Introduction
Neuronal cell apoptosis is associated with various
factors that induce neurological damage, including radiation
exposure (1). Free radicals produced
by the interaction between ionizing radiation and the biological
system attack the components of cells, resulting in cellular damage
and apoptosis (2,3). To date, two apoptotic pathways have
been extensively characterized, one of which is triggered by the
engagement of cell surface death receptors with their specific
ligands, and the other is a mitochondrial pathway, triggered by
alterations in internal cellular integrity induced by numerous
stimuli (4,5). Subsequently, these two pathways induce
the activation of caspases (6),
which hydrolyze important structural and functional proteins of the
cell, ultimately leading to apoptosis (7). Caspases are synthesized in the cell as
inactive zymogens and require activation to be functional (7). Radiation induces caspase activation
through the mitochondrial pathway, which includes the mitochondrial
integration of apoptotic signals and the subsequent release of
cytochrome c, Smac and apoptosis-inducing factor into the
cytosol (8). This release
facilitates the assembly of the apoptosome, which activates
caspase-9 and, in turn, leads to the activation of caspases-3, −6
and −7 (9).
Caspase-mediated cell death is associated with the
pathogenesis of neuronal degeneration along with other factors such
as oxidative damage and inflammation. In addition to activating
cell death, previous studies have demonstrated that caspase-3 also
has fundamental roles in signal transduction (10,11).
Reactive oxygen species (ROS) are byproducts of the normal cellular
metabolism of oxygen; however, a dramatic increase in ROS levels
may lead to oxidative stress. It has been demonstrated that ROS
induced by ionizing radiation are capable of triggering oxidative
cellular damage and stimulating the activation of intracellular
signaling pathways (12). The brain,
which is a major metabolizer of oxygen with relatively poor
protective antioxidant mechanisms, is particularly vulnerable to
the ROS. In recent years, research in this field has focused on
antioxidant agents that are suitable as radiation countermeasures
(13–15). An effective protective agent against
irradiation administered prior to exposure to radiation may protect
cells from cellular and molecular injury (16).
Melatonin (N-acetyl-5-methoxytryptamine;
Mel), which is a neurohormone and the major product of the pineal
gland, may be a novel therapeutic agent for the treatment of
various disorders associated with inflammation and oxidative stress
(17). It has been reported that Mel
was able to improve short and long-term neurobehavioral deficits
and attenuate hippocampal impairments following hypoxia in neonatal
mice (18). In addition to
neutralizing ROS species, Mel also acts via the stimulation of
various anti-oxidative systems and stabilizes cell membranes
(19). As such, Mel modulates the
gene expression levels of numerous protective enzymes to reduce
apoptosis and lipid peroxidation (20,21). Mel
has previously been demonstrated to improve the survival rates of
mice when administered prior to irradiation exposure (22). The hippocampus is a region of active
proliferation and neurogenesis within the brain. It has previously
been demonstrated that ionizing radiation induces the apoptosis of
neural cells within the subventricular zone of the lateral
ventricles and the subgranular zone of the hippocampus in the adult
brain (23). Furthermore, previous
studies have demonstrated that the caspase-dependent cytotoxicity
of ionizing radiation in hippocampal neurons is induced by
oxidative stress (24,25).
The present study aimed to investigate whether Mel
inhibits the caspase cell death pathway to protect hippocampal
neurons from irradiation-induced apoptosis. Furthermore, the
underlying mechanisms of this phenomenon within cells were studied
in order to elucidate whether Mel may be a novel therapeutic agent
for the prophylactic treatment of irradiation.
Materials and methods
Animals and reagents
A total of 18 male Sprague-Dawley rats, weighing
200–220 g and aged 6–8 weeks, were maintained in individual cages
for 1 week at 22±2°C, 40–60% humidity and under a 12-h light-dark
cycle, with ad libitum access to food and water. All animal
experiments were conducted in accordance with a protocol approved
by the Institutional Animal Care and Use Committee of the Institute
of Radiation Medicine, Chinese Academy of Medical Sciences
(Tianjin, China). Animals were randomly assigned into three groups
(n=6/group): Irradiation (IR) group, irradiation with Mel (IR +
Mel) group and control (Con) group. Mel was purchased from
ImmunoWay Biotechnology Company (Newark, DE, USA).
