoride (Sangon Biotech Co., Ltd.), then 50
µg total protein was quantitated using a BCA protein assay kit
(P0009; Beyotime Institute of Biotechnology) and subjected to 6–10%
SDS-PAGE. After being resolved by electrophoresis, the proteins
were transferred to a polyvinylidene fluoride membrane (EMD
Millipore, Billerica, CA, USA), blocked for 3 h at room temperature
in Tris-buffered saline (TBS) with 5% bovine serum albumin
(10099–141; Gibco; Thermo Fisher Scientific, Inc.), and incubated
with anti-HCN4 (1:100; ab69054; Abcam), anti-Cx43 (1:200;
sc-9059; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-Cx45 (1:200; sc-25716) rabbit and GAPDH (1:200; sc-48166;
Santa Cruz Biotechnology, Inc.) goat polyclonal antibodies
overnight at 4°C, with gentle agitation. After washing three times
with TBS with Tween-20, the membranes were incubated with
corresponding horseradish peroxidase-conjugated mouse anti-rabbit
polyclonal IgG (1:5,000; sc-2357; Santa Cruz Biotechnology, Inc.)
at room temperature for 2 h. The specific bands of the target
proteins were visualized using an enhanced chemiluminescence
detection kit (P0018A; Beyotime Institute of Biotechnology),
according to the manufacturer's recommendations. Finally, the
target signals were normalized against the GAPDH signal and
