Introduction
The incidence of diabetes mellitus (DM) has
increased year upon year. Diabetic nephropathy (DN) is the most
common serious microvascular complication associated with DM, and
is the predominant cause of end-stage renal disease and mortality
(1,2). However, the underlying pathogenesis of
DN is complex (3,4), and has yet to be fully elucidated,
although previous studies have associated genetic factors, glucose
metabolism, hemodynamic alterations, oxidative stress and cell and
inflammatory factors with its occurrence and development (5,6). DN has
been shown to be a type of inflammatory process, and thus various
anti-inflammatory therapies have been used for its treatment
(7). In a previous study, glycogen
synthase kinase-3β (GSK-3β) exerted regulatory effects on
inflammation via the tumor necrosis factor-α-induced nuclear factor
(NF)-κB signaling pathway (8); thus
suggesting that the NF-κB signaling pathway may be involved in DN.
Previous studies reported that the receptor activator of NF-κB
(RANK) and its ligand (RANKL) have a key role in the
differentiation, development and maturation of osteoclast cells
(9–11). In addition, RANK and RANKL have been
implicated in the pathogenesis of various diseases, including
autoimmune thyroiditis, Crohn's disease and rheumatoid arthritis
(12,13). Previous studies have reported that
rat models of glomerulosclerosis with 5/6 nephrectomy and podocyte
injury caused by puromycin amino exhibited elevated expression
levels of RANK and RANKL (14,15).
However, to the best of our knowledge, the underlying mechanism by
which GSK-3β may regulate the NF-κB signaling pathway in DN has yet
to be investigated. The characteristic lesions of DN have also been
associated with podocyte injury (16). Therefore, the present study aimed to
investigate the effects and associations of GSK-3β, RANK, RANKL and
NF-κB in a rat model of DN. In addition, lithium chloride (LiCl)
treatment was used to inhibit GSK-3β, and alterations in the mRNA
and protein expression levels of RANK, RANKL and NF-κB were
detected in the renal tissues of the rats, in order to devise novel
strategies for the treatment of DN.
Materials and methods
Establishment of an animal model of DN
and grouping
A total of 24 healthy Sprague-Dawley rats (age, 8
weeks; weight, 200–250 g), including 12 female and 12 male rats,
from the Laboratory Animal Center at the Guizhou Medical University
(Guiyang, China), were fed a normal diet for 2 weeks, after which
tail vein blood glucose concentrations were measured. A total of 8
rats were randomly selected to form the normal control (NC) group.
Diabetes was induced in the remaining 16 rats by intraperitoneal
injection with 55 mg/kg streptozotocin solution (Sigma-Aldrich, St.
Louis, MO, USA) following fasting. The rats in the NC group were
injected with an identical volume of solvent (citric acid-sodium
citrate buffer). Venous blood was collected after 72 h, and a
glucose concentration of >16.7 mmol/l was as an indicator of
diabetes (17). The two groups were
fed for 10 weeks, during which the rats were weighed and the blood
glucose levels were measured weekly. After 10 weeks, the rats were
fasted, receiving only water, for 24 h. Next the rats underwent
measurement of urine protein concentration; a protein concentration
of ≥30 mg or >10-times that of the NC group was considered to
indicate DN in the diabetic rats (18). The 16 rats in the successfully
established DN group were randomly divided into the DN model (DNM)
group and the DN model lithium chloride (LiCl) intervention (DNI)
group (n=8 rats per group). The DNI group was injected with 15
mg/kg LiCl (Sigma-Aldrich) for 10 days, whereas the DNM and NC
groups received an equal volume of saline. At 1 day prior to
sacrifice, the urine samples were collected over 24 h, after which
the rats were anaesthetized by abdominal injection with 0.2%
propofol in medium- and long-chain triglyceride emulsion (1 ml/100
g body weight; Guangdong Jiabo Pharmaceutical Co., Ltd., Guangzhou,
China), followed by sacrifice by cervical dislocation on day 12.
The rat kidneys were harvested, fixed with 10% neutral buffered
formalin, embedded in paraffin and cut into 3-µm sections, in order
to conduct hematoxylin and eosin (HE) staining (Fuzhou Maixin
Biotechnology Development Co., Ltd., Fuzhou, China) and
immunohistochemical analyses. The present study was conducted in
accordance with the recommendations outlined in the Guide for the
Care and Use of Laboratory Animals (National Institutes of Health,
Bethesda, MA, USA). The animal use protocol was reviewed and
approved by the Institutional Animal Care and Use Committee of the
Guizhou Province, China.
Detection of general index
The concentration of urinary protein was determined
by Coomassie Blue staining (Sangon Biotech Co., Ltd., Shanghai,
China) and the blood glucose was measured using a glucose meter
(MHY-26413; Beijing Meihua Instrument Technology Co., Ltd.,
Beijing, China).
Samples of renal tissues
All rats were anaesthetized by abdominal injection
with 0.2% propofol in medium- and long-chain triglyceride emulsion,
prior to sacrifice by cervical dislocation. Subsequently, the
kidneys were harvested, the renal capsule was removed, and the
kidneys were flushed with saline, fixed and preserved at 4°C for HE
staining and immunohistochemical analyses. The left kidney was
stored in an ice box maintained at −80°C prior to reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
HE staining
Paraffin-embedded 3-µm kidney sections were baked at
55°C in the oven for 10 min, dewaxed, and immersed in xylene for 10
min. After removing the xylene, the kidney sections were washed
with water and incubated with 1% hematoxylin solution for 5 min.
Subsequently, the tissue sections were washed with tap water and 1%
hydrochloric acid, followed by incubation with 0.5% eosin stain for
2 min. After rinsing with tap water, the kidney sections were
placed in double distilled water, dehydrated and mounted with
neutral glue. Alterations in the morphologies of the glomerulus,
tubules and interstitium of the renal tissues of the rats were
observed in the Q550CW Image Acquisition and Analysis System (Leica
Microsystems, GmbH, Wetzlar, Germany).
Immunohistochemistry
Paraffin-embedded kidney sections were baked and
dehydrated, followed by washing with phosphate-buffered saline
(PBS; Sangon Biotech Co., Ltd.). The sections were repaired for 10
min using 0.01M citrate repair solution (pH 6.0), cooled to room
temperature, and washed with PBS. Following incubation with 3%
hydrogen peroxide for 20 min at room temperature, the kidney
sections were incubated with the following primary antibodies:
Rabbit anti-rat GSK-3β (1:100; BV4582-100), NF-κB (1:200;
BV8453-002), RANK (1:200; BV6545-221) and RANKL (1:200; BV9023-300)
polyclonal antibodies (all BioVision, Inc., Milpitas, CA, USA),
overnight at 4°C. On the following day, the kidney sections were
incubated with peroxidase-conjugated secondary antibodies at
20–37°C for 60 min. The antibody complexes were visualized by
staining with diaminobenzidine (Sigma-Aldrich), after washing with
PBS. The reaction was quenched using distilled water, after which
the kidney sections were counterstained with hematoxylin for 2 min
and differentiated by alcohol hydrochloride (Sigma-Aldrich). The
sections were dehydrated conventionally using alcohol
(Sigma-Aldrich), made transparent with xylene (Sigma-Aldrich) and
mounted using neutral gum (Fuzhou Maixin Biotechnology Development
Co., Ltd.). Finally, the sections were examined under a DVM6
optical microscope (Leica Microsystems, GmbH).
