Introduction
Sarcoidosis is a multisystemic disease of unknown
origin characterized by the formation of non-caseating granulomas.
Sarcoidosis occurs worldwide and should be diagnosed upon the
clinical presentation for non-caseating granulomas, which are also
associated with other infectious diseases, autoimmune disorders and
neoplasia. Sarcoidosis is often asymptomatic (1); however, its symptoms may be systemic
such as fatigue, fever or weight loss, or organ-specific, such as
shortness for breath or cough. Thoracic involvement is the most
common presentation, reported in up to 90% of patients (2). However, sarcoidosis can involve almost
any other organ, including the skin, eyes and abdominal organs
(2). Extra-thoracic disease can
occur in association with or in the absence of intra-thoracic
disease (3). Involvement of
abdominal viscera is seldom, which may resemble certain common
infectious or neoplastic conditions. Involvement of the liver and
spleen is observed in 40–60% of autopsies (4,5).
However, an isolated extrathoracic sarcoidosis accounts for only
10% of patients (6). To the best of
our knowledge there have been only 10 cases of splenic sarcoidosis
reported in the English literature, data from which are summarized
in Table I. Accessory spleens (AS)
might be formed during embryonic development as separated or
ectopic splenic tissue. They are usually near the splenic hilum,
but may be located in the greater omentum (7). There are no previous reports of
accessory splenic sarcoidosis. Herein, we report a case of isolated
sarcoidosis of an accessory spleen in the greater omentum, which
was identified postoperatively in a 44-year-old female.
 | Table I.Reports of splenic sarcoidosis. |
Table I.
Reports of splenic sarcoidosis.
| Case | Author, year | Gender | Age (years) | Symptoms | Lesion number | Nodular size
(mm) | Laboratory tests | Abdominal CT | MRI | PET-CT | Surgical
treatment | Refs. |
|---|
| 1 | Zia et al,
2005 | F | 47 | Nausea, epigastric
pain | Multiple | Unknown | Elevated bilirubin