analyzed using Quantity One software (version 4.62; Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Standard curve
concentrations were 0.025, 0.05, 0.1, 0.2, 0.3, 0.4 and 0.5 µg/µl,
respectively with the wavelength set to 562 nm in UV. Experiments
were performed >3 times to verify quantification values.
Statistical analysis
All values are presented as the mean ± standard
error of the mean. Statistical comparisons were analyzed using
Student's unpaired t-test with SPSS 19.0 software (IBM SPSS,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
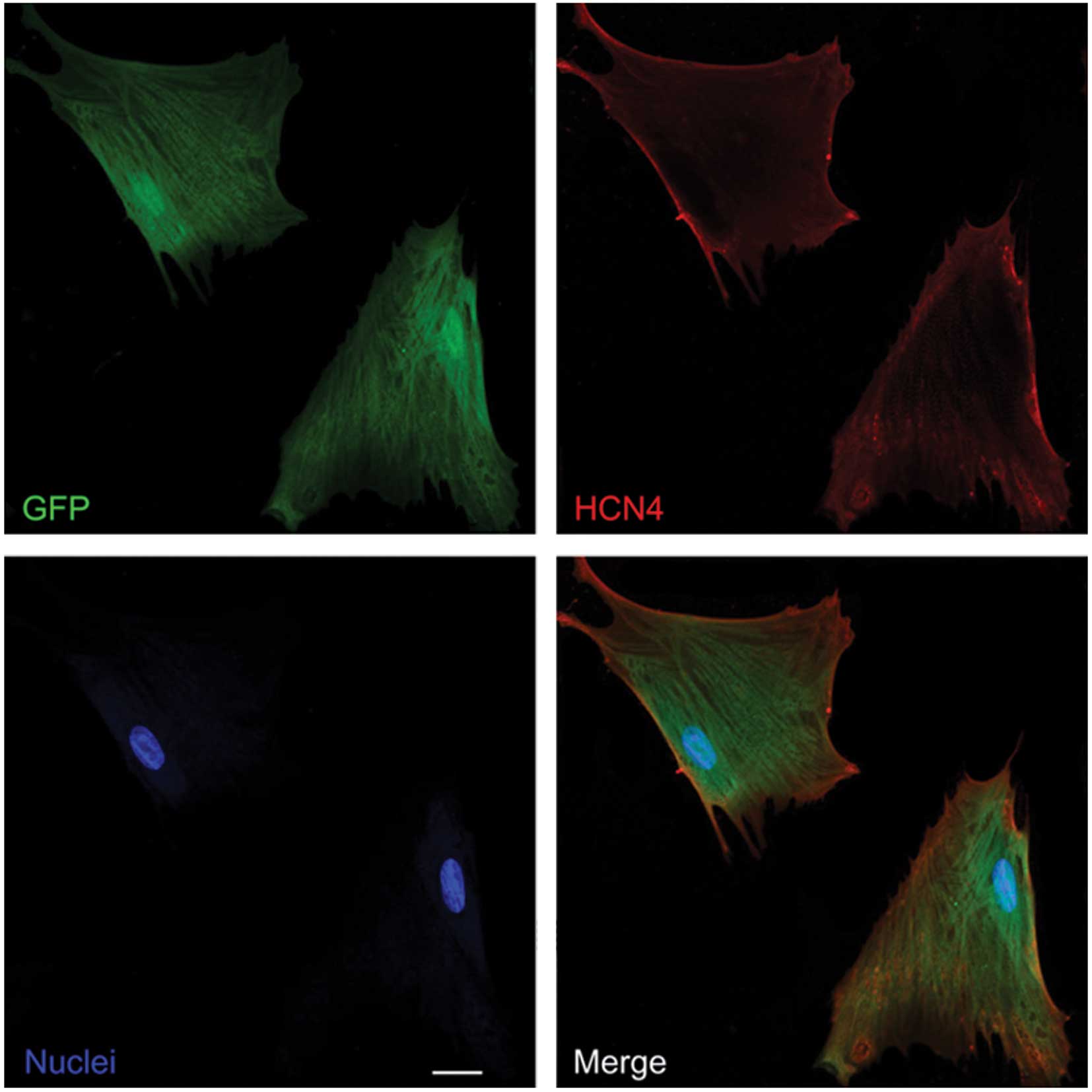
Demonstration of gene transfer
At an MOI of 20, the transfection rate of the cMSCs
was 95±3.7%, which was confirmed by confocal laser microscope
images in at least two different random fields. The
mHCN4-GFP-transfected cMSCs in groups A and B expressed GFP
and HCN4 protein (Fig.
1).
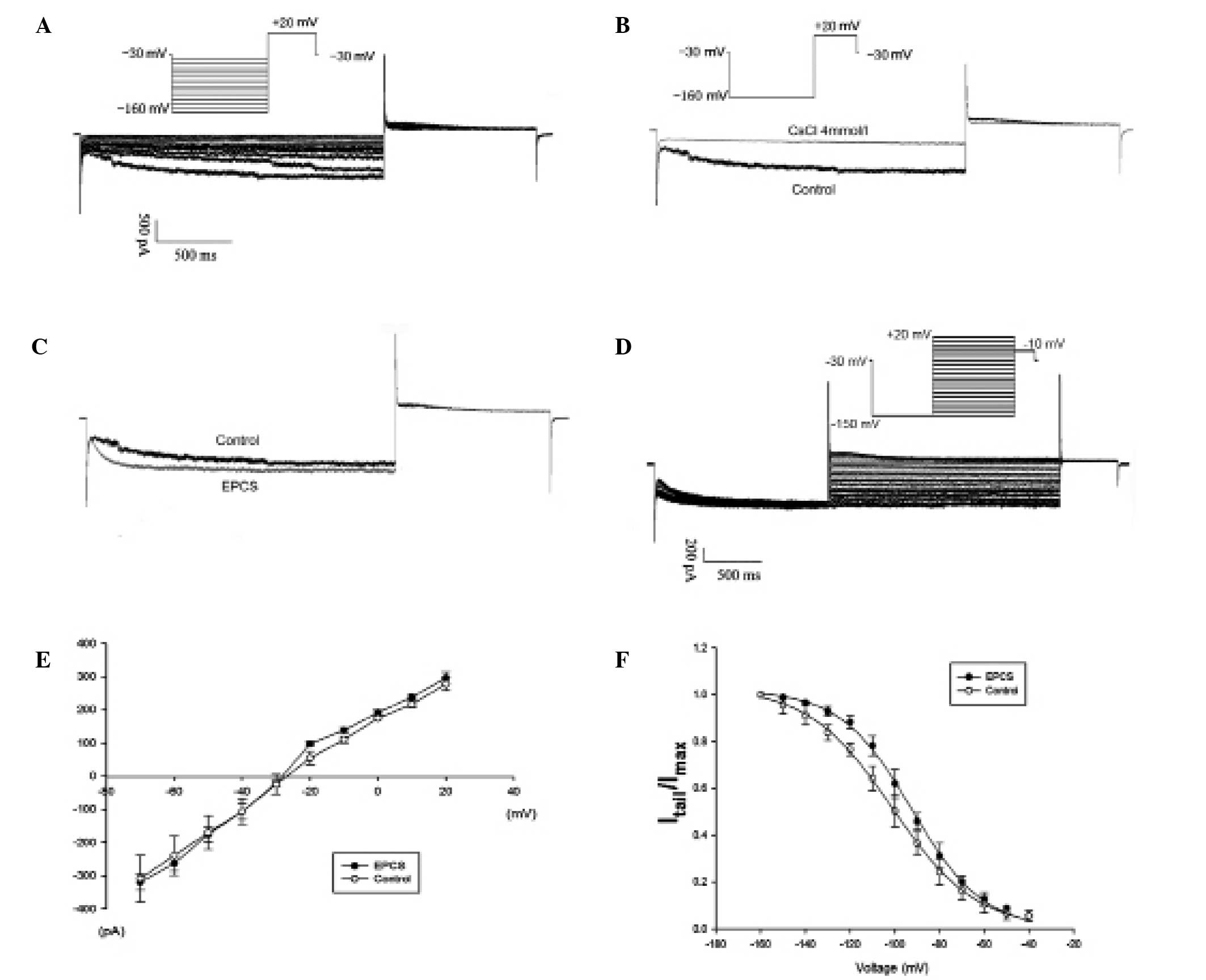
Characteristics of If in
transfected cMSCs
The If characteristics were recorded in
whole cell patch-clamp mode. The mHCN4-GFP transfected cMSCs
in group A showed a time and voltage dependent inward current
(Fig. 2A). The current amplitude was
−1,081.5±15.4 pA at −160 mV command voltage, and the current