Analysis of immunohistochemical
results
The immunohistochemical results were analyzed using
the PHMIAS2008CSVER3.0 Automatic Image Analysis system (Phenix
Optical (Guangdong), Co., Ltd., Zhongshan, China). Eight image
fields were randomly selected in each tissue section under an
optical microscope, after which grayscale images were captured and
the tubular/spherical positive immunohistochemical staining was
observed. The relative protein expression levels were calculated
and analyzed using the BIOMIAS-2001 High-Resolution Image Analysis
system (Institute of Image and Graphics, Sichuan University,
Chengdu, China). Five non-overlapping fields (magnification, ×400)
were randomly selected in each section for the determination of
integrated optical density (IOD) values for GSK-3β, NF-κB, RANK and
RANKL. The IOD value was used to represent the expression
intensity, where the larger IOD value reflected upregulated
expression.
RT-qPCR
Total RNA was extracted from the kidney sections
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and was treated with RNase-free
DNase (Promega Corporation, Madison, WI, USA). The concentration of
RNA was quantified using the Nano-200 Nucleic Acid analyzer
(Hangzhou Allsheng Instruments Co., Ltd., Hangzhou, China). The
reverse transcription reaction with 13 µl total RNA was performed
using the PrimeScript First Strand cDNA Synthesis kit (Takara
Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's protocol. PCR was conducted using the CFX96 Touch
Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA), with a reaction mixture consisting of 5 µl SYBR
Green 1 dye (Thermo Fisher Scientific Inc.), 7.5 µl 2X Premix Ex
Taq (Sangon Biotech Co., Ltd.), 0.25 µl forward and reverse primer,
3 µl cDNA (5 ng/µl) and 4 µl dH2O (Sangon Biotech Co.,
Ltd.). The primers used in the were as follows: GSK-3β forward,
5′-GCTTCAACCCCTTCAAATGC-3′ and reverse,
5′-GACGCAGAAGCGGTGTTATTG-3′; RANK forward,
5′-TTAAGCCAGTGCTTCACGGG-3′ and reverse, ACGTAGACCACGATGATGTCGC-3′;
RANKL forward, 5′-GCTCACCTCACCATCAATGCT-3′ and reverse,
5′-GGTACCAAGAGGACAGACTGACTTTA-3′; NF-κB forward,
5′-ATAGAAGAGCAGCGTGGGGACT-3′ and reverse,
5′-GGATGACGTAAAGGGATAGGGC-3′; and β-actin forward,
5′-GGAGATTACTGCCCTGGCTCCTA-3′, and reverse,
5′-GACTCATCGTACTCCTGCTTGCTG-3′ (Generay Biotech, Co., Ltd.,
Shanghai, China). The PCR cycling conditions were as follows: 95°C
for 15 min, followed by 40 cycles of 95°C for 15 sec, 93°C for 30
sec and 55°C for 40 sec, and a final extension step at 72°C for 5
min.
Analysis of RT-qPCR results
The StepOnePlus Real-Time PCR system (Thermo Fisher
Scientific, Inc.) was used to amplify the fluorescence signal from
each cycle, which was analyzed using the Applied Biosystems
Sequence Detection Systems software, version 14.0 (Thermo Fisher
Scientific, Inc.). The quantification cycle (CQ) and relative
quantification (RQ) values were analyzed as follows: RQ=2-CQ. The
mRNA expression levels of GSK-3β, RANK, RANKL, and NF-κB, relative
to β-actin, were calculated using the 2−ΔΔCq method
(19).
Statistical analysis
Data are presented as the mean ± standard error of
the mean of ≥3 independent experiments. Statistical analyses were
conducted using Microsoft Excel with unpaired Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
General data
Prior to the establishment of the DN model, all rats
received standard diets, and had normal faeces and urine. All rats
had clean fur, and there was no significant difference in the body
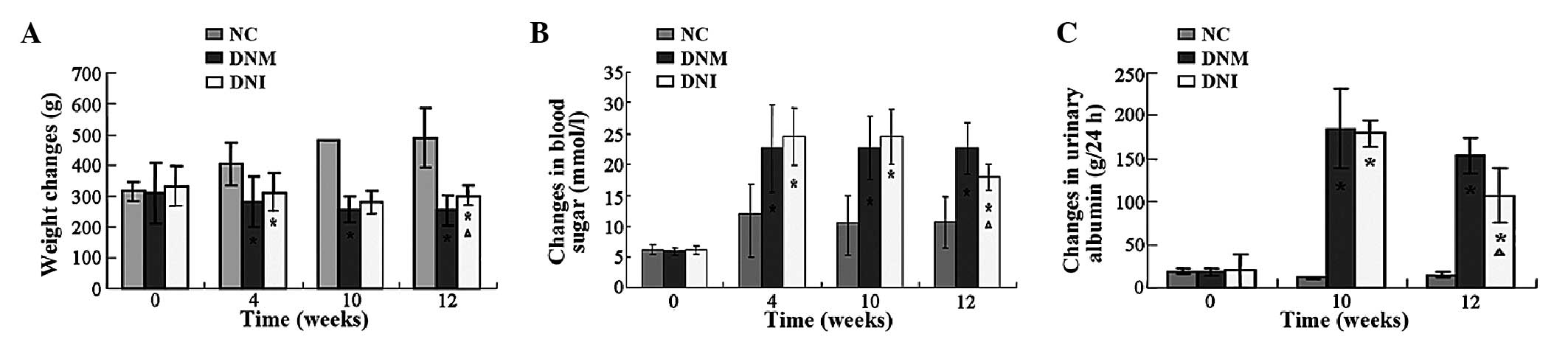
weight of the rats between the groups (P>0.05; Fig. 1A). After modeling, the rats exhibited
varying degrees of polyuria, polydipsia, polyphagia, weight loss
and reduced movement. In addition, the fur of the rats in the DNM
and DNI groups was messy and dull in appearance. At 4 and 10 weeks
post-modeling, the body weights of the rats in the DNM and DNI
groups were significantly reduced, as compared with the body
weights of the rats in the NC group (P<0.05; Fig. 1A). At 12 weeks, the body weights of
the rats in the DNM and DNI groups were significantly lower, as
compared with the NC group (P<0.05; Fig. 1A); however, the body weights of the
rats in the DNI group were significantly higher, as compared with
the DNM group (P<0.05; Fig. 1A).