and AST | Confirmed multiple
splenic nodules | – | Five focal areas of
increased uptake in the splenic parenchyma | LS | (12) |
| 2 | Chen et al,
2009 | M | 50 | – | Multiple | Unknown | Thrombopenia | Primary
lymphoma | – | – | LS | (17) |
| 3 | Givoinale et
al, 2009 | F | 32 | Epigastric
pain | Multiple | 15 | Mild anemia,
elevated ACE | – | Splenomegaly with
multiple splenic lesions | – | LS | (16) |
| 4 | Joglekar et
al, 2009 | F | 46 | Back and leg
pain | Multiple | Unknown | Raised serum
calcium, urea, creatinine and ESR | Borderline enlarged
spleen, multiple low density coalescent nodular lesions | Posterocentral and
right posterolateral disc protrusion at L5/S1 with compression of
thecal sac | – | OS | (11) |
| 5 | Ogiwara et
al, 210 | F | 74 | Cold sweats,
palpitation | Single | 60×57×60 | Low plasma glucose,
low serum potassium, serum immunoreactive insulin and C-peptide
level undetectable | Splenic mass with
central contrast enhancement | Normal pituitary
gland and hypothalamic region | Normal pituitary
gland and hypothalamic region | OS | (18) |
| 6 | Cuilliere-Dartigues
et al, 2010 | M | 18 | Night sweats,
severe neutropenia | Multiple | 10–30 | ACE normal | Mild splenomegaly
with diffuse nodular lesions | – | Intense splenic
uptake | LS | (19) |
| 7 | Palade et
al, 2012 | F | 66 | Splenomegaly,
hypersplenism, secondary anemia | Unknown | Unknown | Mild
hypochromia | – | – | – | OS | (13) |
| 8 | Bauones et
al, 2013 | F | 37 | Chronic abdominal
discomfort | Multiple | 14–60 | Normal | Multiple hypodense
splenic nodules with inhomogenous enhancement | Multiple splenic
nodules with heterogenous enhancement | – | LS | (10) |
| 9 | Souto et al,
2014 | F | 29 | – | Multiple | 10, mostly | Normal | Multiple lesions in
the spleen with homogeneous enhancement | Multiple splenic
lesions with homogeneous enhancement, low signal on T1 and high
signal on T2 | No
fluorodeoxyglucose uptake in the spleen | LS | (9) |
| 10 | Dennis et
al, 2014 | M | 65 | Headache, weakness,
