Introduction
Helicobacter pylori (H. pylori) are
Gram-negative bacteria that adhere to the surface of gastric mucosa
and cause inflammation, but do not invade gastric epithelial cells
(1). H. pylori infect ~50% of
the world population and the main risk factors include age,
ethnicity, gender, geographic location and socioeconomic status
(2). Infection with H. pylori
is considered the major cause of active chronic gastritis and also
serves an notable role in peptic ulcers (3).
Since the discovery of H. pylori, it has been
strongly associated with the development of gastric cancer
(4). H. pylori was the first
bacterial species to be recognized by the International Agency for
Research on Cancer as a group I carcinogen (5). The development of cancer in H.
pylori infected individuals may be through the following
possible mechanisms: i) The production of mutagenic radicals as an
inflammatory response to H. pylori infection; ii) the
reduction of antioxidants in mucosa; and iii) the induction of a
hyperproliferative state (6,7). However, only a small percentage of
infected individuals will develop neoplasia (1–3%) due to specific
interactions between the host and pathogen, which are dependent on
specific bacteriological factors and/or inflammatory responses
regulated by host genes (8,9).
H. pylori-induced gastritis can lead to other
types of cellular damage (10). The
most prevalent form of gastric neoplasia is intestinal-type
adenocarcinoma, which evolves through a series of events initiated
by the transition of normal mucosa to superficial chronic
gastritis, which subsequently results in atrophic gastritis,
intestinal metaplasia, and eventually to dysplasia and neoplasia
(11). Various inflammatory
biomarkers, including the tumor necrosis factor-α (TNF-α), nuclear
factor-κB (NF-κB) and interleukins (ILs), can be used to track the
progress of diseases, as well as assist in the development of novel
anti-inflammatory drugs for the treatment and prevention of cancer
(8). Pathogenic stimuli induce the
expression of TNF-α, which in turn induces proteases and
other mediators responsible for inflammatory responses. TNF-α has
been associated with the various steps involved in tumorigenesis,
including cell transformation, invasion, proliferation, survival,
promotion, angiogenesis and metastasis (8). The activation of TNF receptor 1 (TNFR1)
by TNF-α can result in the activation of NF-κB as a result of
cellular responses (12).
NF-κB serves an essential role in inflammation and
innate immunity, and it is becoming increasingly recognized for its
crucial role in the initiation and progression of cancer, operating
in numerous cell signaling pathways (13). NF-κB regulates the expression of
various substances associated with inflammation and is inactive in
the majority of cells, but it exists in an activated form in cancer
cells (14). This activation is
induced by a wide variety of carcinogens and inflammatory stimuli
(14,15). In the classic NF-κB signaling
pathway, lipopolysaccharides, TNF-α or IL-1 activate TNFR and IL-1
receptors (13).
p38 is a mitogen-activated protein kinase (MAPK)
involved in the regulation of inflammatory cytokine biosynthesis
(16) and stress responses, such as
heat shock or infection (17,18).
Protein kinases are the main regulators in pathways of
embryogenesis, cell differentiation, proliferation and cell death
(19). The p38 MAPK family consists
of four identified isoforms, including p38α, p38β, p38γ and p38δ,
encoded by the genes MAPK14, MAPK11, MAPK12
and MAPK13, respectively. In human tissues, the most
abundant MAPK is p38α (16,20). Although the activation of p38α is
normally associated with anti-proliferative functions, studies
indicate that it has the ability to upregulate cell proliferation
in human tumors and cancer cell lines (20,21).
The use of quantitative polymerase chain reaction
(qPCR) to compare mRNA levels between biopsies from different
individuals and disease states requires meticulous normalization.
For the correction of results from different samples and
experimental conditions, the use of an endogenous reference is
essential (22). In a number of
studies, it has been demonstrated that although common reference
genes are considered to be stable and secure in various tissues,
this is often not the case (23,24).
Only one previous study has validated reference genes for gastric
samples in a Western population of patients with adenocarcinoma
(25).
Currently, the association of inflammation, innate
immunity and cancer is widely accepted (26); however, the cellular and molecular
mechanisms that mediate these processes remain unknown. The present
study aimed to evaluate the mRNA levels of TNF-α,
NFKB1 and p38α in human gastric mucosa samples, and
to investigate the influence of H. pylori on the expression
of these genes in a southern Brazilian population. In order to
perform gene expression analysis, the suitability of five reference
genes were assessed to determine their suitability for use in qPCR
normalization. Although the five genes have been widely studied
(27,28), there are presently no studies
evaluating their association with H. pylori infection and
human gastric inflammation.
Materials and methods
Sample acquisition
Human gastric tissues were obtained by upper
endoscopy from 79 adult patients, including 49 women and 30 men
(aged 47.33±14.31 years), who were admitted to the Endoscopy
Service at Hospital Bruno Born (Lajeado, Brazil) between October
2013 and April 2014 with symptoms of gastritis, including
epigastric pain, nausea, vomiting, bloating, belching, heartburn,
halitosis and flatulence (29). The
present study was approved by the local ethics committee (Univates,
Lajeado, Brazil; CEP 353.624) and written informed consent was
obtained from each patient prior to sample collection. Exclusion
criteria included coagulation disorders (including patients with
problems preventing gastric biopsy), and the use of
anti-inflammatory drugs, antibiotics or proton pump inhibitors.
Two biopsy samples (~3 mm) were obtained from the
lesser curvature of the gastric antrum, one of which was used for
the rapid urease test (RNA Laboratórios, Cascavel, Brazil) and the
other was placed in RNAlater Stabilization Solution (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for posterior RNA
extraction, cDNA synthesis and gene expression analysis. In
addition, two further samples of ~3 mm were collected by the
gastroenterologist for routine histological analyses. Briefly, the
samples were formalin-fixed, paraffin-embedded (both Allkimia,
Campinas, SP, Brazil) and stained with Giemsa (Quimica
Especializada Erich Ltda, São Paulo, SP, Brazil) for H.
pylori detection under the Leica DM500 microscope (Leica
Microsystems GmbH, Wetzlar, Germany).
