Introduction
Radiation therapy is one of the main therapeutic
methods used in the treatment of gliomas following surgical
excision, despite the fact that it often causes radiation necrosis
(reported incidence rate, 2–24%), the most severe type of radiation
injury (1,2). Following treatment, frequent monitoring
is required for the evaluation of the therapy, and magnetic
resonance imaging (MRI) is the preferred modality.
Glioma growth is often accompanied by the breakdown
of the blood brain barrier (BBB) and a higher cerebral blood volume
(CBV), which is due to tumor angiogenesis (3,4);
however, BBB leakage also occurs in radiation injury. Both types of
lesions appear hyperintense on T2-weighted images and show strong
contrast enhancement with surrounding edema and mass effect, which
makes it impossible to differentiate between glioma recurrence and
radiation injury using the conventional enhanced MRI (5–7);
therefore, there is an urgent requirement for the development of
new, functional imaging modalities for the evaluation of the
effectiveness of glioma treatment.
Dynamic susceptibility contrast-weighted (DSC)
magnetic resonance (MR) perfusion imaging allows for the
measurement of cerebral blood flow (CBF) and CBV, which are known to
correlate with both the histologic tumor grade and individual
histological features (8–10). A previous study has shown that
DSC-MRI is useful for the diagnosis of glioma recurrence and
radiation necrosis (11). However,
there are several disadvantages to using this technique. First, it
requires an intravenous injection of a gadolinium contrast agent
(12,13). Secondly, in DSC-MRI, contrast agent
extravasation causes T2-weighted signal intensity loss, which can
in turn result in a decreased relative CBV (rCBV) for high-grade
tumors (14). Thirdly, this
technique is mainly based on gradient-echo or echo planar imaging
(EPI), which have been shown to be highly sensitive to
susceptibility, which may decrease the rCBV ratio (15).
Arterial spin labeling (ASL) MR perfusion imaging
utilizes labeled blood water as the endogenous tracer, and is
recognized as a non-invasive method of measuring CBF. Several
studies have used this technique to evaluate glioma grading and
tumor angiogenesis (16–18). The aim of the present study was to
assess the ability of ASL perfusion imaging to differentiate
between glioma recurrence and radiation necrosis and compare it
with the DSC-MRI technique.
Materials and methods
Subjects
The present study was approved by the Ethics
Committee of the Northern Jiangsu Province Hospital (Yangzhou,
China).
Between March 2012 and March 2014, 57 surgically
treated patients with pathologically confirmed primary gliomas
underwent follow-up MRI. Using contrast-enhanced T1-weighted
imaging, contrast-enhancing lesions were observed in 21 of these
cases and were included in the present study. Sixteen patients
received radiotherapy with 50–60 Gy, while 5 received
radiochemotherapy (50–60 Gy) combined with temozolomide. Patients
to whom temozolomide was administered concurrently received a daily
temozolomide dosage of 75 mg/m2 during
intensity-modulated radiation therapy, and then at 150
mg/m2 for 5 days in each of six 28-day treatment
cycles.
Follow-up MRI scans were performed every 3 months
and included T2-weighted, diffusion weighted (DW) and ASL perfusion
imaging, followed by DSC-MRI.
Materials and methods
Conventional MR images were acquired using a 3.0T MR
imaging system (Discovery MR750; GE Healthcare, Fairfield, CT, USA)
with a 16-channel coil specifically for imaging the head.
Conventional sequences included axial, sagittal and coronal
T2-weighted, axial T1-weighted, fluid-attenuated inversion recovery
sequence and DW imaging.
ASL imaging used a 3-dimensional (3D)
pseudocontinuous ASL method and was performed prior to DSC-MRI as
follows: Repetition time (TR), 4,632 msec; echo time (TE), 1.5
msec; acquisition matrix, 96×61; field of view (FOV), 24 cm; slice
thickness, 4 mm; interslice gap, 0 mm and post label delay, 1,535
msec with spiral acquisition along with 3D proton density-weighted
fast spin echo (FSE) EPI sequence as follows: TR, 3.9 msec, TE, 1.9
msec, parallel imaging factor, 2; acquisition matrix, 96×61; FOV,
24 cm; slice thickness, 4 mm and interslice gap, 0 mm. The dosage
of contrast agent (Magnevist; Bayer HealthCare Pharmaceuticals,
Berlin, Germany) was 0.05 mmol/kg. The automatic bolus injection
(Medrad Spectris Solaris; Bayer HealthCare, Saxonburg, PA, USA) of
the contrast agent (rate, 3 ml/sec) was followed by an injection of
20 ml saline. The total acquisition time for perfusion measurement
and bolus tracking was 1:20 min.
Image evaluation
Qualitative assessment
All images were reviewed by two neuroradiologists
who were blinded to the clinical and histopathological history of
the cases. The images were evaluated using the FuncTool Performance
software package with the Advantage 4.5 Workstation (GE
Healthcare).
Since the regions of the gliomas with maximum
perfusion are suggestive of malignancy and aggressiveness, three
maximally perfusion regions of the entire lesions were located by
drawing regions of interest (ROIs) of area 0.5–2.0 cm2.
The average of the values from these regions was calculated and
normalized to the contralateral normal white matter.