gait instability, impaired cognition, weight loss | Unknown | Unknown | Hypercalcium,
hypoparathyroid, decreased 25- hydroxyvitamin D hormone | Mild splenomegaly
without focal lesions | – | Intense uptake in
the spleen, malignancy? | HALS | (20) |
Case report
A 44-year-old female presented to the Department of
General Surgery of Lishui Central Hospital (Lishui, China)
complaining of pain beneath the xiphoid process for two years. The
patient denied any personal history of surgery or family history of
cancer. Physical examination presented with normal results. The
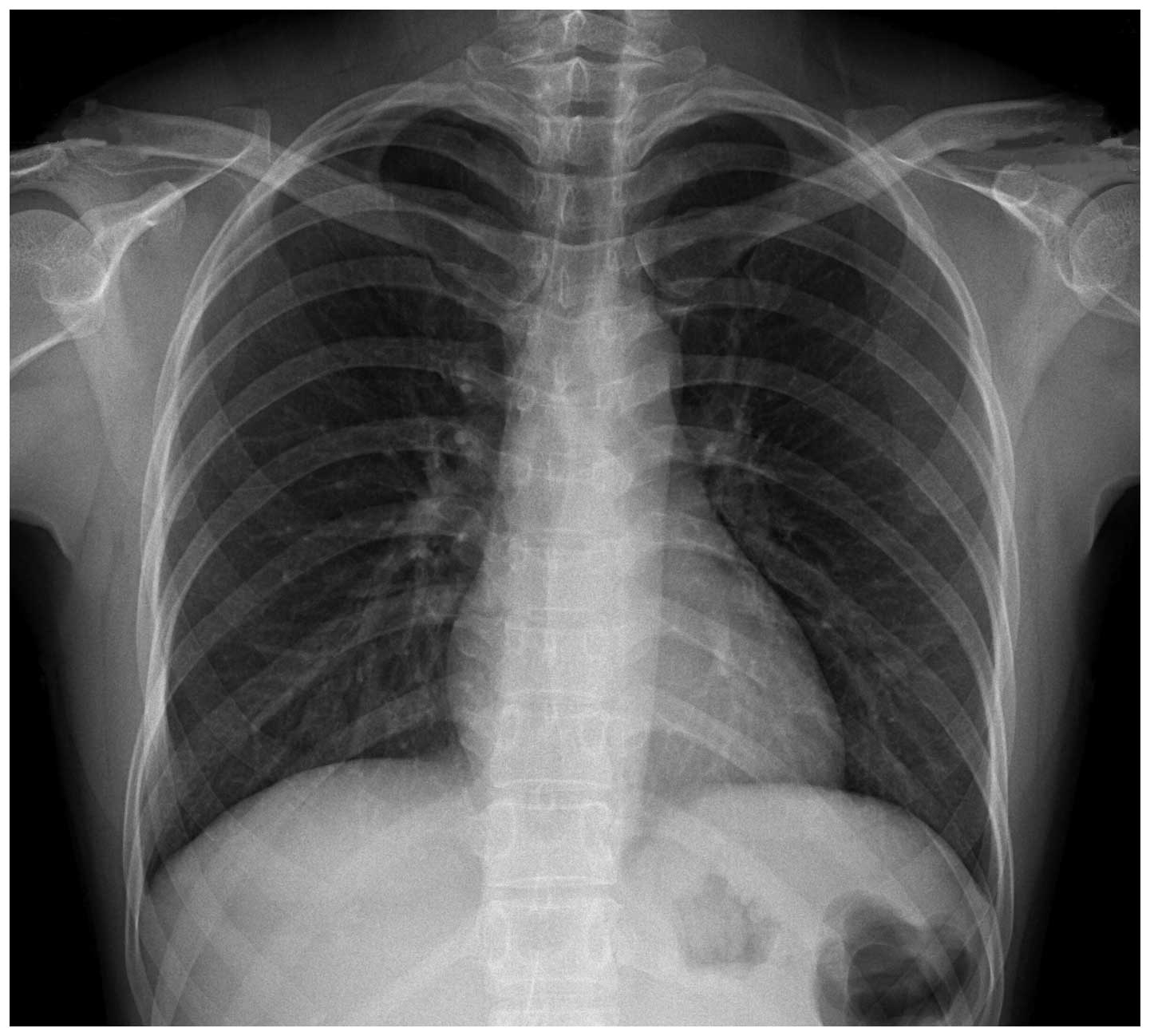
liver and spleen presented normally. Chest X-ray results were
normal (Fig. 1). Gastric endoscopy
showed an ulcer in the antrum measuring 1.5×1.5 cm, which was
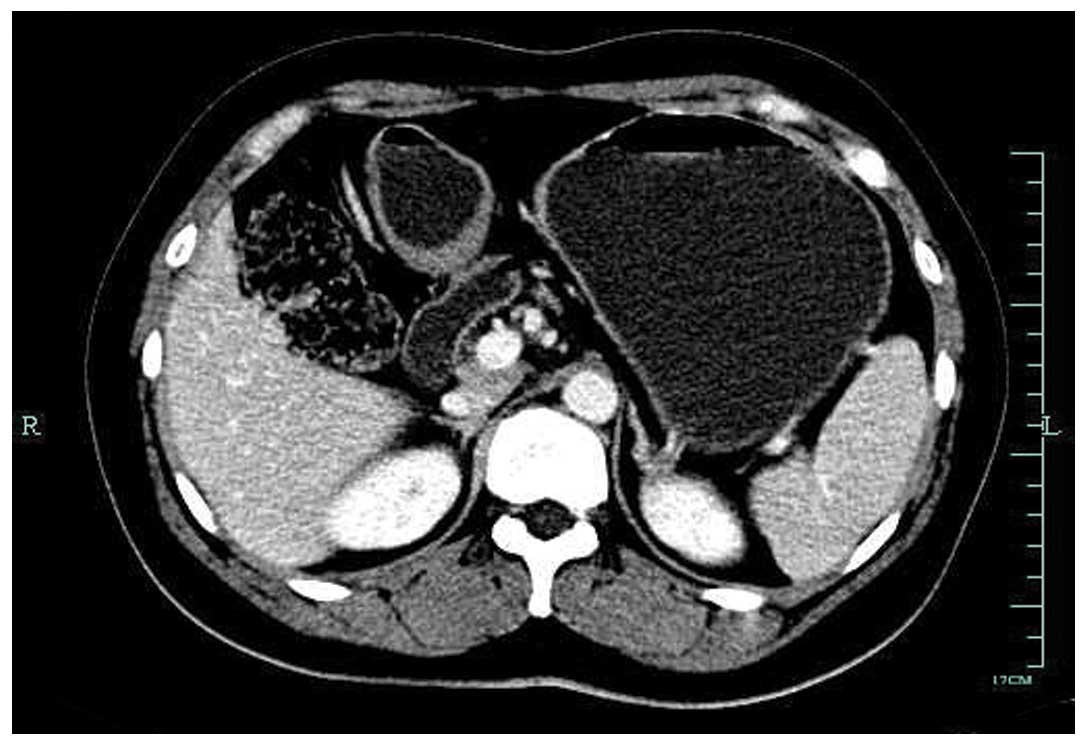
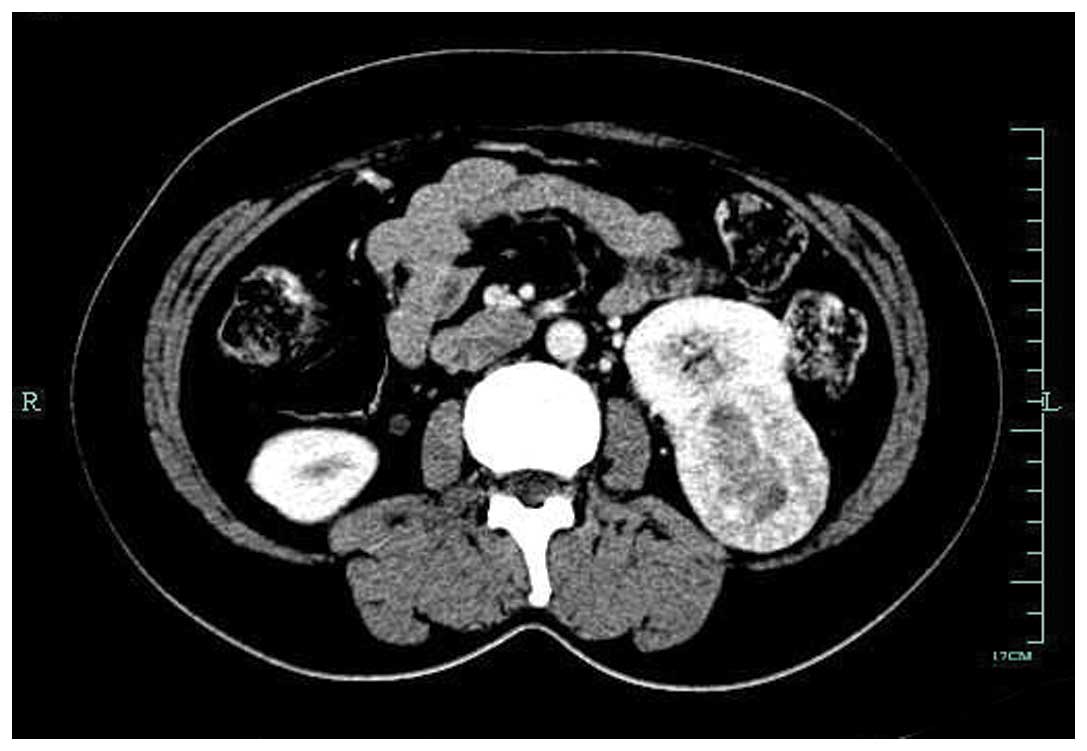
confirmed to be a signet-ring cell carcinoma by biopsy. Computed
tomography (CT) of the abdomen revealed mild thickening of the
posterior antrum, and a mass measuring 5×4 cm in the inferior pole
of the left kidney. No abnormal foci could be found in the greater
omentum or other organs. There was no associated splenomegaly
(Figs. 2 and 3). Mycobacterial, fungal, bacterial and
parasitic infections were all excluded. After four days of
preoperative preparation, including liver function, renal function
and blood coagulation function blood tests and the exclusion of
surgical contraindications, the patient underwent surgical
exploration. Intraoperatively, a solid ulcer was detected in the
antrum measuring 1×1 cm, and a solid mass measuring 5×4 cm was
identified in the inferior pole of left kidney. The accessory
spleen in the greater omentum was not explored. There were no
evident masses in the liver and spleen. In the abdominal cavity,
pelvic cavity, mesenteric and para-aorta, no enlarged lymph nodes
were detected. Radical distal gastrectomy and left radical
nephrectomy, with a midline incision from the xiphoid to umbilicus,
were performed.