H. pylori infection diagnosis and
histological analysis
The rapid urease test is based on the principle that
the enzyme urease produced by H. pylori hydrolyses urea into
ammonia (30). A biopsy sample was
introduced immediately after collection into a tube containing the
rapid urease test. The consequent rise in the pH of the medium in
the tube as a result of ammonia production was detected by a phenol
red indicator, changing the medium color and indicating the
presence of H. pylori. When the color change occurred during
the first 24 h, the test was considered positive (31). H. pylori diagnosis was
performed based on the results of the rapid urease, and the samples
were considered positive following confirmation with the modified
Giemsa staining procedure (32). The
samples were classified according to a histological analysis and
observation under the Leica DM500 microscope, using the Sydney
classification system (33). The
samples were divided into groups according to the severity of
changes observed in the histological examination, as follows: i)
normal (absence of inflammation); ii) inactive chronic gastritis
(inflammation without neutrophils); iii) chronic gastritis
(inflammation with neutrophils); and iv) the presence of intestinal
metaplasia (a precancerous lesion).
Total RNA extraction
Samples were processed in a Turrax-like tissue
homogenizer (Ultra Stirrer; Stanhope-Seta, Chertsey, Surrey, UK)
with TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
The total RNA extraction method by TRIzol was an adaptation from
the original method described by Chomczynski and Sacchi (34). Following extraction, the RNA was
purified using the RNAspin Mini kit (GE Healthcare Life Sciences,
Little Chalfont, UK) according to the manufacturer's instructions
and stored at −80°C. The concentration and purity of the RNA was
assessed by L-Quant spectrophotometer (Loccus Biotecnologia, Cotia,
Brazil) at 260 and 280 nm wavelengths. A ratio of ~2.0 was
considered appropriate for RNA purity.
Synthesis of cDNA
cDNA was synthesized from 1 µg total RNA using the
Superscript III First Strand Synthesis System SuperMix (Invitrogen;
Thermo Fisher Scientific, Inc.) and oligo-dT primers, according to
the manufacturer's instructions, and stored at −20°C.
Primer design
The primers used for the amplification of cDNA
fragments specific for p38α, TNF-α and NFKB1
were designed using the published sequence of each gene and the
online tool Primer3 (35). To select
a reference gene, five reference genes commonly used in gene
expression studies were examined to identify the most appropriate
gene for the current experiment. The primers were supplied by
Laboratory of Molecular Biology, Endocrinology and Tumors (Federal
University of Rio Grande do Sul, Porto Alegre, Brazil) (27). Each primer was synthesized by
Invitrogen Brazil, Ltd. (São Paulo, Brazil). A dissociation curve
was created for each pair of primers in order to confirm reaction
specificity. The melting temperature (Tm) is specific to each
amplicon. The primer sequences, product length and mean Tm of each
gene are presented in Table I.
 | Table I.Primer sequences, product lengths and
mean Tm of the studied genes. |
Table I.
Primer sequences, product lengths and
mean Tm of the studied genes.
| Gene symbol | Primer sequence
(5′-3′) | NCBI no. | Product length
(bp) | Mean Tm (°C) |
|---|
| NFKB1 |
|
|
|
|
|
Sense |
ACACCGTGTAAACCAAAGCC | NM_003998.3 | 209 | 82.71 |
|
Antisense |
CAGCCAGTGTTGTGATTGCT |
|
|
|
| p38α |
|
|
|
|
|
Sense |
CAGTGGGATGCATAATGGCC | NM_001315.2 | 243 | 82.12 |
|
Antisense |
GCATCTTCTCCAGCAAGTCG |
|
|
|
| TNF-α |
|
|
|
|
|
Sense |
CCCTGGTATGAGCCCATCTATC | NM_000594.3 | 120 | 84.82 |
|
Antisense |
AAAGTAGACCTGCCCAGACTCG |
|
|
|
| SDHA |
|
|
|
|
|
Sense |
TGGTTGTCTTTGGTCGGG | NM_004168.2 | 85 | 81.69 |
|
Antisense |
GCGTTTGGTTTAATTGGAGGG |
|
|
|
| ACTB |
|
|
|
|
|
Sense |
CTGGAACGGTGAAGGTGACA | NM_001101.3 | 140 | 82.31 |
|
Antisense |
AAGGGACTTCCTGTAACAATGCA |
|
|
|
| B2M |
|
|
|
|
|
Sense |
CTATCCAGCGTACTCCAAAG | NM_004048.2 | 165 | 78.88 |
|
Antisense |
ACAAGTCTGAATGCTCCACT |
|
|
|
| GAPDH |
|
|
|
|
|
Sense |
CTTTGTCAAGCTCATTTCCTGG | NM_002046.3 | 133 | 84.70 |
|
Antisense |
TCTTCCTCTTGTGCTCTTGC |
|
|
|
| HPRT1 |
|
|
|
|
|
Sense |
AGATGGTCAAGGTCGCAAG | NM_000194.2 | 128 | 79.78 |
|
Antisense |
GTATTCATTATAGTCAAGGGCATATCC |
|
|
|
qPCR conditions
The amplification of cDNA and relative
quantification was performed by qPCR with the StepOnePlus Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). A
Platinum SYBR Green qPCR SuperMix-UDG kit (Invitrogen; Thermo
Fisher Scientific, Inc.) with a total volume of 25 µl, including
12.5 µl SuperMix, 0.5 µl 50 µmol/l Rox reference dye, 0.3 µl of
each primer (10 µmol/l forward and 10 µmol/l reverse), 9.4 µl
H2O and 2.0 µl 1:20-diluted template cDNA. Duplicate
measurements were recorded according to the following protocol:
Initial incubation for 3 min at 94°C, followed by 45 cycles of 30
sec denaturation at 94°C, 30 sec annealing at 55°C and 30 sec
extension at 60°C. Standard curves were constructed by plotting the
cycle threshold values of the qPCR performed on a five-fold
dilution series of cDNA standards.