The indices of blood glucose and urine protein concentrations in
the DNM and DNI groups were significantly higher, as compared with
those of the NC group (P<0.05; Fig.
1B and C); however, treatment with LiCl was able to
significantly reduce the blood glucose and urine protein
concentrations in the DNI rats, as compared with the DNM group
(P<0.05; Fig. 1B and C). These
results suggested that the rat model of DN had been successfully
established, and that LiCl treatment was able to mitigate the DN in
rats.
HE staining
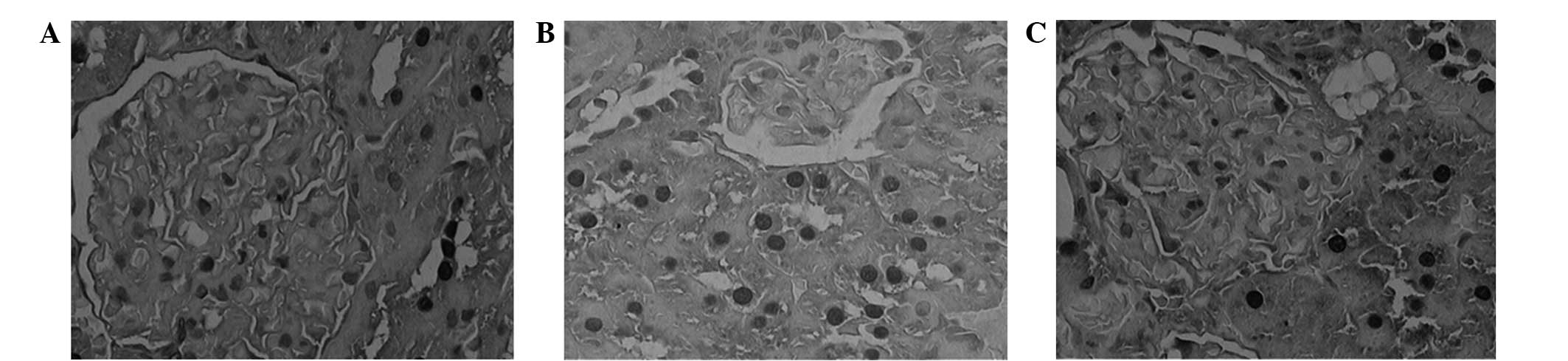
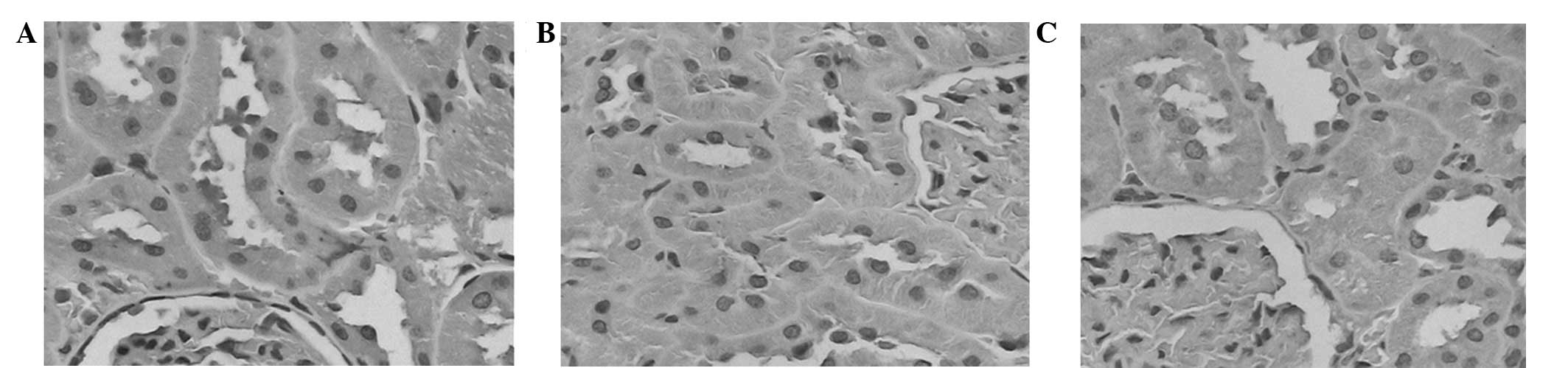
In the NC group, the sizes of the glomeruli appeared
uniform, cell proliferation was not detected, the capillary
basement membrane was uniform and thin, the mesangium appeared
narrow and the cavity of the capillary was open (Fig. 2A and B). Furthermore, the epithelial
cells of the renal tubules were intact, the basement membrane was
thin, the peritubular capillaries exhibited no fibrosis and there
was no obvious interstitial edema (Fig.
2A and B). Conversely, in the DNM group, the rats exhibited
glomerular hypertrophy, the mesangial matrix was increased in size,
the capillary basement membrane was thickened and luminal occlusion
and renal arteriosclerosis was detected (Fig. 2C and D). In addition, the tubular
basement membrane appeared thickened, and stenosis and occlusion,
interstitial edema and fibrosis were detected (Fig. 2C and D). In the DNI group, the volume
of the glomeruli was slightly increased, and the capillary basement
membrane was only marginally thickened, as compared with the DNM
group (Fig. 2E and F). In addition,
the mesangial matrix was only slightly thickened, the degree of
degeneration of tubular epithelial cells was improved, and luminal
narrowing was not obvious, as compared with the DNM group (Fig. 2C and D).