Mel administration
Rats in the IR + Mel group were administered Mel
(100 mg/kg body weight) by intraperitoneal injection; the IR and
Con groups were treated with an equal volume of isotonic NaCl
solution (Fuyu Fine Chemical Co., Ltd., Tianjin, China) as a
vehicle, with and without the proceeding irradiation, respectively.
All treatments were performed 30 min prior to radiation exposure in
red light at 6 p.m.
Irradiation
Rats were placed in ventilated plexiglass containers
(30×25×30 cm; Nanfang Organic Glass Factory, Tianchang, China) and
administered total body irradiation (TBI) using 137 Cs γ rays
(Cammacell-40; Atomic Energy, Mississauga, ON, Canada) at a dosage
of 1.0 Gy/min (26). Rats in the IR
and IR + Mel groups received a total of 4.0 Gy TBI. Rats in the
control group were placed in identical containers for the same
period without irradiation.
Tissue preparation
At 24 h post-experimental intervention, the rats
were sacrificed by an overdose with intraperitoneally administered
sodium pentobarbital (50 mg/kg; Beijing Biosynthesis Biotechnology
Co., Ltd., Beijing, China) and immediately treated with a cardiac
perfusion of 4% paraformaldehyde (CellChip Biotechnology Co., Ltd.,
Beijing, China). The hippocampi were harvested and cut into 12-µm
coronal sections (3 rats/group) using a CM 3000 cryostat (Leica
Microsystems GmbH, Wetzlar, Germany) and were subsequently placed
on glass slides and stored at −80°C (27).
Immunohistology, terminal
deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) and
cresyl violet (CV) staining
A standard immunohistochemical analysis was
conducted according to a previous study (28). Briefly, coronal sections were air
dried for 15 min, post-fixed in 10% formalin (Hangzhou Norming
Biological Technology Co., Ltd., Hangzhou, China) for 15 min,
washed twice in phosphate-buffered saline and then processed for
immunostaining with rabbit anti-active caspase-3 polyclonal
antibody (1:1,000; cat. no. ab2302; Abcam, Cambridge, MA, USA).
This was followed by incubation with horseradish
peroxidase-conjugated goat anti-rabbit IgG (1:3,000; cat. no.
ta140003; OriGene Technologies, Inc., Beijing, China) and then
3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St. Louis,
MO, USA). Subsequently, the sections were visualized under a light
microscope (LSM-510; Carl Zeiss AG, Oberkochen, Germany).
DNA fragmentation was detected using a TUNEL kit
(In Situ Cell Death Detection Kit, POD; Roche Diagnostics,
Indianapolis, IN, USA) according to the manufacturer's protocol and
as described previously (29).
Briefly, sections were incubated for 90 min at 37°C with TUNEL
reaction mixture. Positive control sections were incubated with 200
U/ml DNase I (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) for 5 min prior to fixation. Negative control sections
underwent the same procedure but terminal deoxynucleotidyl
transferase was omitted from the reaction buffer to evaluate
nonspecific labeling. TUNEL cell counts were performed on brain
sections (n=3) from the hippocampi. TUNEL-positive cells were
averaged from the counts of three adjacent brain sections of a rat.
The sections were visualized using the Eclipse Ti-U inverted
microscope (Nikon Corporation, Tokyo, Japan) with an
excitation/emission wavelength of 500/550 nm (green).
CV staining was performed in order to detect the
Nissl body in the neuronal cytoplasm and to identify the basic
neuronal structure of necrotic neurons in the brain and spinal
cord. Sections were rinsed in tap and distilled water, and
subsequently stained in 0.1% CV solution (CellChip Biotechnology
Co., Ltd.) for 3–10 min. Following rinsing in distilled water, the
sections were differentiated in 95% ethyl alcohol (Sangon Biotech
Co., Ltd., Shanghai, China), dehydrated in 100% alcohol and cleared
with xylene (Sangon Biotech Co., Ltd.), prior to mounting with
permanent mounting medium (Yantuo Biological Technology Co., Ltd.,
Shanghai, China). The Nissl body was stained purple-blue (28).