Radical distal gastrectomy was completed as follows:
i) The greater omentum was separated from the transverse colon,
followed by resection of the superior leaf of the transverse
mesocolon; ii) the gastroepiploic vein and artery were separately
ligated, and the anterior pancreatoduodenal and infrapyloric nodes,
retroduodenopancreatic lymph nodes and nodes of inferior portion of
the hepatododenal ligament were resected; iii) the right gastric
artery was ligated, and the duodenum was transected and closed ~3
cm from the pylorus; iv) the lymph nodes and the tissue surrounding
the celiac trunk were resected, and the left gastric vein was
ligated at its origin followed by the left artery; v) dissection
continued up the lesser curvature of the stomach, where the right
paracardial zone was dissected and two short gastric veins were
ligated; vi) the upper portion of the stomach was transected using
a Proximate linear cutter (Ethicon Endo-Surgery, LLC, Guaynabo,
Puerto Rico), and the right broken line of the stomach was 3 cm
below the cardia, whereas the left was above the origin of the
second resected gastric short vein; and vii) Hofmeister's
gastrojejunal anastomosis (Billroth II) was performed (8). Left radical nephrectomy was performed
as follows: i) Left posterior peritoneum and perirenal fascia were
incised; ii) after the left renal pedicle was divided, the left
renal artery and vein were separately ligated; iii) the left renal,
perirenal fat and fascia were divided; and iv) after the left
ureter was resected below the iliac vessels, the specimen was
removed.
In addition to signet-ring cell carcinoma in the
antrum and the left renal clear cell cancer, postoperative
pathology demonstrated a red lesion measuring 0.5×0.5 cm in the
greater omentum, which was similar to the spleen in the normal
splenic cavity and was thus regarded as an accessory spleen.
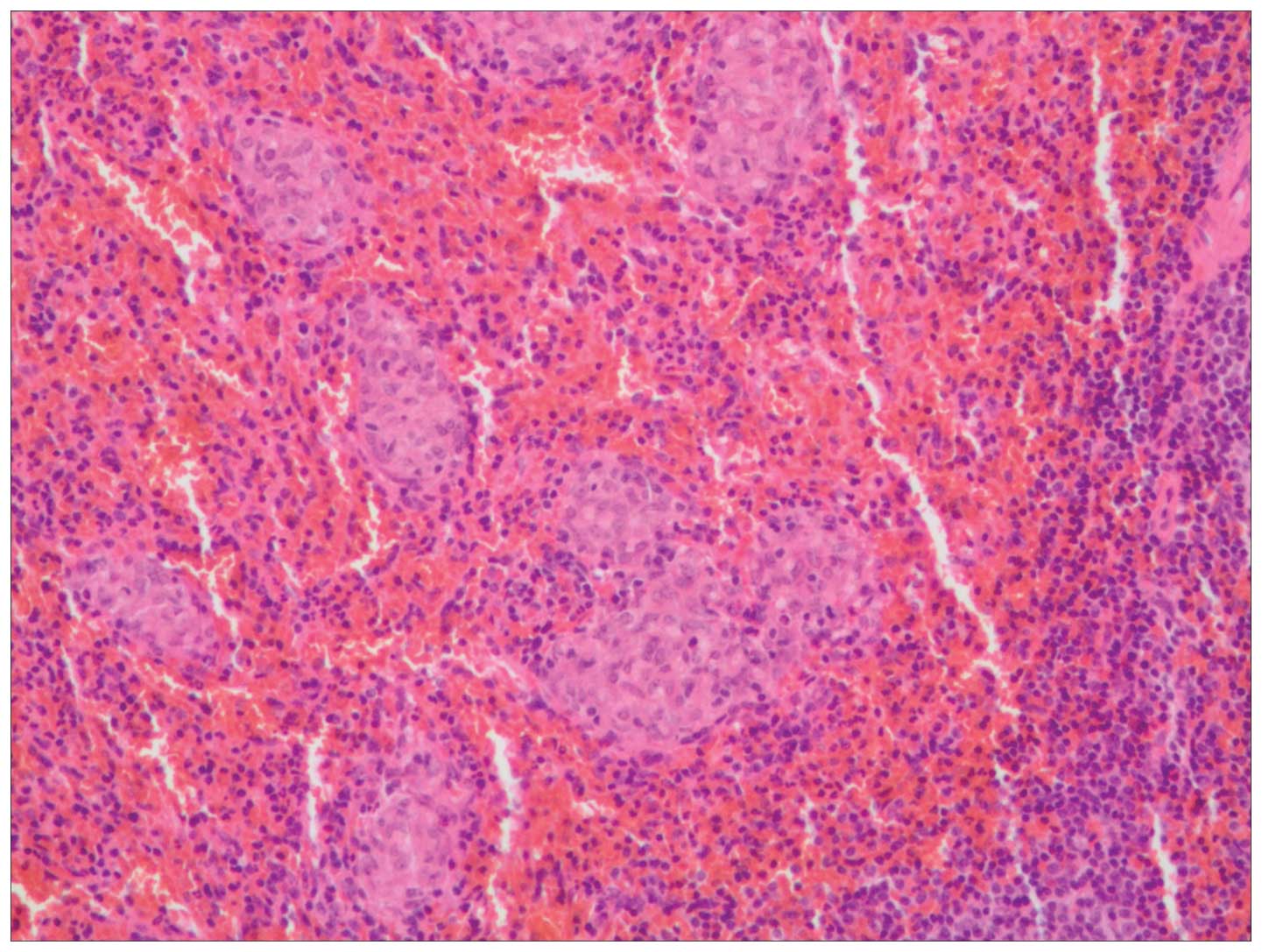
Histological examination of the lesion in the greater omentum
revealed small and dense epithelioid non-caseating granulomas, with
clear margins surrounded by a small quantity of lymphocytic
infiltrates (Fig. 4). Fungi were
excluded using periodic acid-Schiff and hexamine silver staining.
Acid-fast bacilli were excluded by acid-fast staining. Acid-Schiff
and hexamine silver staining were performed using 0.5% potassium