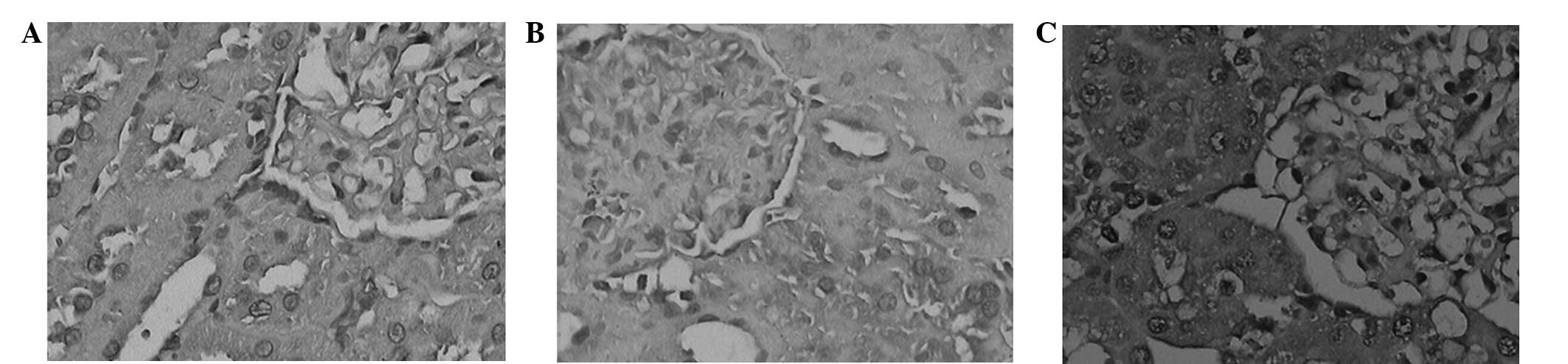
 | Figure 2.Renal cells of rats in the normal
control (NC), diabetic nephropathy (DN) model (DNM) and DN model
lithium chloride intervention (DNI) groups. (A) NC group
(magnification, ×200); (B) NC group (magnification, ×400); (C) DNM
group (magnification, ×200); (D) DNM group (magnification, ×400);
(E) DNI group (magnification, ×200); (F) DNI group (magnification,
×400). (A and B) In the NC group, the structure of glomerular
appeared normal, the size was uniform, there was no evidence of
hypertrophy of endothelial cells, mesangial cells or the glomerular
cell wall, the mesangial matrix appeared normal in size and the
morphology of the tubules was normal. (C and D) In the DNM group,
glomerular hypertrophy was detected, the mesangial matrix was
increased in size, the basement membrane was thickened, edema of
the epithelial cells of the renal tubules was detected, and the
lumen appeared narrowed or occluded. (E and F) In the DNI group,
the cells of the mesangial had proliferated, the mesangial matrix
was only slightly increased, the basement membrane was thickened
only mildly, and edema of tubular epithelial cells was abated. |
Immunohistochemistry
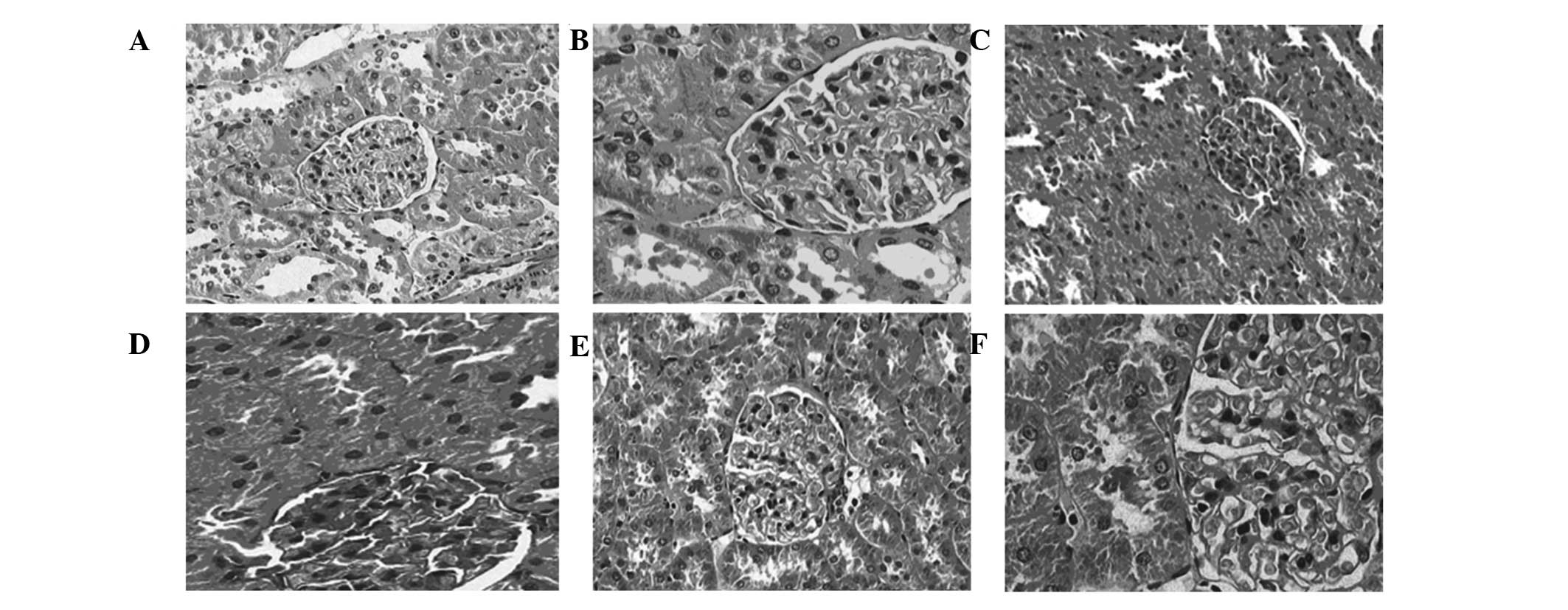
The sites that stained positively for GSK-3β were
predominantly in the cytoplasm of renal tubular epithelial cells,
although its expression was also detected in the glomeruli. The
protein expression levels of GSK-3β were markedly increased in the
renal tubular epithelial cells of the DNM group, as compared with
the NC group (Fig. 3), and this
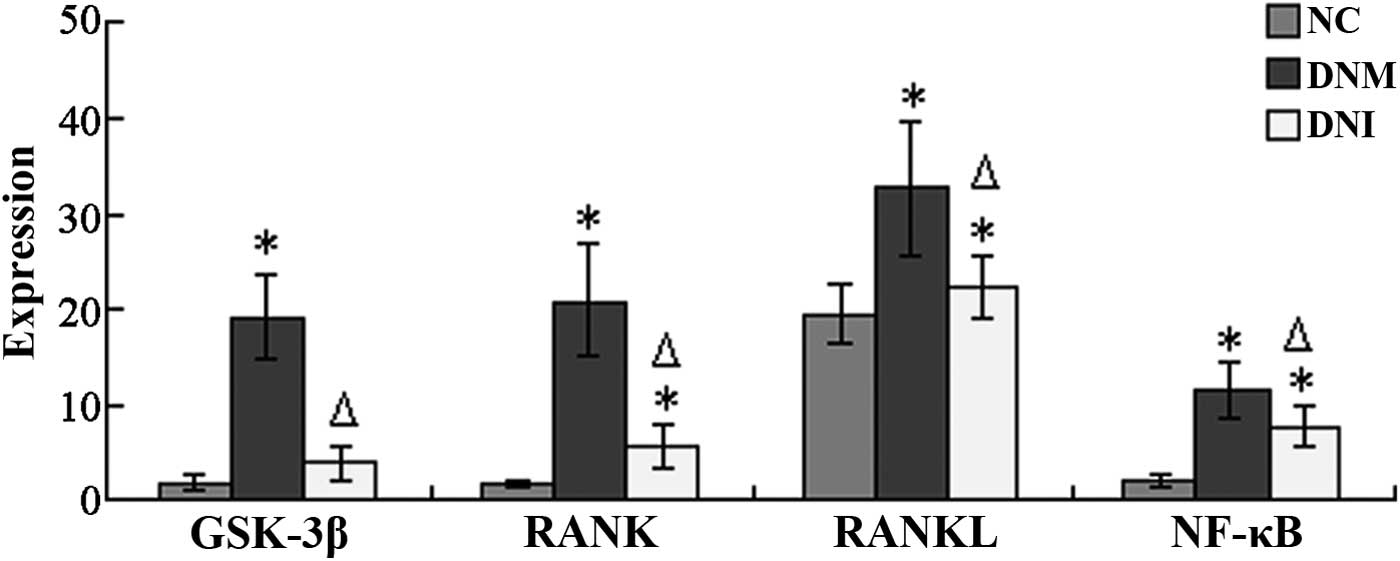
difference was shown to be significant (P<0.05; Fig. 4). Conversely, the expression levels
of GSK-3β were markedly decreased in the DNI group, as compared
with the DNM group (Fig. 3), and
this difference was shown to be significant (P<0.05; Fig. 4). These results suggested that LiCl
may attenuate DN-induced increases in the expression levels of
GSK-3β.