Western blot analysis
Western blotting was performed according to a
standard procedure as described previously (29). Briefly, the rats were sacrificed at
24 h following irradiation, and the hippocampi (n=3/group) were
obtained. Total protein was isolated from the hippocampi using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Jiangsu, China), according to the manufacturer's
protocol. The homogenates were centrifuged at 21,890 × g for 30 min
at 4°C and the protein concentration in the supernatant was
determined spectrophotometrically by measuring the absorbance at
595 nm (A595 nm). Equal volumes (20 µg) of protein were mixed with
loading buffer containing 5% 2-mercaptoethanol (Hangzhou Norming
Biological Technology Co., Ltd.), heated for 5 min at 95°C and
separated by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis, followed by immunoblotting onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). After
blocking with 5% milk in Tris-buffered saline supplemented with
0.1% Tween-20, the membranes were incubated overnight at 4°C with
rabbit anti-cleaved caspase-3 polyclonal antibody (1:1,000; cat.
no. ab2302; Abcam) and rabbit anti-β-actin monoclonal antibody
(1:500; cat. no. 4967L; Cell Signaling Technology, Inc., Danvers,
MA, USA, followed by incubation with HRP-conjugated goat
anti-rabbit IgG (1:1,000; cat. no. ta140003; OriGene Technologies,
Inc.) for 2 h at room temperature. Proteins were detected using
enhanced chemiluminescence (ECL reagents; GE Healthcare Life
Sciences, Little Chalfont, UK) and exposed to radiographic film
(Hyperfilm ECL; GE Healthcare Life Sciences). The relative amount
of protein was normalized to β-actin and analyzed using the Gel-Pro
Analyzer software, version 4.0 (Media Cybernetics, Inc., Rockville,
MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Tissue samples were initially homogenized and total
RNA was extracted from the tissues using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. RNA was treated with DNase (TURBO
DNA-free™ kit; Thermo Fisher Scientific, Inc.) and 3 µg RNA was
used for cDNA synthesis, as previously described (29). Briefly, RNA was reverse transcribed
into cDNA using a using an iScript™ Select cDNA Synthesis kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). PCR was performed
using the 2X PCR Master Mix (Beyotime Institute of Biotechnology),
an ABI PRISM® 7500 Sequence Detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and the following
primers: Caspase-3, forward 5′-AATTCAAGGGACGGGTCATG-3′ and reverse
5′-GCTTGTGCGCGTACAGTTTC-3′; and GAPDH, forward
5′-ATGACATCAAGAAGGTGGTG-3′ and reverse 5′-CATACCAGGAAATGAGCTTG-3′
(Sangon Biotech Co., Ltd.). The PCR cycling conditions were as
follows: 95°C for 20 sec, followed by 50 cycles at 95°C for 3 sec
and 60°C for 30 sec, and then a final extension step at 72°C for 5
min. The cycle threshold (Cq) values of caspase-3 were normalized
to the Cq values of the GAPDH housekeeping gene using the
2−∆∆Cq method (30) All
samples were analyzed in triplicate A negative control and an
RT-minus control were used to verify the results of the first
strand cDNA synthesis step.
ROS analysis and caspase-3 activation
assay
A 2′,7′-dichlorodihydrofluorescein diacetate (DCF)
fluorogenic probe (Hangzhou Boda Biological Technology Co., Ltd,
Hangzhou, China) was used to assess the production of ROS, as
previously described (31). The
activities of caspase-3 were analyzed using a fluorogenic caspase
assay with Ac-DEVD-AFC (BD Pharmingen, San Diego, CA, USA) as the
substrate. The results were expressed as the fold change, compared
with the control, according to technique described by Li et
al (28).