permanganate solution, 8% chromic acid solution, 5% silver nitrate
solution, 3% hexamethylene tetramine solution, 5% sodium
tetraborate solution, 0.1% gold chloride solution and 5% sodium
thiosulfate (Sinopharm Chemical Reagent Co., Ltd., Shanghai,
China). Phenol fuchsine fluid and Mayer's hemalum solution (Fuzhou
Maixin Biotech. Co., Ltd., Fuzhou, China) were used for acid-fast
bacilli staining.
In consideration of the aforementioned tests,
sarcoidosis of accessory spleen in the greater omentum was
confirmed. The patient recovered uneventfully and was discharged on
day 8 postoperation. The patient remained alive after two-year
follow-up without sarcoidosis and malignant tumor recurrence.
Discussion
In the present study a rare case of sarcoidosis of
accessory spleen in the greater omentum was reported. Sarcoidosis
is observed worldwide, the incidence rate of the disease, organs
involved, manifestations and prognosis vary widely with geography
and ethnicity. A diagnosis of sarcoidosis is based on clinical and
radiological results (6), in
addition to the histology of epithelioid granulomas (9). However, granulomas are not a specific
finding of sarcoidosis, and other diseases such as infections
caused by bacteria or fungi and environmental agents must be
excluded. The lungs are involved in 90% of patients with
sarcoidosis; however, almost any other organ may be involved, yet
the digestive tract is rarely involved (2,5,10,11).
Gastrointestinal symptoms might exist and are variable depending on
the location of disease (8,12,13). The
frequency of splenomegaly in sarcoidosis may range between 1 and
40% (14,15). Correlation between pulmonary
abnormalities and hepatosplenic involvement has not yet been
established (16). Autopsy studies
have revealed splenic involvement in 38–77% of patients with
sarcoidosis (1). Isolated splenic
sarcoidosis is rare (1). Sarcoidosis
of the spleen without clinical or radiographic pulmonary disease is
exceedingly rare, with only sporadic cases reported. There have
been only 10 cases of isolated splenic sarcoidosis, and no reports
of sarcoidosis in the accessory spleen (Table I). Due to the popularity and small
size of the accessory spleen, the operator ignored it
intraoperatively. Splenic sarcoidosis exhibits a female preference
(7:3 female to male ratio), with a median age of 46.5 years.