Statistical analysis
Data were tabulated and analyzed with descriptive
statistics using SPSS software version 20.0 (IBM SPSS, Armonk, NY,
USA). To confirm the normality of gene expression values, the
Kolmogorov-Smirnov test was performed. The Mann-Whitney U test was
performed to compare two groups, and the Kruskal-Wallis test
followed by Dunn's multiple comparison test were performed to
compare more than two groups. P<0.05 was considered to indicate
a statistically significant difference.
Gene expression variability for reference gene
selection was evaluated using NormFinder algorithm, which is a
Visual Basic application for Microsoft Excel that calculates the
average expression stability of each studied gene (36).
Results
Classification according to H. pylori
infection
The samples were classified as positive
(HP+) or negative (HP−) for H. pylori
infection, according to the rapid urease test and histological exam
results (Fig. 1). Table II presents the principal
characteristics of the study population with regards to the H.
pylori infection groups. The participants were from the region
of Taquari Valley, State of Rio Grande do Sul (Brazil) and their
mean age was 47.33±14.31 year.
 | Table II.Main characteristics of the study
population (n=79) according to HP infection. |
Table II.
Main characteristics of the study
population (n=79) according to HP infection.
| Parameter | HP+
(n=27) | HP−
(n=52) |
|---|
| Age (years) |
|
|
| Mean ±
standard deviation | 47.93±12.17 | 47.02±15.40 |
|
Range | 22–64 | 19–81 |
| Gendera |
|
|
| Female
(n=49) | 15 (30.61) | 34 (69.39) |
| Male
(n=30) | 12 (40.00) | 18 (60.00) |
| Previous HP
treatmenta (n=29) | 6 (20.69) | 23 (79.31) |
Classification according to
histological analysis
According to the severity of changes observed on
histological examination, the samples were classified into the
following groups: Normal; inactive chronic gastritis (ICG); active
chronic gastritis (ACG); and intestinal metaplasia (IM). The group
classification is presented in Table
III.
 | Table III.Main characteristics of the study
population according to histological analysis. |
Table III.
Main characteristics of the study
population according to histological analysis.
| Parameter | Normal (n=17) | ICG (n=29) | ACG (n=25) | IM (n=8) |
|---|
| Age (years) |
|
|
|
|
| Mean ±
standard deviation | 45.00±15.47 | 46.90±15.95 | 46.80±11.93 | 55.50±11.67 |
|
Range | 21–75 | 19–81 | 22–64 | 35–70 |
| Gendera |
|
|
|
|
|
Female | 13 (76.47) | 19 (65.52) | 15 (60.00) | 2 (25.00) |
|
Male | 4 (23.53) | 10 (34.48) | 10 (40.00) | 6 (75.00) |
| Previous HP
treatmenta | 5 (29.41) | 8 (27.59) | 6 (24.00) | 5 (62.50) |
| HP+ | 0 (0.00) | 0 (0.00) | 25 (100.0) | 2 (25.00) |
All samples classified as ACG were diagnosed as
HP+. In the IM group, 62.5% (n=5) of patients received
previous HP treatment, 25.0% (n=2) were HP+, while 12.5%
of this group (n=1) had no prior report of H. pylori
infection. The groups classified as normal and ICG were diagnosed
as HP−.
Reference gene selection
For reference gene selection, qPCR was performed on
39 samples (this is the number of samples that had been collected
at the time and was deemed sufficient for this analysis), divided
into the following groups: Normal (n=11), ICG (n=11), ACG (n=12)
and IM (n=5).
The expression stability of a candidate gene was
indicated by its stability value, with a smaller value indicating a
more stable gene. The stability values of the candidate genes
obtained using NormFinder software are presented in Table IV. NormFinder analysis demonstrated
that the succinate dehydrogenase complex, subunit A, flavoprotein
(SDHA) was the most stable gene, with the lowest stability
value and smallest intergroup variation (Fig. 2), and thus SDHA was selected
for normalization of the subsequent data. The most stable
combination of the two genes was SDHA plus ACTB
(stability value, 0.112). The qPCR assay of each gene was analyzed
in the linear phase, and a linear function of the relative
fluorescence vs. cycle number was fitted with a typical
R2 value of >9.