density was −45.6±7.7 pA/pF at the same voltage. The V1/2 was
−101.2±4.6 mV, the time constant of activation was 324±41 msec at
−160 mV, similar to values for HCN4 expression in cardiac
myocytes (14). The voltage protocol
(Fig. 2D) enabled the measurement of
the reversal potential (Fig. 2E),
which was −26.4±3.8 mV. The detection rate of this inward current
was 76% (16/21). It was also investigated whether Cs+, a specific
pacemaker current blocker, was able to block this expressed
current. This inward current was blocked following the external
addition of 4 mmol/l Cs+ (Fig. 2B),
consistent with Cs+ inhibition of If as previously
reported (3–5).
Under the same recording conditions, the detection
rate of the If current was similar in the EPCS inductive
group B (77%, 17 of 22). The channel activation curve shifted to
right (Fig. 2F), which indicated
that the current curve depicted a positive forward reaction. The
V1/2 changed to −92.4±4.8 from −101.2±4.6 mV (P<0.05; Table I), while the absolute values of
If channel reversal potential increased (−28.9±3.0 vs.
−26.4±3.8 mV, P<0.05). The time constant of activation decreased
to 251±44 msec at −160 mV (P<0.05).
 | Table I.Comparative data measured for
If in mHCN4-transfected canine mesenchymal stem cells in
groups A and B. |
Table I.