Patients may be asymptomatic (9,17), or
exhibit abdominal pain (12,16). Other atypical symptoms include sweats
(18,19), weakness and weight loss (20). The incidence of splenomegaly
associated with sarcoidosis is variable, but may be has high as 40%
(21). Massive splenomegaly is quite
rare; Fordice et al (22)
reviewed 6,074 cases of sarcoidosis, 628 patients had quantified
splenomegaly and 20 (3%) had massive splenomegaly. Splenic
infarction due to sarcoidosis was has been reported (23). Symptoms related to hypersplenism with
anemia, thrombocytopenia and leucopenia have been reported
(6). However, massive splenomegaly
in sarcoidosis may not result in a pathophysiological effect
(17). As the present case exhibited
two malignant tumors. Whether the incidence of splenic sarcoidosis
is related to the tumors requires confirmation.
Routine laboratory tests are not usually helpful in
the diagnosis of sarcoidosis as these tests are nonspecific.
However, peripheral lymphopenia with CD4 depletion, elevated levels
of serum-angiotensin converting enzyme (S-ACE), lysozyme, β-2
microglobulin, hypercalcemia and hypercalciuria can be helpful to
the diagnosis (24). ACE (25) is elevated in 60% of cases,
hypercalcemia or hypercalciuria occur in about 10–40% of patients
(6). Lymphocytic alveolitis with a
high CD4/CD8 ratio in bronchoalveolar lavage (BAL) fluid is highly
suggestive of the disease (24).
Warshauer et al (1) observed
a linear association between serum ACE level and spleen size as
well as between ACE level and the presence of hepatosplenic
nodules; however, no correlation between the degree of chest
abnormality and the involvement of liver and spleen was identified
(2). In these cases, the diagnosis
of sarcoidosis could be difficult as sarcoidosis is often not
considered due to the nonspecific nature of laboratory tests.
The imaging features of splenic sarcoidosis are
variable. Radiographic results of isolated splenic lesions are
nonspecific and own many differential diagnoses including lymphoma,
metastatic disease, hematomas, abscesses, hamartomas, and
angiosarcomas (26). Certainly, a
histopathologic diagnosis for definitive diagnosis is needed. The
spleen is often enlarged; and hypodense splenic nodules can be seen
in approximately 15% of patients (1). Lesions are often multiple and diffused
distributed; however, they may be undetectable (20). The majority of nodules are between
0.1 and 3.0 cm, with a mean of ~1.0 cm. Notably, Bauones et
al (10) observed capsular
retraction on ultrasound and magnetic resonance images (MRI), and
confirmed at gross examination. This unreported imaging feature in
splenic sarcoidosis may be associated with the presence of
significant collagen fibrosis that surrounded the granulomas.
Notably, in the spleen capsular retraction is rarely reported, in
relation to splenic infarction or neoplastic disease (27). In particular, there was no thoracic
and lymphatic system involvement to suggest sarcoidosis in this
patient. Kessler et al (28)
concluded that focal lesions in spleen in sarcoidosis patients can
occasionally be detected by ultrasound; however, diffuse increased
homogeneous or heterogeneous echogenicity is more common.
Pérez-Grueso et al (5)
initially adopted contrast enhanced ultrasound (CEUS) to diagnose
splenic sarcoidosis. Grzelak et al (29) implemented CEUS to diagnose two cases
of hepatosplenic sarcoidosis, and concluded that CEUS has the
potential to become a reliable tool; replacing more expensive and
less available imaging methods, such as CT and MRI. However,
certain cases exhibited a tumor-like pattern under CEUS; consisting
of slight but diffuse enhancement of the lesions in the arterial
phase followed by progressive hypoenhancement in the parenchymal
phase (30). Tana et al
(31) speculated that this may be
related to different vascularization of the lesions and suggested
the need for further studies before making definite conclusions. In
one report, lesions visible on contrast-enhanced CT were not
observed under sonography, suggesting that the acoustic impedance
of the granulomas was similar to that of normal splenic tissue
(11,32). Dynamic gadolinium-enhanced MRI is
useful in characterizing splenic lesions and narrowing the
differential diagnosis. Classically sarcoidosis-related splenic
lesions are T1 and T2 hypointense and relatively hypoenhancing
(33). Positron emission
tomography-CT is a useful imaging method for the evaluation of
disease activity and the identification of areas that are
undetectable by ultrasound, CT and MRI, as well as in monitoring
response to treatment in patients with sarcoidosis. Among the
reviewed cases, which employed PET-CT, intense fluorodeoxyglucose
uptake in the spleen was detected in 75% of patients (9,12,19,20).
Kataoka et al (34) suggested
multiple imaging modalities, including dynamic CT and MRI for
detecting splenic lesions of sarcoidosis. Due to the risk of
bleeding, tract seeding and variable results, a percutaneous biopsy
via ultrasound or CT was not suggested. Without lung or other
systemic involvement, the symptoms of splenic sarcoidosis may
resemble more serious neoplastic or infectious disease (6), and in this context only splenectomy
allows the establishment of a diagnosis. Following diagnosis,
patients require continual follow-up for systemic manifestations
and associated complications of sarcoidosis. None of the presently
reviewed 10 cases were diagnosed preoperatively. The present case
was accidently identified postoperative pathology.