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Statistical comparison of
the results was performed using paired Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Mel administration attenuates
irradiation-induced neuronal damage
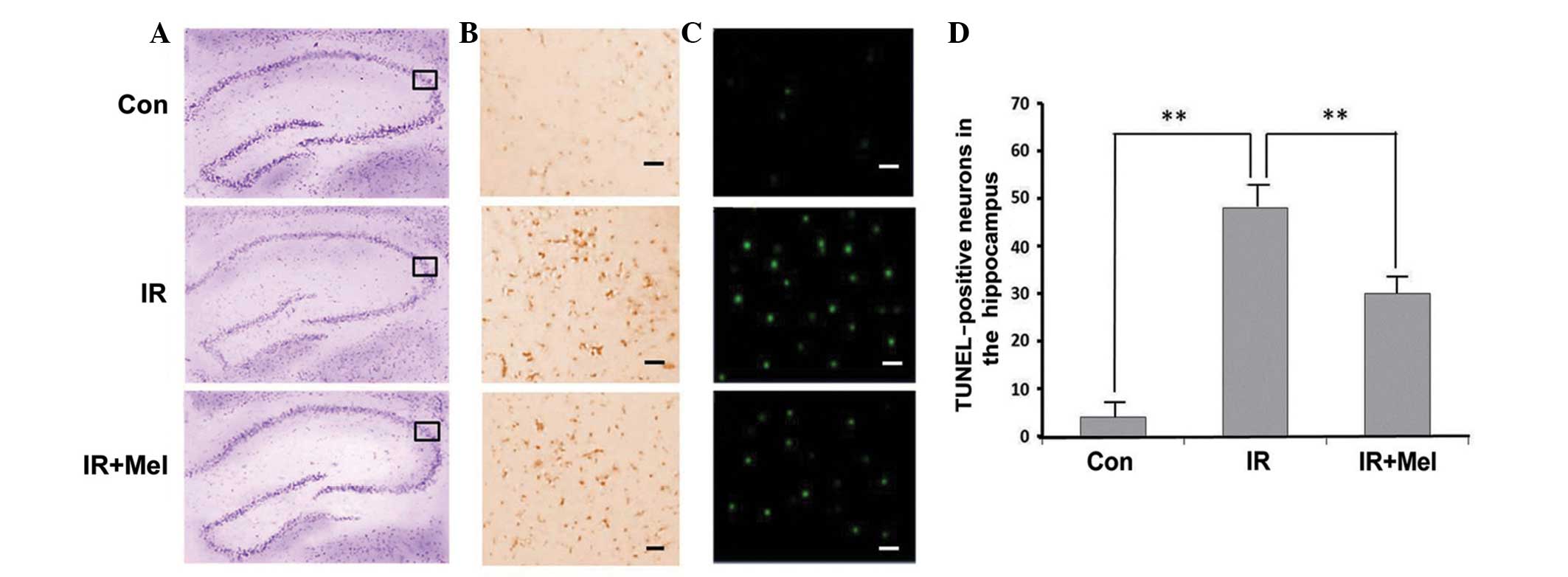
The Nissl body was successfully stained purple-blue
(Fig. 1A). Microscopic examination
of the hippocampal sections stained with CV showed pathological
changes in the IR and IR ± Mel groups, as compared with the control
group (Fig. 1A). Viable neurons had
a deeply stained cytoplasm and lightly stained nucleus.
Immunohistochemical labeling of caspase-3 was more intense in the
hippocampi of the IR group than in the IR + Mel group; whereas
staining was more intense in the IR + Mel group than the control
group in the cytoplasm (Fig. 1B).
TUNEL-positive cells were not clearly observed in the CA3 region of
the control group, whilst TUNEL-positive staining was detected in
the nuclear region of cells in the IR group with condensed
chromatin and fragmented DNA (Fig.
1C). The IR + Mel group exhibited a decrease in TUNEL
positivity compared with the IR group (Fig. 1C). Upon quantification of viable
cells in the hippocampi, the IR and IR + Mel groups exhibited a
decrease in the mean number of surviving neurons, as compared with
the control group. However, the number of surviving neurons were
significantly increased in the IR + Mel group, as compared with the
IR group (P<0.01; Fig. 1D). These
results suggest that Mel administration attenuates
irradiation-induced damage.