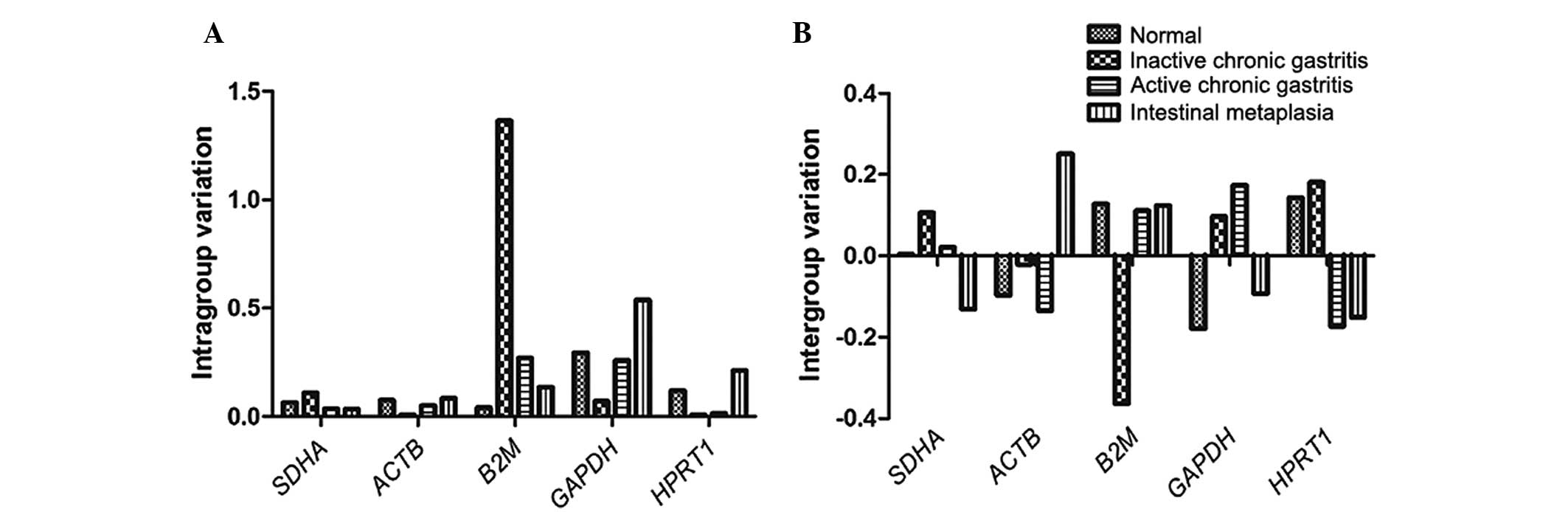 | Figure 2.(A) Intra- and (B) intergroup
variation of five reference genes in human gastric samples as
calculated by NormFinder, identifying SDHA as the gene with
the smallest, and B2M with the highest, variation.
SDHA, succinate dehydrogenase complex, subunit A,
flavoprotein; ACTB, β-actin; B2M,
β2-microglobulin; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; HPRT1,
hypoxanthine phosphoribosyltransferase 1. |
 | Table IV.Candidate reference genes for
normalization of quantitative polymerase chain reaction in human
gastric non-neoplastic tissues, according to their stability, as
calculated by NormFinder software. |
Table IV.
Candidate reference genes for
normalization of quantitative polymerase chain reaction in human
gastric non-neoplastic tissues, according to their stability, as
calculated by NormFinder software.
| Rank | Gene | Stability
value |
|---|
| 1 | SDHA | 0.149 |
| 2 | ACTB | 0.180 |
| 3 | HPRT1 | 0.231 |
| 4 | GAPDH | 0.265 |
| 5 | B2M | 0.270 |
Expression of NFKB1, p38α and
TNF-α
As presented in Fig.
3, no significant differences in NFKB1 and p38α
levels were observed among the groups with regards to the H.
pylori infection status (P>0.05; Mann-Whitney test; Fig. 3A and C) and histological analysis
(P>0.05; Kruskal-Wallis followed by Duncan's multiple
comparisons test; Fig. 3B and D).
However, a statistically significant difference was observed
between the expression of TNF-α in groups with and without
H. pylori infection (P<0.0001; Fig. 3E). Considering the results of
histological analysis, the ACG group demonstrated higher expression
levels of TNF-α when compared with the other groups
(P<0.01; Fig. 3F).
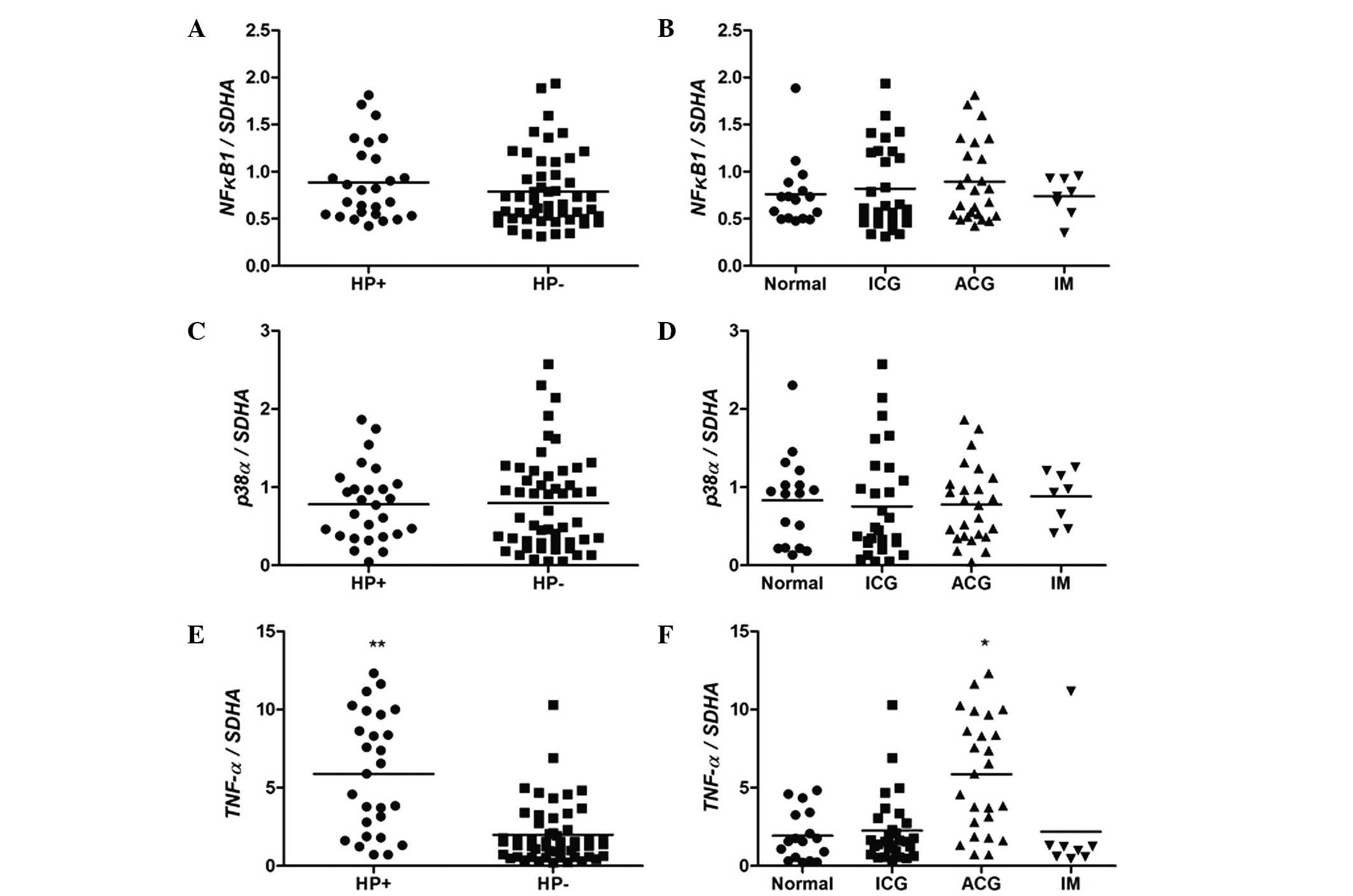 | Figure 3.mRNA expression levels of (A and B)
NFKB1, (C and D) p38α and (E and F) TNF-α in
human gastric mucosa, according to HP infection and histological
analysis statuses. *P<0.01 vs. normal (Kruskal-Wallis followed
by Duncan's multiple comparisons test), **P<0.0001 vs.
HP− (Mann-Whitney U test). ICG, inactive chronic
gastritis; ACG, active chronic gastritis; IM, intestinal
metaplasia; SDHA, succinate dehydrogenase complex, subunit
A, flavoprotein; HP, H. pylori. |
Discussion
In the present study, the expression levels of
TNF-α were increased in H. pylori-positive samples,
regardless of the patient gender. The group classified as ACG, when
compared with other groups, also demonstrated an increase in
TNF-α expression levels. This may be explained by the fact
that all samples in this group tested positive for H. pylori
infection. These results are in accordance with the study conducted
by Pimentel-Nunes et al (37), who observed increased levels of
TNF-α mRNA in gastric biopsies infected with H.
pylori. It is known that H. pylori infection leads to
inflammation of the stomach, associated with the production of
inflammatory cytokines, such as TNF-α, as demonstrated by studies
observing increased levels of TNF-α in infected patients (38,39).
However, a study by Abbas et al (40) conducted on patients with liver
cirrhosis observed no statistically significant difference in the
levels of TNF-α expression when comparing positive and
negative H. pylori groups, or groups with moderate and