Splenectomy is considered as a treatment option for
hypersplenism, prophylaxis for splenic rupture and neoplastic
exclusion (16). Splenectomy might
not alter the course of disease and is merely an adjunct to medical
therapy in order to resolve complications of sarcoidosis (35). With the improvements in the
technologies, laparoscopic splenectomy has gradually become the
gold standard for surgical removal of the spleen, and there have
been reported laparoscopic splenectomies performed for sarcoidosis
(9,12). Laparoscopic splenectomy is a
minimally invasive procedure that provides superior aesthetic
results, decreased blood loss and decreased postoperative
complications and faster recovery, which allows the earlier
initiation of chemotherapy. If a splenectomy is to be performed,
comprehensive exploration should be conducted to exclude the
accessory spleen, which may also suffer from sarcoidosis. In the
presently reviewed cases, hypercalcemia and hypersplenism
disappeared after splenectomy (11,13). As
isolated splenic disease may be only a precursor to future systemic
disease, and thus patients must be followed over their lifetimes.
Asymptomatic patients do not require the immunosuppressive drugs;
however, if symptoms do occur, steroids or even methotrexate and
azathioprine can be used. As many as 66% of patients spontaneously
remit (6). The lack of response or
the appearance of complications may require splenectomy (12). In the majority of cases the
recommended therapy is using glucocorticoid, which should be
resumed in the instance of a relapse.
In summary, one rare case of isolated sarcoidosis of
accessory spleen in the greater omentum is reported. Symptoms of
splenic sarcoidosis are atypical, requiring multiple imaging
modalities for detection Splenectomy was indicated for symptoms,
hypersplenism, prophylaxis for splenic rupture and neoplastic
exclusion. Intraoperatively, comprehensive exploration should be
conducted to exclude the accessory spleen, which may also suffer
from sarcoidosis.
References
|
1
|
Warshauer DM and Lee JK: Imaging
manifestations of abdominal sarcoidosis. AJR Am J Roentgenol.
182:15–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jundson MA: Hepatic, splenic and
gastrointestinal involvement with sarcoidosis. Semin Respir Crit
Care Med. 23:529–541. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kataoka M, Nakata Y, Hiramatsu J, Okazaki
K, Fujimori Y, Ueno Y, Tanimoto Y, Kanehiro A, Tada S and Harada M:
Hepatic and splenic sarcoidosis evaluated by multiple imaging
modalities. Intern Med. 37:449–453. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vagal AS, Shipley R and Meyer CA:
Radiological manifestations of sarcoidosis. Clin Dermatol.
25:312–325. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pérez-Grueso MJ, Repiso A, Gómez R,
Gonzalez C, de Artaza T, Valle J, García A and Carrobles JM:
Splenic focal lesions as manifestation of sarcoidosis:
Characterization with contrast-enhanced sonography. J Clin
Ultrasound. 35:405–408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Warshauer DM: Splenic sarcoidosis. Semin
Ultrasound CT MR. 28:21–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Unver Dogan N, Uysal II, Demirci S, Dogan
KH and Kolcu G: Accessory spleens at autopsy. Clin Anat.
24:757–762. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thompson JC: The stomach and duodenum.
Textbook of Surgery: The Biological Basis of Modern Surgical
Practice. Sabiston DC: (Philadephia, PA). WB Saunders Company.
810–853. 1986.
|
|
9
|
Soutto MM, Tempes BC, Lambert BF, Trindade
EN and Trindade MR: Laparoscopic splenectomy for isolated splenic
sarcoidosis. JSLS. 18:155–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bauones S, Le Corroller T, Durieux O,
Guenoun D, Del Grande J, Pirro N and Champsaur P: Splenic
sarcoidosis mimicking neoplastic disease. J Clin Ultrasound.
42:38–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Joglekar SP, Hudson RL, Lgasundaram R and
Pereira JH: ‘Surgical cure’ for non parathyroid hypercalcemia.
World J Surg Oncol. 7:232009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zia H, Zemon H and Brody F: Laparoscopic
splenectomy for isolated sarcoidosis of the spleen. J Laparoendosc
Adv Surg Tech A. 15:160–162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Palade R, Voiculescu1 D, Suliman E and
Simion G: Splenic sarcoidosis - a case report. Chirurgia (Bucur).
107:670–674. 2012.PubMed/NCBI
|
|
14
|
Salazar A, Mañá J, Corbella X, Albareda JM
and Pujol R: Splenomegaly in sarcoidosis: A report of 16 cases.
Sarcoidosis. 12:131–134. 1995.PubMed/NCBI
|
|
15
|
Madaule S, Lauque D, Sailler L, Arlet P
and Carles P: Splenomegaly in sarcoidosis: Clinical features and
outcome. Analysis of 17 cases. Rev Med Interne. 25:348–356.