Level of caspase-3 activity and mean
ROS accumulation
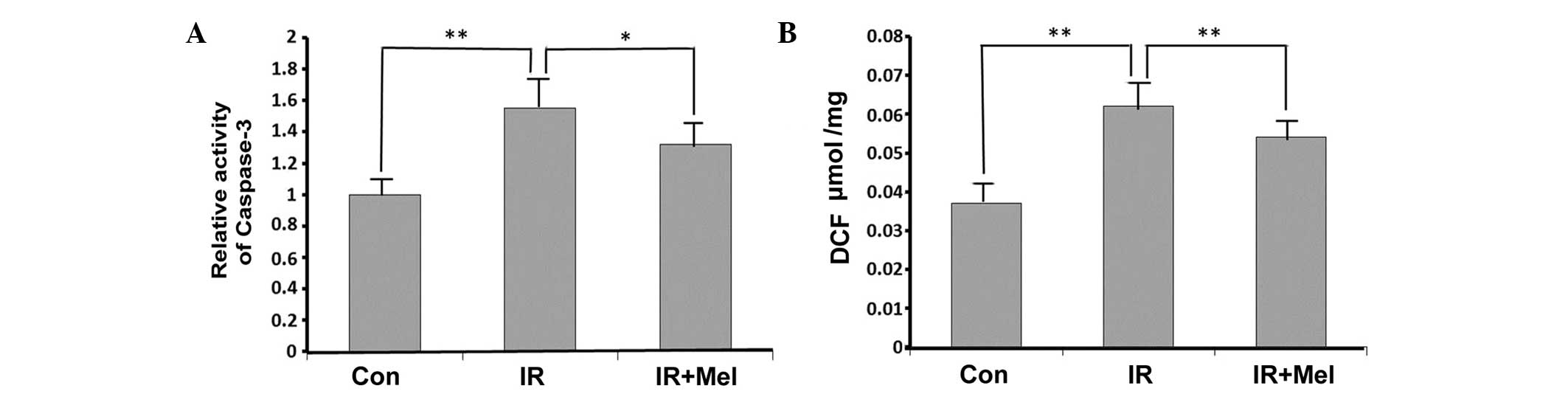
Caspase-3 activity levels were significantly
increased in the IR group compared with the control group
(P<0.01; Fig. 2A). Treatment with
Mel significantly attenuated caspase-3 activity levels in the IR +
Mel group compared with the IR group (P<0.05; Fig. 2A). As detected by a DCF probe, the
mean ROS accumulation was significantly increased in the hippocampi
of rats in the IR group compared with the control group (P<0.01;
Fig. 2B). Furthermore, Mel
administration significantly reduced ROS accumulation, and thus
attenuated irradiation-induced damage, in the IR + Mel group
compared with the IR group (P<0.01; Fig. 2B).
Mel administration attenuates
caspase-3 expression levels
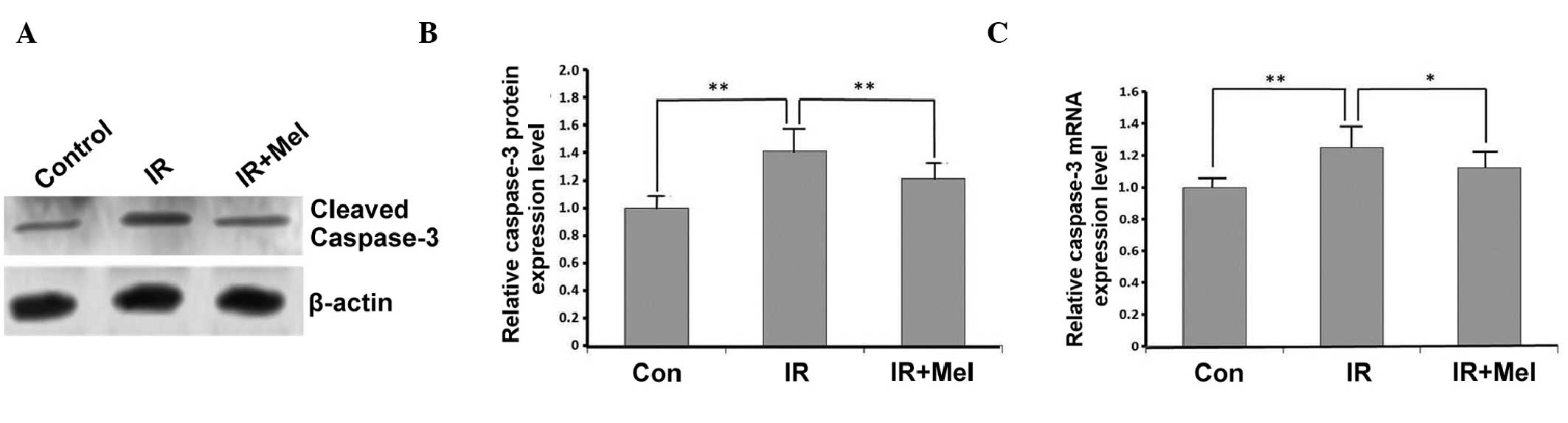
The expression levels of caspase-3 were detected by
western blot and RT-qPCR analyses. In the IR group, caspase-3
expression levels were significantly increased compared with the
control group, at the protein (P<0.01; Fig. 3A and B) and mRNA (P<0.01; Fig. 3C) levels. Caspase-3 protein
expression levels were significantly decreased in the IR+ Mel group
compared with the IR group (P<0.01; Fig. 3A and B), as were caspase-3 mRNA
expression levels (P<0.05; Fig.