 | Figure 3.Immunohistochemical analysis of
glycogen synthase kinase-3β in the renal tissues of rats in the (A)
normal control (NC) group (magnification, ×200), (B) NC group
(magnification, ×400), (C) diabetes nephropathy model (DNM) group
(magnification, ×200), (D) DNM group (magnification, ×400), (E)
diabetic nephropathy group treated with lithium chloride (DNI)
group (magnification, ×200) and (F) DNI group (magnification,
×400). |
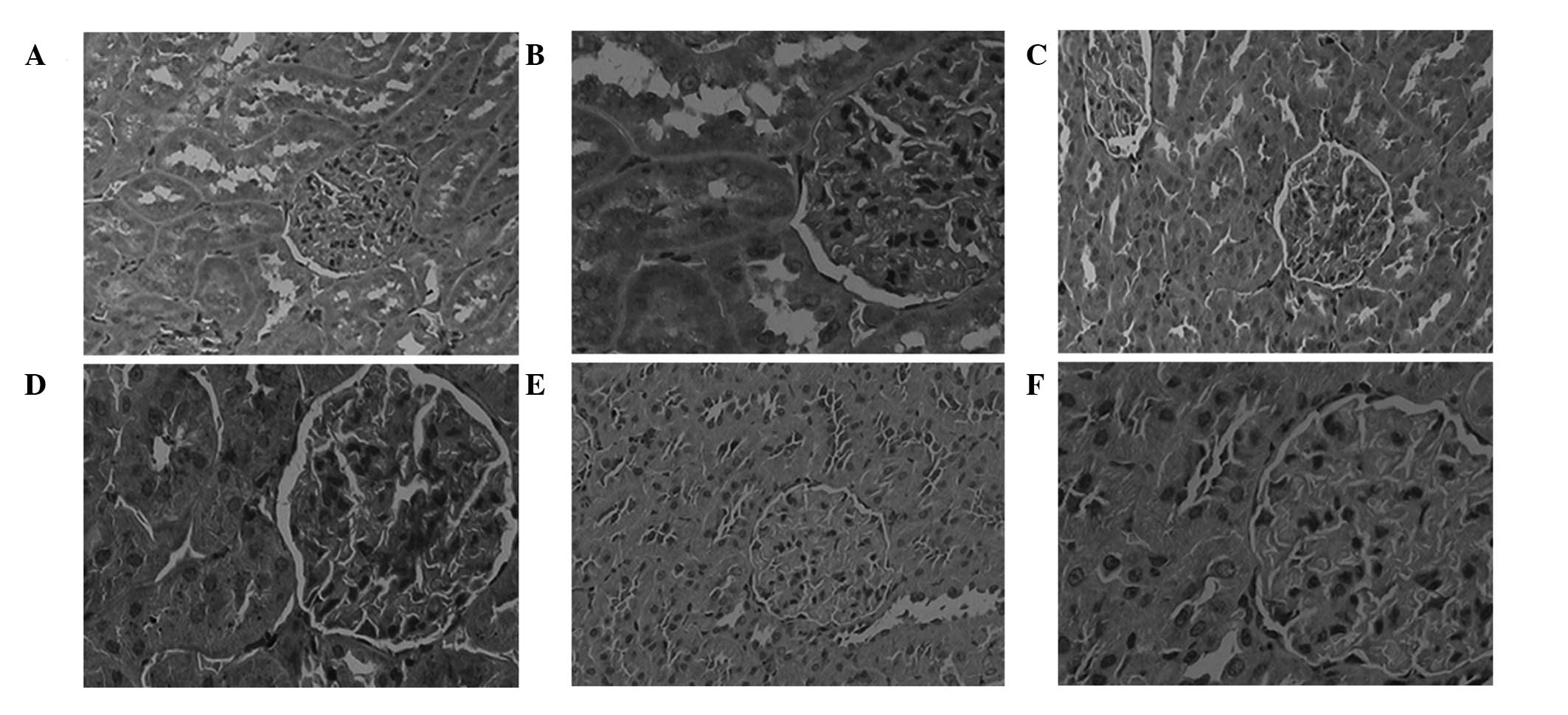
Positive sites of RANK protein expression were
detected in the glomeruli and certain tubules. The protein
expression levels of RANK were markedly increased in the DNM group,
as compared with the NC group (Fig.
5), and this difference was shown to be significant (P<0.05;
Fig. 4). However, the protein
expression levels of RANK were markedly decreased in the DNI group,
as compared with the DNM group (Fig.
5), and this difference was shown to be significant (P<0.05;
Fig. 4).
The RANKL protein was detected in the tubules and
glomeruli, and appeared as yellow granules. The protein expression
levels of RANKL were markedly increased in the DNM group, as
compared with the NC group (Fig. 6),
and this difference was shown to be significant (P<0.05;
Fig. 4). Conversely, the protein
expression levels of RANKL were markedly decreased in the DNI
group, as compared with the DNM group (Fig. 6), and this difference was shown to be
significant (P<0.05; Fig. 4).
Positive sites of NF-κB occurred predominantly in
the cytoplasm and nucleus of the renal tubules. In the NC group,
low levels of NF-κB protein expression were detected in the
cytoplasm of tubular epithelial cells only, whereas, in the DNM
group, high levels of NF-κB were detected in the cytoplasm and low
levels were detected in the nucleus (Fig. 7). As compared with the same period in
the NC group, the protein expression levels of NF-κB were
significantly increased in the DNM group (P<0.05; Fig. 4). Conversely, the protein expression
levels of NF-κB were markedly decreased in the DNI group, as
compared with the DNM group (Fig.
7), and this difference was shown to be significant (P<0.05;
Fig. 4).