2004.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giovinale M, Fonnesu C, Soriano A,
Cerquaglia C, Curigliano V, Verrecchia E, De Socio G, Gasbarrini G
and Manna R: Atypical sarcoidosis: Case reports and review of the
literature. Eur Rev Med Pharmacol Sci. 13(Suppl 1): S37–S44.
2009.
|
|
17
|
Chen MY, Cai JT, Du Q and Wang LJ:
Sarcoidosis of spleen presenting with solitary thrombopenia. Eur J
of Intern Med. 20:e122009. View Article : Google Scholar
|
|
18
|
Ogiwara Y, Mori S, Iwama M, Sawabe M,
Takemoto M, Kanazawa N, Furuta K, Fukuda I, Kondo Y, Kimbara Y, et
al: Hypoglycemia due to ectopic secretion of insulin-like growth
factor-I in a patient with an isolated sarcoidosis of the spleen.
Endocr J. 57:325–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cuilliere-Dartigues P, Meyohas MC,
Balladur P, Gorin NC and Coppo P: Splenic sarcoidosis: An unusual
aetiology of agranulocytosis. Am J Hematol. 85:8912010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dennis BA, Jajosky RP and Harper RJ:
Splenic sarcoidosis without focal nodularity: A case of
1,25-dihydroxyvitamin D-mediated hypercalcemia localized with FDG
PET/CT. Endocr Pract. 20:e29–e33. 2014. View Article : Google Scholar
|
|
21
|
Xiao GQ, Zinberg JM and Unger PD:
Asymptomatic sarcoidosis presenting as massive splenomegaly. Am J
Med. 113:698–699. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fordice J, Katras T, Jackson RE, Cagle PT,
Jackson D, Zaleski H and Asimacopoulos PJ: Massive splenomegaly in
sarcoidosis. South Med J. 85:775–778. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patel I, Ismaji M and Steuer A:
Sarcoidosis presenting as massive splenic infarction. Case Rep
Rheumatol. 2012:8347582012.PubMed/NCBI
|
|
24
|
Bergoin C, Lamblin C and Wallaert B:
Biological manifestations of sarcoidosis. Ann Med Interne (Paris).
152:34–38. 2001.(In French). PubMed/NCBI
|
|
25
|
Baudin B: Angiotensin I-converting enzyme
(ACE) for sarcoidosis diagnosis. Pathol Biol (Paris). 53:183–188.
2005.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Folz SJ, Johnson CD and Swensen SJ:
Abdominal manifestations of sarcoidosis in CT studies. J Comput
Assist Tomogr. 19:573–579. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fiorention F, Bribani A and Rosselli A: A
rare cause of the left upper quadrant abdominal pain. Intern Emerg
Med. 6:55–57. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kessler A, Mitchell DG, Israel HL and
Goldberg BB: Hepatic and splenic sarcoidosis: Ultrasound and MR
imaging. Abdom Imaging. 18:159–163. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grzelak P, Augsburg L, Majos A, Stefanczyk
L, Gorski P, Piotrowski W and Antczak A: Use of contrast-enhanced
ultrasonography in hepatosplenic sarcoidosis: Report of 2 cases.
Pol J Radiol. 77:60–63. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stang A, Keles H, Hentschke S, von
Seydewitz CU, Dahlke J, Malzfeldt E and Braumann D: Differentiation
of benign from malignant focal splenic lesions using sulfur
hexafluoride-fillled microbubble contrast-enhanced pulse-inversion
sonography. AJR Am J Roentgenol. 193:709–721. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tana C, Iannetti G, Mezzetti A and
Schiavone C: Splenic sarcoidosis remains a diagnostic challenge. J
Clin Ultrasound. 42:1562014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Franquet T, Oteo JA, Cozcoluella R and
Casas JM: Multinodular splenic sarcoidosis: Discordant CT and
sonographic findings. AJR Am J Roentgenol. 156:1113–1114. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Raber EL, Haba J and Beck P: Splenic
sarcoidosis: A case report and review of the imaging findings of
multiple incidental splenic lesios as the initial presentation of
sarcoidosis. Can J Gastroenterol. 25:477–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kataoka M, Nakata Y, Hiramatsu J, Okazaki
K, Fujimori Y, Ueno Y, Tanimoto Y, Kanehiro A, Tada S and Harada M:
Hepatic and splenic sarcoidosis evaluated by multiple imaging
modalities. Internal Med. 37:449–453. 1998. View Article : Google Scholar
|
|
35
|
Sharma OP, Vucinic V and James DG:
Splenectomy in sarcoidosis: Indications, complications and
long-term followup. Sarcoidosis Vasc Diffuse Lung Dis. 19:66–70.
2002.PubMed/NCBI
|