Comparative data measured for
If in mHCN4-transfected canine mesenchymal stem cells in
groups A and B.
| Parameter | Group A | Group B |
|---|
| Detection rate | 76% (16/21) | 77% (17/22) |
| Current amplitude
(pA, −160 mV) | −1,081.5±15.4 | −1,
343.4±18.6a |
| Cell membrane
capacitance (pF) |
24.3±4.8 | 25.0±5.6 |
| Current density
(pA/pF, −160 mV) |
−45.6±7.7 |
−53.7±4.0a |
| Half-maximal
activation (mV) | −101.2±4.6 |
−92.4±4.8a |
| Slope factor |
−16.0±1.6 |
−18.8±1.8a |
| Time constant of
activation (msec, −160 mV) |
324±41 |
251±44a |
| Reversal potential
(mV) |
−26.4±3.8 |
−28.9±3.0a |
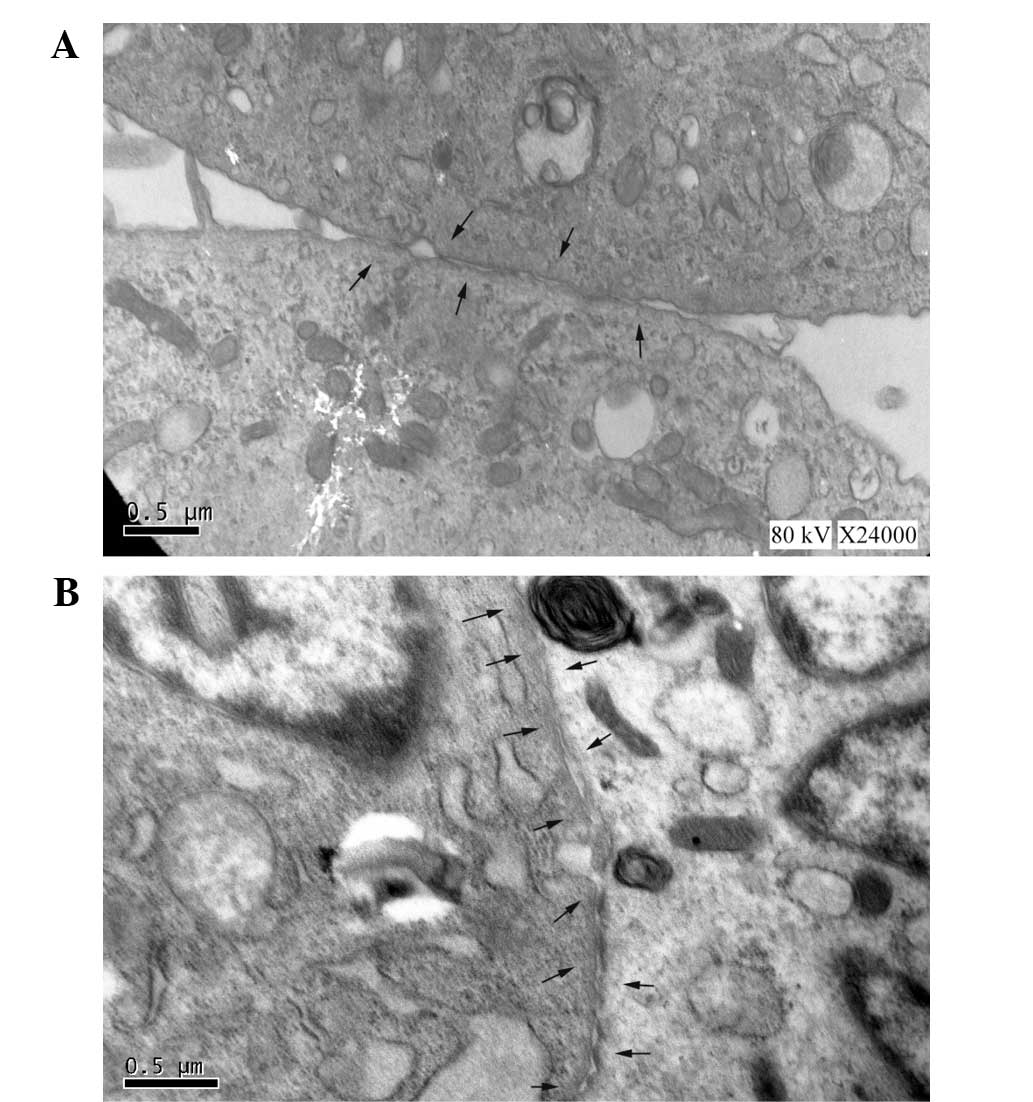
Expression of gap junction
proteins
For consistent comparisons in mHCN4
transfected cMSCs with or without EPCS induction, the gap junctions
between adjacent cells were analyzed using TEM (Fig. 3). Following EPCS induction, the cMSCs
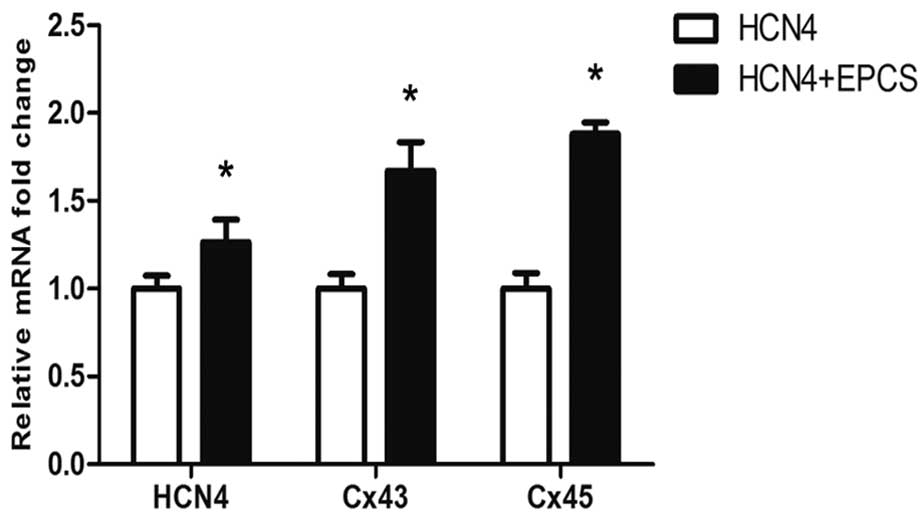
showed an increased expression of gap junctions as Fig. 3B. In addition, the mRNA and protein
expression levels of Cx43 and Cx45 in each group were evaluated
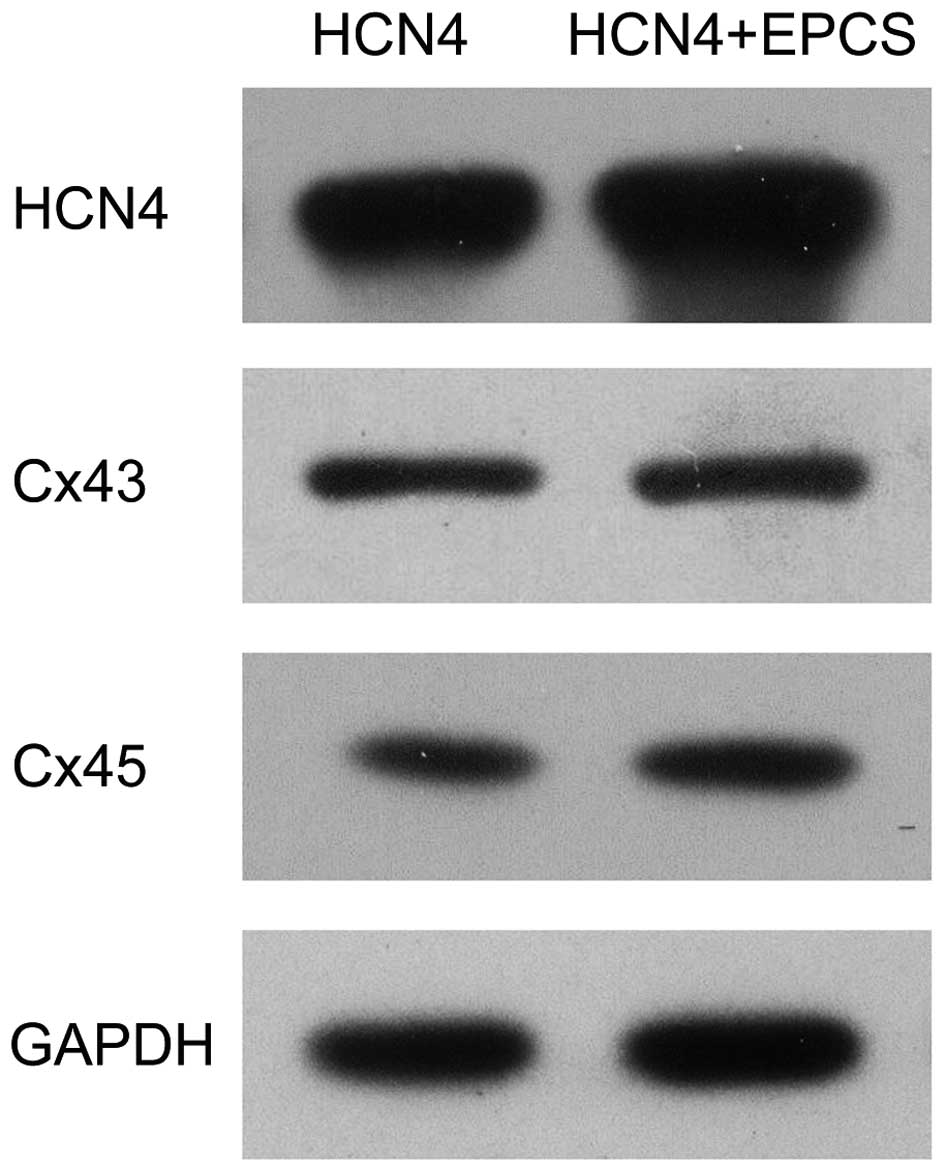
using RT-qPCR and western blot analyses. As shown in Fig. 4, the mRNA expression levels of
HCN4, Cx43 and Cx45 were significantly increased in EPCS
inductive group B compared with group A (P<0.05). The protein
expression levels of HCN4, Cx43 and Cx45 were also
upregulated in group B, as shown in Fig.
5.