RT-qPCR
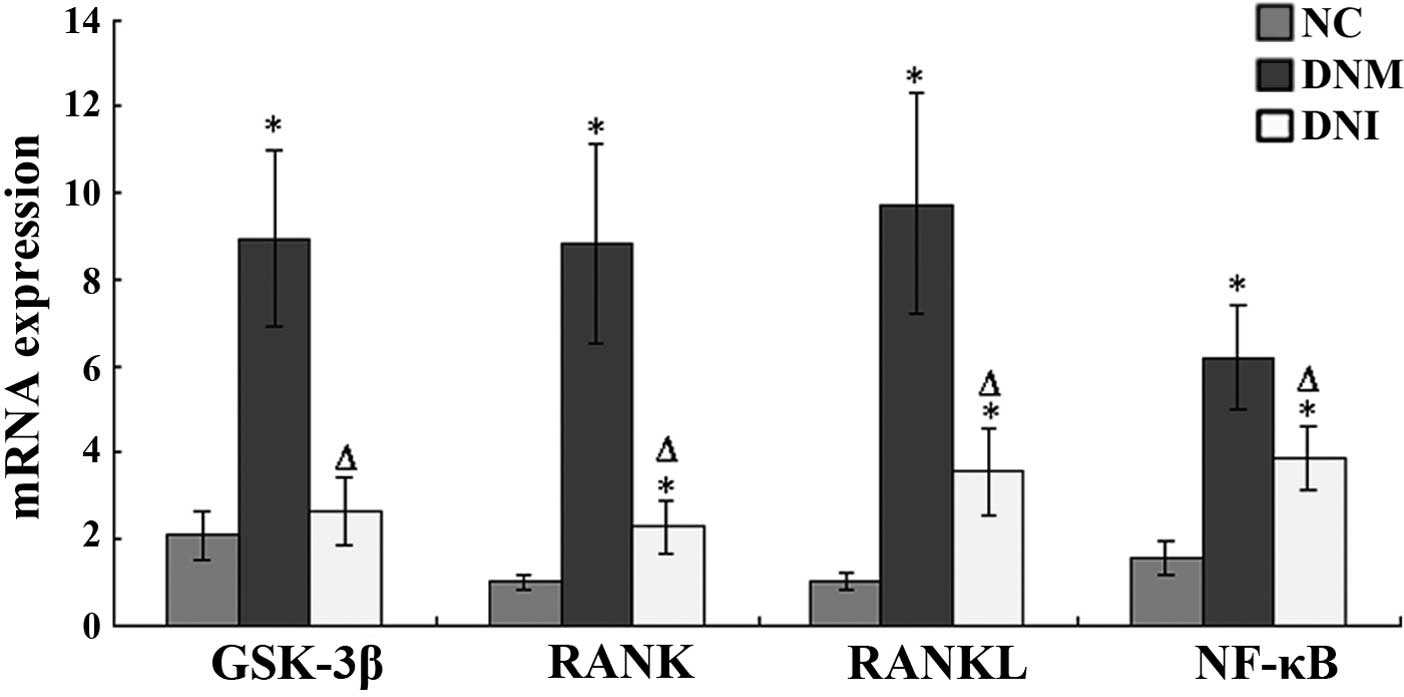
The mRNA expression levels of GSK-3β in the renal
tissue sections from the DNM rats were significantly increased, as
compared with the NC rats (P<0.05; Fig. 8), whereas the mRNA expression levels
of GSK-3β in the DNI rats were significantly reduced, as compared
with the DNM rats (P<0.05; Fig.
8).
The mRNA expression levels of RANK in the renal
tissue sections from the DNM and DNI groups were significantly
increased, as compared with the NC rats (P<0.05; Fig. 8). However, the mRNA expression levels
of RANK were significantly lower in the DNI group, as compared with
the DNM group (P<0.05; Fig.
8).
The mRNA expression levels of RANKL in the renal
tissue sections from the DNM and DNI groups were significantly
higher, as compared with the NC group (P<0.05; Fig. 8). However, the mRNA expression levels
of RANKL were significantly lower in the DNI group, as compared
with the DNM group (P<0.05; Fig.
8).
The mRNA expression levels of NF-κB in the renal
tissue sections from the DNM and DNI groups were significantly
higher, as compared with the NC group (P<0.05; Fig. 8). However, the mRNA expression levels
of NF-κB in the DNI group were significantly lower, as compared
with the DNM group (P<0.05; Fig.
8).
Discussion
DN is a common complication of DM, and a key cause
of disability and mortality in patients with DM. The pathogenesis
underlying DN is currently unclear, although previous studies have
reported multifactorial results, including a disorder of glucose
metabolism (20), hemodynamic
alterations (21) and inflammatory
cytokines (22). GSK-3β is a
serine/threonine kinase, which has a variety of functions and
exists in all eukaryotic cells (23). Previous studies have demonstrated
that, in addition to catalyzing the phosphorylation of glycogen
synthase, GSK-3β is involved in signaling pathways, including the
insulin (24), Wnt/β-catenin
(25), Hedgehog (26) and Notch (27) pathways. GSK-3β has also been shown to
have a regulatory role in cell differentiation, metabolism,
apoptosis and inflammation (28). A
positive regulatory role for GSK-3β was initially detected in
fibroblasts (29). Subsequently, it
was demonstrated that inhibiting the activity of GSK-3β reduced the
transcriptional activity of NF-κB target genes and the expression
of its target proteins (30). NF-κB
is a transcription factor that is expressed by a variety of cells
and has been shown to have numerous regulatory roles (31). In the resting state, NF-κB is held in
an inactive form in the cytoplasm by its inhibitor IκB; however,
when a cell encounters one of various stimulators, including oxygen
free radicals, cytokines and viruses, IκB is rapidly degraded,
NF-κB and IκB dissociate and NF-κB enters the nucleus and binds to
specific sequences, in order to regulate the expression of target
genes (32).
In a previous study, stimulation of RANKL was
associated with activation of the NF-κB signaling pathway (33). The signal from RANKL is transduced to
TNF receptor-associated factor 6 (TRAF6) via RANK, after which
TRAF6 may activate NF-κB via the NF-κB-inducible kinase and the
NF-κB inducing kinase agent (34,35). In
previous studies, RANK expression has not only been in detected in
lymphocytes, but also in the precursor cells of osteoclasts, mature
osteoclasts and myofibroblasts (35–38).
Inhibitors of GSK-3β have previously been shown to
regulate glucose metabolism, the epithelial-mesenchymal transition
and insulin resistance, thereby preventing the occurrence of DN
(39). Furthermore, previous studies
have reported that GSK-3β inhibitors may inhibit the
phosphorylation of the p65 carboxy-terminal, thereby inhibiting the
activation of NF-κB and reducing the transcription of the ICAM-1
gene (40,41). As an inhibitor of GSK-3β, LiCl is
commonly used for the treatment of mania and various other diseases
and its target enzymes include GSK-3β and inositol phosphatase. A
previous study demonstrated in vitro and in vivo that
GSK-3β regulated the production of various inflammatory mediators
by the action of its downstream substrates, including NF-κB and
cAMP response element-binding protein, thereby balancing pro- and
anti-inflammatory responses (42).