3C). These results suggested that treatment with Mel
significantly attenuated the expression levels of caspase-3.
Discussion
In the present study, a modified protocol was used
to induce hippocampal neurodegeneration by irradiation in
vivo in order to investigate the potential protective mechanism
of Mel on the hippocampi via decreased caspase-3 expression and
activity levels. Radiation damage to cells is caused by oxidative
stress (32). The increased
caspase-3 levels detected in the IR group of the present study
demonstrated the role of oxidative mechanisms in
irradiation-induced tissue injury. Free oxygen radicals are
molecules released from macrophages and neutrophils, which are
efficient in the early period of inflammation, that target DNA
proteins and lipids (33).
Appropriate antioxidation intervention, via the inhibition or
reduction of free radicals, offers protection against
radiation-induced damage. Mel is a highly efficient free radical
scavenger and a general antioxidant that has previously been
demonstrated to protect DNA, lipids and proteins (32,34,35).
Furthermore, antioxidant enzyme activities have been shown to
exhibit circadian rhythms that correspond to Mel rhythmicity and
the total antioxidant status (36).
Mel has been reported to increase the activity of important
antioxidant enzymes at the molecular level, including superoxide
dismutase and glutathione peroxidase (37). The present study investigated the
antioxidant and immunoenhancing actions of Mel via the inhibition
of caspase-3 in vivo.
As previous studies have demonstrated, Mel acts as a
stimulant under immunosuppressive conditions or as an
anti-inflammatory compound during immune responses, including acute
inflammation (38–41). Advantageously, Mel is ubiquitously
distributed in all the cellular compartments, and is capable of
quickly passing through all the biological membranes (42). The results of a previous study
demonstrated that no significant changes were identified in the Mel
levels among the IR, IR + Mel and control groups 24 h after
treatment (43). These results may
be due to the ability of Mel to stimulate antioxidative enzymes
which may maintain their enzyme activity levels following the
metabolic decomposition of Mel. Therefore, signal transduction and
the expression levels of antioxidant enzymes following treatment
with radiation and Mel should be investigated in future
studies.
In conclusion, the results of the present study
demonstrated that Mel may protect hippocampal neurons from
apoptosis via the inhibition of caspase-3 following irradiation. A
significant decrease in caspase-3 mRNA (P<0.05) and protein
(P<0.01) expression levels was detected at 24 h after Mel
treatment. Therefore, caspase-3 inhibition may have a
neuroprotective and antioxidant role in the interventional
treatment of Mel, and these results demonstrate the potential
therapeutic effect of Mel against irradiation. Further studies are
required in order to elucidate the underlying effects and
mechanisms of Mel on irradiation-induced alterations in
caspase-3.
Acknowledgements
The present study was supported by the National
Science and Technology Pillar Program during the 12th Five-year
Plan Period (grant no. 2012BA127B02), by the Special Foundation of
the Ministry of Health (grant no. 201002009), National Natural
Science Foundation of China (grant nos. 31170804, 31240052,
31200634 and 81560025), the Natural Science Foundation of Tianjin
(grant nos. 13JCYBJC23500, 13JCQNJC11600, 11ZCGYSY02400,
12JCYBJC15300 and 12JCYBJC32900) and the PUMC Youth Fund and
Fundamental Research Funds for the Central Universities (grant nos.
2012G01 and 2012J05).
References
|
1
|
Li T, Lu C, Xia Z, Xiao B and Luo Y:
Inhibition of caspase-8 attenuates neuronal death induced by limbic
seizures in a cytochrome c-dependent and
Smac/DIABLO-independent way. Brain Res. 1098:204–211. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mansour HH, Hafez HF, Fahmy NM and Hanafi
N: Protective effect of N-acetylcysteine against radiation induced
DNA damage and hepatic toxicity in rats. Biochem Pharmacol.