Discussion
Due to the limited ethical restrictions and immune
privilege, MSCs are currently the optimal candidate for biological
pacemaker reconstruction (3–5). In our previous studies, we demonstrated
that mHCN4-transfected cMSCs can express HCN4 protein
stably and produce an If pacemaker current in
vitro and in vivo (3,4). Wen
et al previously reported that cMSCs are able to
differentiate into cardiomyocytes by treatment with EPCS (10). The aim of the present study was to
investigate the effects of EPCS on the If channel
reconstructed in mHCN4-transfected cMSCs.
The HCN gene family is responsible for the
generation of the pacemaker If current. The isoform
HCN4 has been demonstrated to be important for the proper
function of pacemaking (3). The
heart is the largest source of bioelectricity in the human body,
and numerous studies have investigated the effects of applying
electrical stimulation signals to heart (6–10). In
cardiac tissue engineering studies, biomimetic systems mimicking
electrical signals in native heart have been used to enhance
functional coupling of the cells and to increase the amplitude of
synchronous construct contractions (6–10). An
electric stimulation system had been designed in the Department of
Cardiology at Southwest Hospital, Third Military Medical University
to deliver electrical signals mimicking those in the native heart.
This EPCS system had been shown to be safe for use in cMSCs, and
successfully promoted the cardiogenesis of cMSCs in our previous
study (10). The present study
demonstrated that mHCN4-expressing cMSCs are capable of
generating a time- and voltage- dependent inward current. This
current is quite sensitive to extracellular Cs+ and has similar
channel kinetic characteristics as physiological HCN4
If current (14).
Following EPCS conduction, the channel activation curve shifted to
the right and the time constant of activation decreased. TEM images
showed more gap junctions in the EPCS inductive group. Therefore,
it was hypothesized that these changes in the If current
may be associated with the variation of gap junctions.
Gap junctions can establish communication channels
between adjacent cells and allow direct intercellular transfer of
ions, small molecules and electrical coupling (15). Gap junctions are composed of Cx
protein subunits, and there are 21 members in the Cx gene family in
the human genome and 20 members in the mouse to date (16). Cx40, Cx43 and Cx45 are expressed in
cardiac tissues with distinct distributions. Cx45 is predominantly
expressed in SAN, and is able to assemble gap junction channels
with low conduction. By contrast, Cx40 and Cx43 are primarily
expressed in working myocardium and can form gap junction channels
with high conduction. Notably, a number of studies have suggested
that Cx43 may also be expressed in SAN (16,17).