severe gastropathy (41).
TNF-α is a key cytokine in tumor promotion;
therefore, it is important to clarify how the pro-inflammatory
cytokines induced by H. pylori are involved in the
development of gastric cancer (42).
Previous studies have demonstrated the presence of the gene encoder
of the TNF-α inducer protein (Tipα) in the H. pylori genome,
which functions as a carcinogenic factor by inducing the gene
expression of TNF-α and activating NF-κB (41,42).
In the current study, no significant difference was
observed in NF-κB expression levels between groups, which is
similar to the results observed in the study of Pimentel-Nunes
et al (37). Furthermore,
Naito and Yoshikawa (43) reported
that H. pylori activates the NF-κB gene in epithelial
cells of the gastric mucosa in vitro and in vivo. In
addition, Huang et al (44)
demonstrated that H. pylori induces phosphorylation of the
proteins Iκβα (an NK-κB inhibitor) and RELA (also known as p65)
that activate RELA nuclear translocation, making H. pylori
an activator of the NF-κB signaling pathway. Considering the
results of the present study, it is not possible to determine
whether the NF-κB pathway is associated with increased levels of
TNF-α, since the methods used could not verify the
phosphorylation levels and, consequently, the protein
activation.
Ferrand et al (45) demonstrated an increase in
TNF-α expression levels in response to H. pylori
infection in mouse gastric epithelial cells in vitro. This
increase was associated with the activation of NF-κB and nuclear
translocation of p65. The same study observed the influence of
H. pylori on the NF-κB pathway and its involvement in the
migration of mesenchymal stem cells, which may be associated with
gastric pathophysiology and carcinogenesis (45). In the current study, a total of 87.5%
of the IM samples, which is considered a precancerous lesion, had a
history of infection with H. pylori (previous HP treatment
or HP+ status), suggesting the involvement of H.
pylori in the development of these lesions.
Kim et al (46) demonstrated that H. pylori
induces the activation of p38 MAPK in vitro, which reduces
the expression of the MucA gene, responsible for the
production of mucus, by promoting apoptosis in gastric epithelial
cells. Seo et al (47)
concluded that MAPKs, such as p38 and ERK, can control the
activation of NF-κB in gastric epithelial cells infected by H.
pylori. However, these studies were analyzed at the protein
level, not the mRNA level. In the present study, no differences in
p38 expression were observed. For the activation of p38
translocation to the nucleus and the stimulation of transcription
factors, p38 must be phosphorylated. Thus, protein analysis is
required to detect this phosphorylation.
Among the p38 isoforms, the p38α gene was
selected for analysis, since it is the most abundant isoform in
tissues and also the most widely studied. However, the isoform p38δ
is detected primarily in endocrine tissue (48), which may form the subject of further
studies due to its presence in gastric epithelium endocrine cells.
O'Callaghan et al (49)
reported the importance of studying p38δ as a result of evidence
demonstrating that it may act as a promoter and as a tumor
suppressor.