Therefore, inhibition of GSK-3β may serve a crucial function in the
regulation of inflammation (43).
Since inflammation is a key factor underlying the pathogenesis of
DN (44–46), GSK-3β inhibition may be considered a
promising therapeutic target.
The present study aimed to investigate the
pathogenesis of DN by detecting the mRNA and protein expression
levels of RANK, RANKL and NF-κB in renal tissues from a rat model
of DN, as well as analyzing whether treatment of the rats with a
GSK-3β inhibitor exerted regulatory effects on the expression
levels of RANK, RANKL and NF-κB. The results of the present study
suggested that the expression levels of RANK, RANKL and NF-κB were
significantly increased in the DNM group, as compared with the NC
group; thus suggesting that these proteins may be involved in the
development of DN. In addition, LiCl treatment was able to
significantly attenuate the DN-induced increase in the mRNA and
protein expression levels of RANK, RANKL and NF-κB. Furthermore,
the urine protein concentrations were significantly decreased in
the DNI group at 24 h, as compared with the DNM group, and HE
staining demonstrated that DN-induced pathological alterations in
the renal tissues of the rats were alleviated in the DNI group.
In conclusion, the present study demonstrated that
RANK, RANKL and NF-κB were involved in the development of DN, and
that LiCl was able to reduce the expression levels of RANK, RANKL
and NF-κB by inhibiting GSK-3β. In addition, LiCl treatment
attenuated inflammation and endothelial cell apoptosis, which in
turn may have delayed the development of DN. These results
suggested that GSK-3β may be considered a potential target of
cytokines for the treatment of DN.
Acknowledgements
The present study was supported by the China
National Natural Science Foundation (grant no. 31360280), the
International Cooperation Projects of Guizhou Province (grant no.
[2012]7039), the Qianren Project of Ministry of Human Resources and
Social Security of the People's Republic of China (grant no.
2015-04), and the China State Administration of Foreign Experts
Affairs Talent Recruitment Project (grant no. 20145200012).
References
|
1
|
Kharroubi AT and Darwish HM: Diabetes
mellitus: The epidemic of the century. World J Diabetes. 6:850–867.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Al-Rubeaan K, Youssef AM, Subhani SN,
Ahmad NA, Al-Sharqawi AH, Al-Mutlaq HM, David SK and AlNaqeb D:
Diabetic nephropathy and its risk factors in a society with a type
2 diabetes epidemic: A Saudi National Diabetes Registry-based
study. PLoS One. 9:e889562014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gluhovschi GH, Gluhovschi C, Vlad A, Timar
R, Bob F, Velciov S, Bozdog GH and Petrica L: Diabetic nephropathy
and multiorgan protection. Part I. Rom J Intern Med. 49:163–177.
2011.PubMed/NCBI
|
|
4
|
Gluhovschi G, Gluhovschi C, Vlad A, Timar
R, Bob F, Velciov S, Bozdog G and Petrica L: Diabetic nephropathy
and multiorgan protection. Part II. Rom J Intern Med. 49:237–249.
2011.PubMed/NCBI
|
|
5
|
Mooyaart AL, Valk EJ, van Es LA, Bruijn
JA, de Heer E, Freedman BI, Dekkers OM and Baelde HJ: Genetic
associations in diabetic nephropathy: A meta-analysis.
Diabetologia. 54:544–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mima A: Inflammation and oxidative stress
in diabetic nephropathy: New insights on its inhibition as new
therapeutic targets. J Diabetes Res. 2013:2485632013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X and Lu FE: Significance of
anti-inflammation and immune regulation in the treatment of
diabetic nephropathy. Zhongguo Zhong Xi Yi Jie He Za Zhi.
30:649–654. 2010.(In Chinese). PubMed/NCBI
|
|
8
|
Chang YT, Chen CL, Lin CF, Lu SL, Cheng
MH, Kuo CF and Lin YS: Regulatory role of GSK-3β on NF-κB, nitric
oxide, and TNF-α in group A streptococcal infection. Mediators
Inflamm. 2013:7206892013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanaka S: Signaling axis in osteoclast
biology and therapeutic targeting in the RANKL/RANK/OPG system. Am
J Nephrol. 27:466–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koya D, Haneda M, Nakagawa H, Isshiki K,
Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, et al:
Amelioration of accelerated diabetic mesangial expansion by
treatment with a PKC beta inhibitor in diabetic db/db mice, a
rodent model for type 2 diabetes. FASEB J. 14:439–447.
2000.PubMed/NCBI
|
|
11
|
Mima A, Ohshiro Y, Kitada M, Matsumoto M,
Geraldes P, Li C, Li Q, White GS, Cahill C, Rask-Madsen C and King
GL: Glomerular-specific protein kinase C-β-induced insulin receptor
substrate-1 dysfunction and insulin resistance in rat models of
diabetes and obesity. Kidney Int. 79:883–896. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma X, Liu Y, Zhang Y, Yu X, Wang W and
Zhao D: Jolkinolide B inhibits RANKL-induced osteoclastogenesis by
suppressing the activation NF-κB and MAPK signaling pathways.
Biochem Biophys Res Commun. 445:282–288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Franchimont N, Reenaers C, Lambert C,
Belaiche J, Bours V, Malaise M, Delvenne P and Louis E: Increased
expression of receptor activator of NF-kappaB ligand (RANKL), its
receptor RANK and its decoy receptor osteoprotegerin in the colon
of Crohn's disease patients. Clin Exp Immunol. 138:491–498. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu S, Shi W, Xiao H, Liang X, Deng C, Ye
Z, Mei P, Wang S, Liu X, Shan Z, et al: Receptor activator of
NF-kappaB and podocytes: Towards a function of a novel
receptor-ligand pair in the survival response of podocyte injury.
PLoS One. 7:e413312012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kelly DJ, Hepper C, Wu LL, Cox AJ and
Gilbert RE: Vascular endothelial growth factor expression and
glomerular endothelial cell loss in the remnant kidney model.