Mutations in Cx43 may result in cardiac malformations and related
to atrial fibrillation and sudden infant death syndrome (18,19).
Recently, Yamada et al reported that Cx45 expression was
markedly increased in patients with heart failure, and the
upregulation of Cx45 in cardiac myocytes may lead to increased
arrhythmias in the failing heart, which may impact the
downregulation of Cx43 (20,21).
In the present study, the expression fold changes of
Cx43 and Cx45 were evaluated in mHCN4 genetically modified
cMSCs in vitro. The mRNA and protein expression levels of
Cx43 and Cx45 were detected in mHCN4-transfected cMSCs.
Following EPCS induction, the expression of Cx43, Cx45 and
HCN4 were significantly increased. Under the same
conditions, reduced Cx43 expression and barely detectable Cx45
expression were detected in GFP-transfected cMSCs, indicating that
mHCN4 is able to promote the expression of gap junction
proteins, and that EPCS enhanced this effect. Collectively, the
present results indicate that the activation and amplification of
mHCN4 may promote the cardiac differentiation progress of
cMSCs. In our previous study, we performed preliminary experiments
using fluorescence recovery after photo bleaching to determine
whether mHCN4-transfected cMSCs could form functional gap
junctions (22). The average
fluorescence recovery rate of mHCN4-transfected cMSCs
co-cultured with neonatal rat atrial cells was obviously increased
compared with those of GFP-transfected cMSCs co-cultured with
neonatal rat atrial cells, and it was same as cultured neonatal rat
atrial cells (data not shown). These results suggest that
mHCN4-transfected cMSCs are able to assemble functional gap
junctions, which is consistent with the findings of Valiunas et
al (23).
In conclusion, the present results suggest that
mHCN4-transfected cMSCs are able to generate pacemaker
If current, and that EPCS enhanced this effect. In
addition to the increased gap junctions and upregulated Cx43 and
Cx45 expression, we infer that EPCS may promote the If
current reconstructed in mHCN4 genetically modified cMSCs
via the incremental gap junctions assembled by Cx43 and Cx45. In
addition, this study may provide a reference for the future studies
involving biological pacemaker reconstruction in canines, and may
assist in the future development of gene-targeted and regenerative
therapeutic remedies for SAN dysfunction in humans.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81270246).
References
|
1
|
Van Mierop LH and Gessner IH: The
morphologic development of the sinoatrial node in the mouse. Am J
Cardiol. 25:204–212. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miake J, Marbán E and Nuss HB: Biological
pacemaker created by gene transfer. Nature. 419:132–133. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tong S, Yao Q, Wan Y, Zhou J, Shu M, Zhong
L, Li Y, Zhang Q, Yindai J and Song Z: Development of functional I
f channels in mMSCs after transfection with mHCN4: Effects on cell
morphology and mechanical activity in vitro. Cardiology.
112:114–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nong Y, Zhang C, Wei L, Zhang Z, Cheng J,
Wen L and Song Z: In situ investigation of allografted mouse HCN4
gene-transfected rat bone marrow mesenchymal stromal cells with the
use of patch-clamp recording of ventricular slices. Cytotherapy.
15:905–919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jun C, Zhihui Z, Lu W, Yaoming N, Lei W,
Yao Q and Zhiyuan S: Canine bone marrow mesenchymal stromal cells
with lentiviral mHCN4 gene transfer create cardiac pacemakers.
Cytotherapy. 14:529–539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hart RA and Gandhi OP: Comparison of
cardiac-induced endogenous fields and power frequency induced
exogenous fields in an anatomical model of the human body. Phys Med
Biol. 43:3083–3099. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tandon N, Cannizzaro C, Chao PH, Maidhof
R, Marsano A, Au HT, Radisic M and Vunjak-Novakovic G: Electrical
stimulation systems for cardiac tissue engineering. Nat Protoc.