The results of the present study demonstrated that
infection with H. pylori is associated with active
inflammation of the gastric tissue, and 100% of the H.
pylori-positive samples were also positive for active
gastritis. The most severe type of lesion observed in the samples
from the current study was intestinal metaplasia. Of these, 25%
tested positive for H. pylori, and only one sample (12.5%)
had no history of infection by H pylori. Although H.
pylori is recognized as the main cause of chronic gastritis,
other factors such as smoking, alcoholism, anxiety, stress, poor
diet and lifestyle may contribute to the onset of clinical
manifestations (50).
In the present study, five candidate genes were
analyzed for their potential to be used as the reference gene in
qPCR with human non-neoplastic gastric samples obtained by upper
endoscopy. NormFinder was selected to compare candidate genes due
to its ability to estimate inter and intragroup variation and
consequently calculate a stability value (36). A value closer to zero indicates
greater stability of gene expression. Considering that an arbitrary
cut-off value of 0.15 indicates acceptable stability of the
reference gene (51), the present
study concluded that SDHA was the most appropriate gene for
qPCR normalization compared with ACTB, B2M,
HPRT1 and GAPDH. Furthermore, the combination of
SDHA and ACTB demonstrated a lower stability value,
suggesting that it is a more stable combination of genes for the
normalization of qPCR sample results.
Although ACTB has a low intragroup variation
(similar to SDHA), its higher intergroup variation raises
the stability value, thus it can not be suggested as a reference
gene for the experiments of the present study. ACTB has been
traditionally regarded as a reference gene in quantifying
expression levels in tumors (23).
However, accumulating evidence indicates that ACTB is
dysregulated in gastric and a number of other types of cancer
(52–54). In the present study, the analysis was
focused on non-neoplastic tissues; however, ACTB intergroup
variation was observed to be higher in the samples with intestinal
metaplasia, which is considered a precancerous lesion (55), thus affecting the gene stability
value. The study by Wisnieski et al (25) analyzing normal and adenocarcinoma
samples of gastric tissue and cell lines, determined that
ACTB was the most appropriate reference gene for all
tissues; however, SDHA was not included in their
analysis.
GAPDH is one of the most commonly used
reference genes and it is considered a ‘classical’ reference gene
in the majority of scientific studies (51,56,57).
Numerous studies of gene expression in human gastric mucosa use
GAPDH as the reference gene (58,59),
despite the knowledge that GAPDH is upregulated in stomach
cancer (24). The present study
demonstrated that GAPDH was highly variable between groups,
and was therefore not recommended as a reference gene.
SDHA has been previously investigated as a
reference gene in numerous studies with different experimental
conditions. These studies determined that SDHA was the best
reference gene compared with other frequently used reference genes
(27,60,61).
According to the results of the present and previous studies,
SDHA may be used as a reference gene for qPCR in the
conditions described, as a result of its stability. Therefore, the
inclusion of SDHA as a candidate gene for further studies of
reference gene selection with different conditions and samples must
be considered.
Chronic gastritis is a prevalent disease in the
world population, and its association with H. pylori
infection is well-described (5). The
study of H. pylori, its virulence factors and resistance to
therapy are crucial in order to improve treatments aiming to
eradicate infection with these bacteria. Understanding the genes
associated with the inflammatory and proliferative pathways in
different populations may facilitate the development of effective
treatments and prevention of gastric disease, aid in the reduction
of side effects and increase the efficacy of current
treatments.
In conclusion, the present study demonstrated that
H. pylori infection increases the expression of TNF-α
mRNA expression levels in human gastric mucosa, but does not have
an effect on the expression of p38α and NFKB1. It
also demonstrated that H pylori infection is associated with
chronic active gastritis and the presence of intestinal metaplasia
in a southern Brazilian population. The SDHA was observed to
be the most appropriate reference gene for qPCR in the current
study, as a result of its stability. Therefore, the results support
the inclusion of SDHA as a candidate gene for further
studies of reference gene selection with different conditions and
samples. Future studies are required to elucidate the association
of NFKB1 and p38α with H. pylori
infection.
Acknowledgements
The present study was supported by the Selection
FAPERGS/CAPES 14/2012 Masters Scholarship Program. The authors also
wish to thank Hospital Bruno Born for collaboration in this
research.
References
|
1
|
Clyne M and Drumm B: Adherence of
Helicobacter pylori to primary human gastrointestinal cells.
Infect Immun. 61:4051–4057. 1993.PubMed/NCBI
|
|
2
|
World Gastroenterology Organisation: World
Gastroenterology Organisation Global Guideline: Helicobacter
pylori in developing countries. J Clin Gastroenterol.
45:383–388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marshall BJ: Helicobacter pylori. Am J
Gastroenterol. 89(Suppl): S116–S128. 1994.PubMed/NCBI
|
|
4
|
Suzuki H, Hibi T and Marshall BJ:
Helicobacter pylori: Present status and future prospects in
Japan. J Gastroenterol. 42:1–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Testerman TL and Morris J: Beyond the
stomach: An updated view of Helicobacter pylori
pathogenesis, diagnosis, and treatment. World J Gastroenterol.
20:12781–12808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Preston-Martin S, Pike MC, Ross RK, Jones
PA and Henderson BE: Increased cell division as a cause of human
cancer. Cancer Res. 50:7415–7421. 1990.PubMed/NCBI
|
|
7
|
Correa P: Helicobacter pylori and
gastric carcinogenesis. Am J Surg Pathol. 19(Suppl 1): S37–S43.