75:773–780. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shirazi A, Mihandoost E, Ghobadi G,
Mohseni M and Ghazi-Khansari M: Evaluation of radio-protective
effect of melatonin on whole body irradiation induced liver tissue
damage. Cell J. 14:292–297. 2013.PubMed/NCBI
|
|
4
|
Ashkenazi A and Dixit VM: Death receptors:
Signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vaux DL and Korsmeyer SJ: Cell death in
development. Cell. 96:245–254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu H, Che X, Zheng Q, Wu A, Pan K, Shao A,
Wu Q, Zhang J and Hong Y: Caspases: A molecular switch node in the
crosstalk between autophagy and apoptosis. Int J Biol Sci.
10:1072–1083. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du C, Fang M, Li Y, Li L and Wang X: Smac,
a mitochondrial protein that promotes cytochrome c-dependent
caspase activation by eliminating IAP inhibition. Cell. 102:33–42.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Srinivasula SM, Hegde R, Saleh A, Datta P,
Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y
and Alnemri ES: A conserved XIAP- interaction motif in caspase-9
and Smac/DIABLO regulates caspase activity and apoptosis. Nature.
410:112–116. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boland K, Flanagan L and Prehn JH:
Paracrine control of tissue regeneration and cell proliferation by
Caspase-3. Cell Death Dis. 4:e7252013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sahoo S, Meijles DN and Pagano PJ: NADPH
oxidases: Key modulators in aging and age-related cardiovascular
diseases? Clin Sci (Lond). 130:317–335. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sochocka M, Koutsouraki ES, Gasiorowski K
and Leszek J: Vascular oxidative stress and mitochondrial failure
in the pathobiology of Alzheimer's disease: A new approach to
therapy. CNS Neurol Disord Drug Targets. 12:870–881. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh VK, Ducey EJ, Brown DS and Whitnall
MH: A review of radiation countermeasure work ongoing at the armed
forces radiobiology research institute. Int J Radiat Biol.
88:296–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weiss JF and Landauer MR: History and
development of radiation-protective agents. Int J Radiat Biol.
85:539–573. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dumont F, Le Roux A and Bischoff P:
Radiation countermeasure agents: An update. Expert Opin Ther Pat.
20:73–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suman S, Jain S and Chandna S: Recent
patents in the field of radioprotector development: Opportunities
and challenges. Recent Pat Biotechnol. 7:219–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Poeggeler B, Saarela S, Reiter RJ, Tan DX,
Chen LD, Manchester LC and Barlow-Walden LR: Melatonin-a highly
potent endogenous radical scavenger and electron donor: New aspects
of the oxidation chemistry of this indole accessed in vitro. Ann NY
Acad Sci. 738:419–420. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Z, Liu D, Zhan J, Xie K, Wang X, Xian
X, Gu J, Chen W and Hao A: Melatonin improves short and long-term
neurobehavioral deficits and attenuates hippocampal impairments
after hypoxia in neonatal mice. Pharmacol Res. 76:84–97. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Cook A, Kim J, Baranov SV, Jiang
J, Smith K, Cormier K, Bennett E, Browser RP, Day AL, et al:
Melatonin inhibits the caspase-1/cytochrome c/caspase-3 cell
death pathway, inhibits MT1 receptor loss and delays disease
progression in a mouse model of amyotrophic lateral sclerosis.
Neurobiol Dis. 55:26–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jang SS, Kim HG, Lee JS, Han JM, Park HJ,
Huh GJ and Son CG: Melatonin reduces X-ray radiation-induced lung
injury in mice by modulating oxidative stress and cytokine
expression. Int J Radiat Biol. 89:97–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Missiry MA, Fayed TA, El-Sawy MR and
El-Sayed AA: Ameliorative effect of melatonin against
gamma-irradiation-induced oxidative stress and tissue injury.
Ecotoxicol Environ Saf. 66:278–286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koc M, Buyukokuroglu ME and Taysi S: The
effect of melatonin on peripheral blood cells during total body
irradiation in rats. Biol Pharm Bull. 25:656–657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim JS, Lee HJ, Kim JC, Kang SS, Bae CS,
Shin T, Jin JK, Kim SH, Wang H and Moon C: Transient impairment of
hippocampus-dependent learning and memory in relatively low-dose of
acute radiation syndrome is associated with inhibition of
hippocampal neurogenesis. J Radiat Res. 49:517–526. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang M, Song MS, Kim SH, Kim JC, Kim JS,
Shin T and Moon C: Cytotoxicity of gamma-ray in rat immature
hippocampal neurons. J Vet Sci. 12:203–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim JS, Yang M, Kim SH, Shin T and Moon C:
Neurobiological toxicity of radiation in hippocampal cells. Histol
Histopathol. 28:301–310. 2013.PubMed/NCBI
|
|
26
|
Fike JR, Rosi S and Limoli CL: Neural
precursor cells and central nervous system radiation sensitivity.