Nephrol Dial Transplant. 18:1286–1292. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berthier CC, Zhang H, Schin M, Henger A,
Nelson RG, Yee B, Boucherot A, Neusser MA, Cohen CD, Carter-Su C,
et al: Enhanced expression of Janus kinase-signal transducer and
activator of transcription pathway members in human diabetic
nephropathy. Diabetes. 58:469–477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nam JS, Cheong YS, Karm MH, Ahn HS, Sim
JH, Kim JS, Choi SS and Leem JG: Effects of nefopam on
streptozotocin-induced diabetic neuropathic pain in rats. Korean J
Pain. 27:326–333. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Flyvbjerg A, Denner L, Schrijvers BF,
Tilton RG, Mogensen TH, Paludan SR and Rasch R: Long-term renal
effects of a neutralizing RAGE antibody in obese type 2 diabetic
mice. Diabetes. 53:166–172. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McDonald SD, Pesarchuk E, Don-Wauchope A,
El Zimaity H and Holloway AC: Adverse metabolic effects of a
hypercaloric, high-fat diet in rodents precede observable changes
in body weight. Nutr Res. 31:707–714. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chirinos JA, Segers P, Gillebert TC, De
Buyzere ML, Van Daele CM, Khan ZA, Khawar U, De Bacquer D and
Rietzschel ER: Asklepios Investigators: Central pulse pressure and
its hemodynamic determinants in middle-aged adults with impaired
fasting glucose and diabetes: The Asklepios study. Diabetes Care.
36:2359–2365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matia-García I, de la Cruz-Mosso U,
Muñoz-Valle JF and Parra-Rojas I: Macrophage migration inhibitory
factor and its relationship with obesity and diabetes. Invest Clin.
55:266–277. 2014.(In Spanish). PubMed/NCBI
|
|
23
|
Gong R, Rifai A, Ge Y, Chen S and Dworkin
LD: Hepatocyte growth factor suppresses proinflammatory NFκB
activation through GSK3β inactivation in renal tubular epithelial
cells. J Biol Chem. 283:7401–7410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bouskila M, Hirshman MF, Jensen J,
Goodyear LJ and Sakamoto K: Insulin promotes glycogen synthesis in
the absence of GSK3 phosphorylation in skeletal muscle. Am J
Physiol Endocrinol Metab. 294:E28–E35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ho C, Lee PH, Hsu YC, Wang FS, Huang YT
and Lin CL: Sustained Wnt/β-catenin signaling rescues high glucose
induction of transforming growth factor-β1-mediated renal fibrosis.
Am J Med Sci. 344:374–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ougolkov AV and Billadeau DD: Inhibition
of glycogen synthase kinase-3. Methods Mol Biol. 468:67–75. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kavanagh D, McKay GJ, Patterson CC,
McKnight AJ, Maxwell AP and Savage DA: Warren 3/UK GoKinD Study
Group: Association analysis of Notch pathway signalling genes in
diabetic nephropathy. Diabetologia. 54:334–338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Holmes T, O'Brien TA, Knight R, Lindeman
R, Symonds G and Dolnikov A: The role of glycogen synthase
kinase-3beta in normal haematopoiesis, angiogenesis and leukaemia.
Curr Med Chem. 15:1493–1499. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aljada A, Ghanim H, Saadeh R and Dandona
P: Insulin inhibits NFkappaB and MCP-1 expression in human aortic
endothelial cells. J Clin Endocrinol Metab. 86:450–453. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen H, Yang S, Yang Z, Ma L, Jiang D, Mao
J, Jiao B and Cai Z: Inhibition of GSK-3beta decreases
NF-kappaB-dependent gene expression and impairs the rat liver
regeneration. J Cell Biochem. 102:1281–1289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baldwin AS Jr: The NF-kappa B and I kappa
B proteins: New discoveries and insights. Annu Rev Immunol.
14:649–681. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Perkins ND: Integrating cell-signalling
pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Otero JE, Chen T, Zhang K and Abu-Amer Y:
Constitutively active canonical NF-κB pathway induces severe bone
loss in mice. PLoS One. 7:e386942012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Darnay BG, Ni J, Moore PA and Aggarwal BB:
Activation of NF-kappaB by RANK requires tumor necrosis factor
receptor-associated factor (TRAF) 6 and NF-kappaB-inducing kinase.
Identification of a novel TRAF6 interaction motif. J Biol Chem.
274:7724–7731. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ruocco MG, Maeda S, Park JM, Lawrence T,
Hsu LC, Cao Y, Schett G, Wagner EF and Karin M: I{kappa}B kinase
(IKK){beta}, but not IKK{alpha}, is a critical mediator of
osteoclast survival and is required for inflammation-induced bone
loss. J Exp Med. 201:1677–1687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Satomi N, Sakurai A and Haranaka K:
Relationship of hypoglycemia to tumor necrosis factor production
and antitumor activity: Role of glucose, insulin, and macrophages.
J Natl Cancer Inst. 74:1255–1260. 1985.PubMed/NCBI
|
|
37
|
Gilbert RE, Kim SA, Tuttle KR, Bakris GL,
Toto RD, McGill JB, Haney DJ, Kelly DJ and Anderson PW: Effect of
ruboxistaurin on urinary transforming growth factor-beta in
patients with diabetic nephropathy and type 2 diabetes. Diabetes
Care. 30:995–996. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ohshiro Y, Ma RC, Yasuda Y,
Hiraoka-Yamamoto J, Clermont AC, Isshiki K, Yagi K, Arikawa E, Kern
TS and King GL: Reduction of diabetes-induced oxidative stress,
fibrotic cytokine expression, and renal dysfunction in protein
kinase Cbeta-null mice. Diabetes. 55:3112–3120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao C, Hölscher C, Liu Y and Li L: GSK3: A
key target for the development of novel treatments for type 2
diabetes mellitus and Alzheimer disease. Rev Neurosci. 23:1–11.
2012. View Article : Google Scholar
|
|
40
|
Hinz M and Scheidereit C: The IκB kinase
complex in NF-κB regulation and beyond. EMBO Rep. 15:46–61. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen Y, Gan H, Ouyang Q, Xu D and Pan YAZ:
The effects of anti-inflammatory on activation of nuclear
factor-kappaB and expression of cell adhesion molecules in patients
with ulcerative colitis. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi.
21:732–736. 2004.(In Chinese). PubMed/NCBI
|
|
42
|
Ali A, Hoeflich K and Woodgett JR:
Glycogen synthase kinase-3: Properties, functions, and regulation.
Chem Rev. 101:2527–2540. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
You W, Min X, Zhang X, Qian B, Pang S,
Ding Z, Li C, Gao X, Di R, Cheng Y and Liu L: Cardiac-specific
expression of heat shock protein 27 attenuated endotoxin-induced
cardiac dysfunction and mortality in mice through a
PI3K/Akt-dependent mechanism. Shock. 32:108–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
García-García PM, Getino-Melián MA,
Domínguez-Pimentel V and Navarro-González JF: Inflammation in
diabetic kidney disease. World J Diabetes. 5:431–443. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Agrawal NK and Kant S: Targeting
inflammation in diabetes: Newer therapeutic options. World J
Diabetes. 5:697–710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Duran-Salgado MB and Rubio-Guerra AF:
Diabetic nephropathy and inflammation. World J Diabetes. 5:393–398.
2014. View Article : Google Scholar : PubMed/NCBI
|