1995.PubMed/NCBI
|
|
8
|
Aggarwal BB, Shishodia S, Sandur SK,
Pandey MK and Sethi G: Inflammation and cancer: How hot is the
link? Biochem Pharmacol. 72:1605–1621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peek RM Jr and Crabtree JE: Helicobacter
infection and gastric neoplasia. J Pathol. 208:233–248. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hahm KB, Song YJ, Oh TY, Lee JS, Surh YJ,
Kim YB, Yoo BM, Kim JH, Han SU, Nahm KT, et al: Chemoprevention of
Helicobacter pylori-associated gastric carcinogenesis in a
mouse model: Is it possible? J Biochem Mol Biol. 36:82–94. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Correa P: Human gastric carcinogenesis: A
multistep and multifactorial process - First American Cancer
Society Award Lecture on Cancer Epidemiology and Prevention. Cancer
Res. 52:6735–6740. 1992.PubMed/NCBI
|
|
12
|
Balkwill F: Tumour necrosis factor and
cancer. Nat Rev Cancer. 9:361–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aggarwal BB: Nuclear factor-kappaB: The
enemy within. Cancer Cell. 6:203–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shishodia S and Aggarwal BB: Nuclear
factor-kappaB: A friend or a foe in cancer? Biochem Pharmacol.
68:1071–1080. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee JC, Laydon JT, McDonnell PC, Gallagher
TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter
SW, et al: A protein kinase involved in the regulation of
inflammatory cytokine biosynthesis. Nature. 372:739–746. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cuenda A and Rousseau S: p38 MAP-kinases
pathway regulation, function and role in human diseases. Biochim
Biophys Acta. 1773:1358–1375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kyriakis JM and Avruch J: Mammalian
mitogen-activated protein kinase signal transduction pathways
activated by stress and inflammation. Physiol Rev. 81:807–869.
2001.PubMed/NCBI
|
|
19
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: Regulation and physiological functions.
Endocr Rev. 22:153–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nebreda AR and Porras A: p38 MAP kinases:
Beyond the stress response. Trends Biochem Sci. 25:257–260. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo C, Liu S, Wang J, Sun MZ and Greenaway
FT: ACTB in cancer. Clin Chim Acta. 417:39–44. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rubie C, Kempf K, Hans J, Su T, Tilton B,
Georg T, Brittner B, Ludwig B and Schilling M: Housekeeping gene
variability in normal and cancerous colorectal, pancreatic,
esophageal, gastric and hepatic tissues. Mol Cell Probes.
19:101–109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wisnieski F, Calcagno DQ, Leal MF, dos
Santos LC, Gigek CO, Chen ES, Pontes TB, Assumpção PP, de Assumpção
MB, Demachki S, et al: Reference genes for quantitative RT-PCR data
in gastric tissues and cell lines. World J Gastroenterol.
19:7121–7128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Souza AF, Brum IS, Neto BS, Berger M and
Branchini G: Reference gene for primary culture of prostate cancer
cells. Mol Biol Rep. 40:2955–2962. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Santin AP, Souza AF, Brum IS and
Furlanetto TW: Validation of reference genes for normalizing gene
expression in real-time quantitative reverse transcription PCR in
human thyroid cells in primary culture treated with progesterone
and estradiol. Mol Biotechnol. 54:278–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schubert TT, Schubert AB and Ma CK:
Symptoms, gastritis, and Helicobacter pylori in patients
referred for endoscopy. Gastrointest Endosc. 38:357–360. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berry V and Sagar V: Rapid urease test to
diagnose Helicobacter pylori infection. JK Science. 8:86–88.
2006.
|
|
31
|
Ornellas LC, Cury M, de Lima VM and
Ferrari Junior AP: Evaluation of rapid urease test stored in
refrigerator. Arq Gastroenterol. 37:155–157. 2000.(In Portuguese).
PubMed/NCBI
|
|
32
|
Wabinga HR: Comparison of
immunohistochemical and modified Giemsa stains for demonstration of
Helicobacter pylori infection in an African population. Afr
Health Sci. 2:52–55. 2002.PubMed/NCBI
|
|
33
|
Dixon MF, Genta RM, Yardley JH and Correa
P: Classification and grading of gastritis. The updated Sydney
System. International Workshop on the Histopathology of Gastritis,
Houston 1994. Am J Surg Pathol. 20:1161–1181. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chomczynski P and Sacchi N: Single-step
method of RNA isolation by acid guanidinium
thiocyanate-phenol-chloroform extraction. Anal Biochem.
162:156–159. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rozen S and Skaletsky H: Primer3 on the
WWW for general users and for biologist programmers. Methods Mol
Biol. 132:365–386. 2000.PubMed/NCBI
|
|
36
|
Andersen CL, Jensen JL and Ørntoft TF:
Normalization of real-time quantitative reverse transcription-PCR
data: A model-based variance estimation approach to identify genes
suited for normalization, applied to bladder and colon cancer data
sets. Cancer Res. 64:5245–5250. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pimentel-Nunes P, Gonçalves N,
Boal-Carvalho I, Afonso L, Lopes P, Roncon-Albuquerque R Jr,
Henrique R, Moreira-Dias L, Leite-Moreira AF and Dinis-Ribeiro M:
Helicobacter pylori induces increased expression of
Toll-like receptors and decreased Toll-interacting protein in
gastric mucosa that persists throughout gastric carcinogenesis.
Helicobacter. 18:22–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Crabtree JE, Shallcross TM, Heatley RV and
Wyatt JI: Mucosal tumour necrosis factor alpha and interleukin-6 in
patients with Helicobacter pylori associated gastritis. Gut.
32:1473–1477. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao C, Lu X, Bu X, Zhang N and Wang W:
Involvement of tumor necrosis factor-alpha in the upregulation of
CXCR4 expression in gastric cancer induced by Helicobacter
pylori. BMC Cancer. 10:4192010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Abbas Z, Yakoob J, Usman MW, Shakir T,
Hamid S and Jafri W: Effect of Helicobacter pylori and its
virulence factors on portal hypertensive gastropathy and
interleukin (IL)-8, IL-10, and tumor necrosis factor-alpha levels.