4:155–173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Radisic M, Marsano A, Maidhof R, Wang Y
and Vunjak-Novakovic G: Cardiac tissue engineering using perfusion
bioreactor systems. Nat Protoc. 3:719–738. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Radisic M, Park H, Chen F, Salazar-Lazzaro
JE, Wang Y, Dennis R, Langer R, Freed LE and Vunjak-Novakovic G:
Biomimetic approach to cardiac tissue engineering: Oxygen carriers
and channeled scaffolds. Tissue Eng. 12:2077–2091. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wen L, Zhang C, Nong Y, Yao Q and Song Z:
Mild electrical pulse current stimulation upregulates S100A4 and
promotes cardiogenesis in MSC and cardiac myocytes coculture
monolayer. Cell Biochem Biophys. 65:43–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Q, Wang H, Yang M, Yang D, Zuo Y and
Wang J: Expression of a tumor-associated gene, LASS2, in the human
bladder carcinoma cell lines BIU-87, T24, EJ and EJ-M3. Exp Ther
Med. 5:942–946. 2013.PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gonen-Korkmaz C, Sevin G, Gokce G, Arun
MZ, Yildirim G, Reel B, Kaymak A and Ogut D: Analysis of tumor
necrosis factor α-induced and nuclear factor κB-silenced LNCaP
prostate cancer cells by RT-qPCR. Exp Ther Med. 8:1695–1700.
2014.PubMed/NCBI
|
|
14
|
Stieber J, Herrmann S, Feil S, Löster J,
Feil R, Biel M, Hofmann F and Ludwig A: The
hyperpolarization-activated channel HCN4 is required for the
generation of pacemaker action potentials in the embryonic heart.
Proc Natl Acad Sci USA. 100:15235–15240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Söhl G and Willecke K: Gap junctions and
the connexin protein family. Cardiovasc Res. 62:228–232. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kwong KF, Schuessler RB, Green KG, Laing
JG, Beyer EC, Boineau JP and Saffitz E: Differential expression of
gap junction proteins in the canine sinus node. Circ Res.
82:604–612. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Verheule S, van Kempen MJ, Postma S, Rook
MB and Jongsma HJ: Gap junctions in the rabbit sinoatrial node. Am
J Physiol Heart Circ Physiol. 280:H2103–H2115. 2001.PubMed/NCBI
|
|
18
|
Van Norstrand DW, Asimaki A, Rubinos C,
Dolmatova E, Srinivas M, Tester DJ, Saffitz JE and Duffy HS:
Connexin43 mutation causes heterogeneous gap junction loss and
sudden infant death. Circulation. 125:474–481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salameh A, Blanke K and Daehnert I: Role
of connexins in human congenital heart disease: The chicken and egg
problem. Front Pharmacol. 4:702013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamada KA, Rogers JG, Sundset R, Steinberg
TH and Saffitz J: Up-regulation of connexin45 in heart failure. J
Cardiovasc Electrophysiol. 14:1205–1212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Betsuyaku T, Nnebe NS, Sundset R,
Patibandla S, Krueger CM and Yamada KA: Overexpression of cardiac
connexin45 increases susceptibility to ventricular tachyarrhythmias
in vivo. Am J Physiol Heart Circ Physiol. 290:H163–H171. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qin Y, Zhiyuan S, Shifei T and Zewen W:
Study of differentiation of rat mesenchymal stem cells with mHCN4
gene into spontaneous cardiomyocyte-like cells in vitro. Zhong Guo
Xin Zang Qi Bo Yu Xin Dian Sheng Li Za Zhi. 21:55–58. 2007.
|
|
23
|
Valiunas V, Doronin S, Valiuniene L,
Potapova I, Zuckerman J, Walcott B, Robinson RB, Rosen MR, Brink PR
and Cohen IS: Human mesenchymal stem cells make cardiac connexins
and form functional gap junctions. J Physiol. 555:617–626. 2004.
View Article : Google Scholar : PubMed/NCBI
|