Semin Radiat Oncol. 19:122–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kesari KK, Kumar S and Behari J:
Pathophysiology of microwave radiation: Effect on rat brain. Appl
Biochem Biotechnol. 166:379–388. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Feng L, Xing Y, Wang Y, Du L, Xu C,
Cao J, Wang Q, Fan S, Liu Q and Fan F: Radioprotective and
antioxidant effect of resveratrol in hippocampus by activating
Sirt1. Int J Mol Sci. 15:5928–5939. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharma S, Haldar C and Chaube SK: Effect
of exogenous melatonin on X-ray induced cellular toxicity in
lymphatic tissue of Indian tropical male squirrel, Funambulus
pennanti. Int J Radiat Biol. 84:363–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
LeBel CP, Ali SF, McKee M and Bondy SC:
Organometal-induced increases in oxygen reactive species: The
potential of 2′,7′-dichlorofluorescin diacetate as an index of
neurotoxic damage. Toxicol Appl Pharmacol. 104:17–24. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yavuz MN, Yavuz AA, Ulku C, Sener M, Yaris
E, Kosucu P and Karslioglu I: Protective effect of melatonin
against fractionated irradiation-induced epiphyseal injury in a
weanling rat model. J Pineal Res. 35:288–294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aritaş Y, Akcan A, Erdoğan AR, Akgün H,
Saraymen R and Akyildiz H: Effects of melatonin and phospholipid on
adhesion formation and correlation with vascular endothelial growth
factor expression in rats. Ulus Travma Acil Cerrahi Derg.
15:416–422. 2009.PubMed/NCBI
|
|
34
|
Weiss JF and Landauer MR: Radioprotection
by antioxidants. Ann NY Acad Sci. 899:44–60. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miller E, Walczak A, Majsterek I and
Kędziora J: Melatonin reduces oxidative stress in the erythrocytes
of multiple sclerosis patients with secondary progressive clinical
course. J Neuroimmunol. 257:97–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Albarrán MT, López-Burillo S, Pablos MI,
Reiter RJ and Agapito MT: Endogenous rhythms of melatonin, total
antioxidant status and superoxide dismutase activity in several
tissues of chick and their inhibition by light. J Pineal Res.
30:227–233. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Majsterek I, Gloc E, Blasiak J and Reiter
RJ: A comparison of the action of amifostine and melatonin on
DNA-damaging effects and apoptosis induced by idarubicin in normal
and cancer cells. J Pineal Res. 38:254–263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hardeland R, Madrid JA, Tan DX and Reiter
RJ: Melatonin, the circadian multioscillator system and health: The
need for detailed analyses of peripheral melatonin signaling. J
Pineal Res. 52:139–166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carrillo-Vico A, Lardone PJ,
Alvarez-Sánchez N, Rodríguez-Rodríguez A and Guerrero JM:
Melatonin: Buffering the immune system. Int J Mol Sci.
14:8638–8683. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Espino J, Pariente JA and Rodríguez AB:
Oxidative stress and immunosenescence: Therapeutic effects of
melatonin. Oxid Med Cell Longev. 2012:6702942012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mate I, Madrid JA and De la Fuente M:
Chronobiology of the neuroimmunoendocrine system and aging. Curr
Pharm Des. 20:4642–4655. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sainz RM, Mayo JC, Rodriguez C, Tan DX,
Lopez-Burillo S and Reiter RJ: Melatonin and cell death:
Differential actions on apoptosis in normal and cancer cells. Cell
Mol Life Sci. 60:1407–1426. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vijayalaxmi Meltz ML, Reiter RJ, Herman TS
and Kumar KS: Melatonin and protection from whole-body irradiation:
Survival studies in mice. Mutat Res. 425:21–27. 1999. View Article : Google Scholar : PubMed/NCBI
|