Saudi J Gastroenterol. 20:120–127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Suganuma M, Kurusu M, Okabe S, Sueoka N,
Yoshida M, Wakatsuki Y and Fujiki H: Helicobacter pylori
membrane protein 1: A new carcinogenic factor of Helicobacter
pylori. Cancer Res. 61:6356–6359. 2001.PubMed/NCBI
|
|
42
|
Suganuma M, Kurusu M, Suzuki K, Nishizono
A, Murakami K, Fujioka T and Fujiki H: New tumor necrosis
factor-alpha-inducing protein released from Helicobacter
pylori for gastric cancer progression. J Cancer Research Clin
Oncol. 131:305–313. 2005. View Article : Google Scholar
|
|
43
|
Naito Y and Yoshikawa T: Molecular and
cellular mechanisms involved in Helicobacter pylori-induced
inflammation and oxidative stress. Free Radic Biol Med. 33:323–336.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang X, Lv B, Zhang S, Dai Q, Chen BB and
Meng LN: Effects of radix curcumae-derived diterpenoid C on
Helicobacter pylori-induced inflammation and nuclear factor
kappa B signal pathways. World J Gastroenterol. 19:5085–5093. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ferrand J, Lehours P, Schmid-Alliana A,
Mégraud F and Varon C: Helicobacter pylori infection of
gastrointestinal epithelial cells in vitro induces
mesenchymal stem cell migration through an NF-κB-dependent pathway.
PLoS One. 6:e290072011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim H, Seo JH and Kim KH: The effect of
p38 mitogen-activated protein kinase on mucin gene expression and
apoptosis in Helicobacter pylori-infected gastric epithelial
cells. Ann NY Acad Sci. 1010:90–94. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Seo JH, Lim JW and Kim H: Differential
role of ERK and p38 on NF-κB activation in Helicobacter
pylori-infected gastric epithelial cells. J Cancer Prev.
18:346–350. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang XS, Diener K, Manthey CL, Wang S,
Rosenzweig B, Bray J, Delaney J, Cole CN, Chan-Hui PY, Mantlo N, et
al: Molecular cloning and characterization of a novel p38
mitogen-activated protein kinase. J Biol Chem. 272:23668–23674.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
O'Callaghan C, Fanning LJ and Barry OP:
p38δ MAPK: Emerging roles of a neglected isoform. Int J Cell Biol.
2014:2726892014.PubMed/NCBI
|
|
50
|
Ddine LC, Ddine CC, Rodrigues CC, Kirsten
VR and Colpo E: Factors associated with chronic gastritis in
patients with presence and absence of Helicobacter pylori.
Arq Bras Cir Dig. 25:96–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pérez R, Tupac-Yupanqui I and Dunner S:
Evaluation of suitable reference genes for gene expression studies
in bovine muscular tissue. BMC Mol Biol. 9:792008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Le PU, Nguyen TN, Drolet-Savoie P, Leclerc
N and Nabi IR: Increased beta-actin expression in an invasive
moloney sarcoma virus-transformed MDCK cell variant concentrates to
the tips of multiple pseudopodia. Cancer Res. 58:1631–1635.
1998.PubMed/NCBI
|
|
53
|
Micheva KD, Vallée A, Beaulieu C, Herman
IM and Leclerc N: Beta-actin is confined to structures having high
capacity of remodelling in developing and adult rat cerebellum. Eur
J Neurosci. 10:3785–3798. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Popow A, Nowak D and Malicka-Blaszkiewicz
M: Actin cytoskeleton and beta-actin expression in correlation with
higher invasiveness of selected hepatoma Morris 5123 cells. J
Physiol Pharmacol. 57:111–123. 2006.PubMed/NCBI
|
|
55
|
Miao XP, Li JS, Li HY, Zeng SP, Zhao Y and
Zeng JZ: Expression of ornithine decarboxylase in precancerous and
cancerous gastric lesions. World J Gastroenterol. 13:2867–2871.
2007.PubMed/NCBI
|
|
56
|
de Jonge HJ, Fehrmann RS, de Bont ES,
Hofstra RM, Gerbens F, Kamps WA, de Vries EG, van der Zee AG, te
Meerman GJ and ter Elst A: Evidence based selection of housekeeping
genes. PLoS One. 2:e8982007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Suzuki T, Higgins PJ and Crawford DR:
Control selection for RNA quantitation. Biotechniques. 29:332–337.
2000.PubMed/NCBI
|
|
58
|
de Souza CR, Leal MF, Calcagno DQ, Costa
Sozinho EK, Borges BN, Montenegro RC, Dos Santos AK, Dos Santos SE,
Ribeiro HF, Assumpção PP, et al: MYC deregulavtion in gastric
cancer and its clinicopathological implications. PLoS One.
8:e644202013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zuk K, Peczek L, Stec-Michalska K, Medrek
M and Nawrot B: SATB1 expression in gastric mucosa in relation to
Helicobacter pylori infection and family history of gastric
cancer. Adv Med Sci. 57:237–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Balogh A, Paragh G Jr, Juhász A, Köbling
T, Törocsik D, Mikó E, Varga V, Emri G, Horkay I, Scholtz B and
Remenyik E: Reference genes for quantitative real time PCR in UVB
irradiated keratinocytes. J Photochem Photobiol B. 93:133–139.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gur-Dedeoglu B, Konu O, Bozkurt B, Ergul
G, Seckin S and Yulug IG: Identification of endogenous reference
genes for qRT-PCR analysis in normal matched breast tumor tissues.
Oncol Res. 17:353–365. 2009. View Article : Google Scholar : PubMed/NCBI
|