Artifacts
A 5-point scale was used to assess the image
artifacts (ranging from 1, severe degradation to 5, little
degradation) caused by motion and susceptibility effects. A score
of 5 was attributed to an excellent image quality, with almost no
artifacts; while a score of 3 indicated degraded image quality
owing to artifacts (sufficiently degraded to interfere with
accurate diagnosis).
Classification of lesions
Six cases of glioma recurrence were classified
according to the histopathological analysis, while 15 lesions (10
lesions of glioma recurrence and 5 of radiation necrosis) were
verified based on the follow-up MRI scan according to the Macdonald
criteria (19,20). Each patient was followed up for a
minimum of 11 months, and the rate of follow-up was 100%.
Statistical analysis
All statistical analyses were performed using SPSS
16.0 (SPSS, Inc., Chicago, IL, USA). The unpaired Student's t-test
was used for the comparison between the CBF and rCBV values of
recurrent gliomas and those radiation necrosis. Artifact scores in
ASL and DSC images were compared using Wilcoxon's sign-rank test.
Linear regression analysis was used to evaluate the correlation
between ASL-CBF and DCS-CBV. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient diagnosis and imaging
results
The present study included 21 patients (11 men and
10 women), with an age range of 32–63 years and a mean age of 51.3
years. Six cases of glioma recurrence were confirmed by surgical
pathology or biopsy, and 10 cases of glioma recurrence were
verified based on the Macdonald criteria (19), since they showed clear radiological
evidence of disease progression despite the use of therapy.
Radiation necrosis was diagnosed in 5 cases by follow-up MRI, in
which enhanced lesions disappeared or decreased in size without any
treatment.
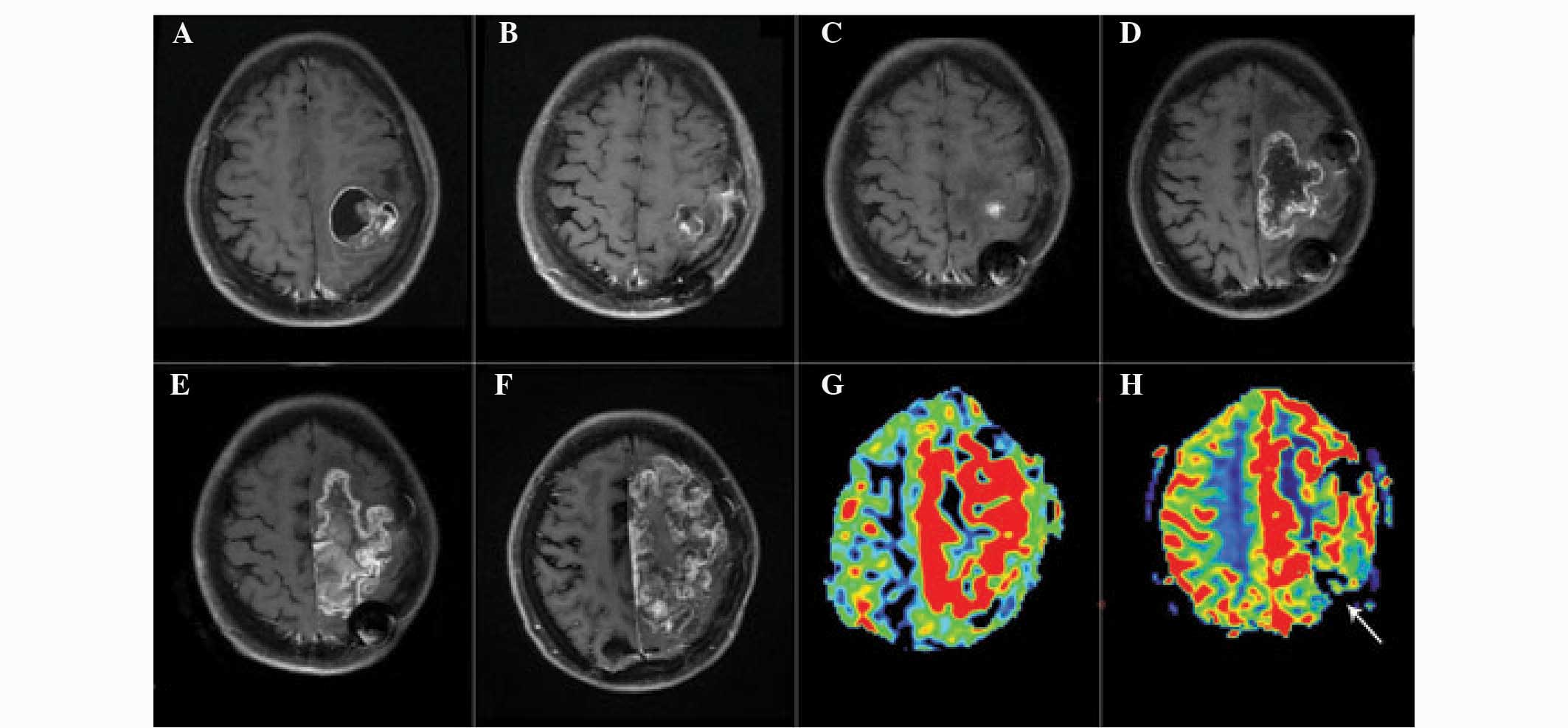
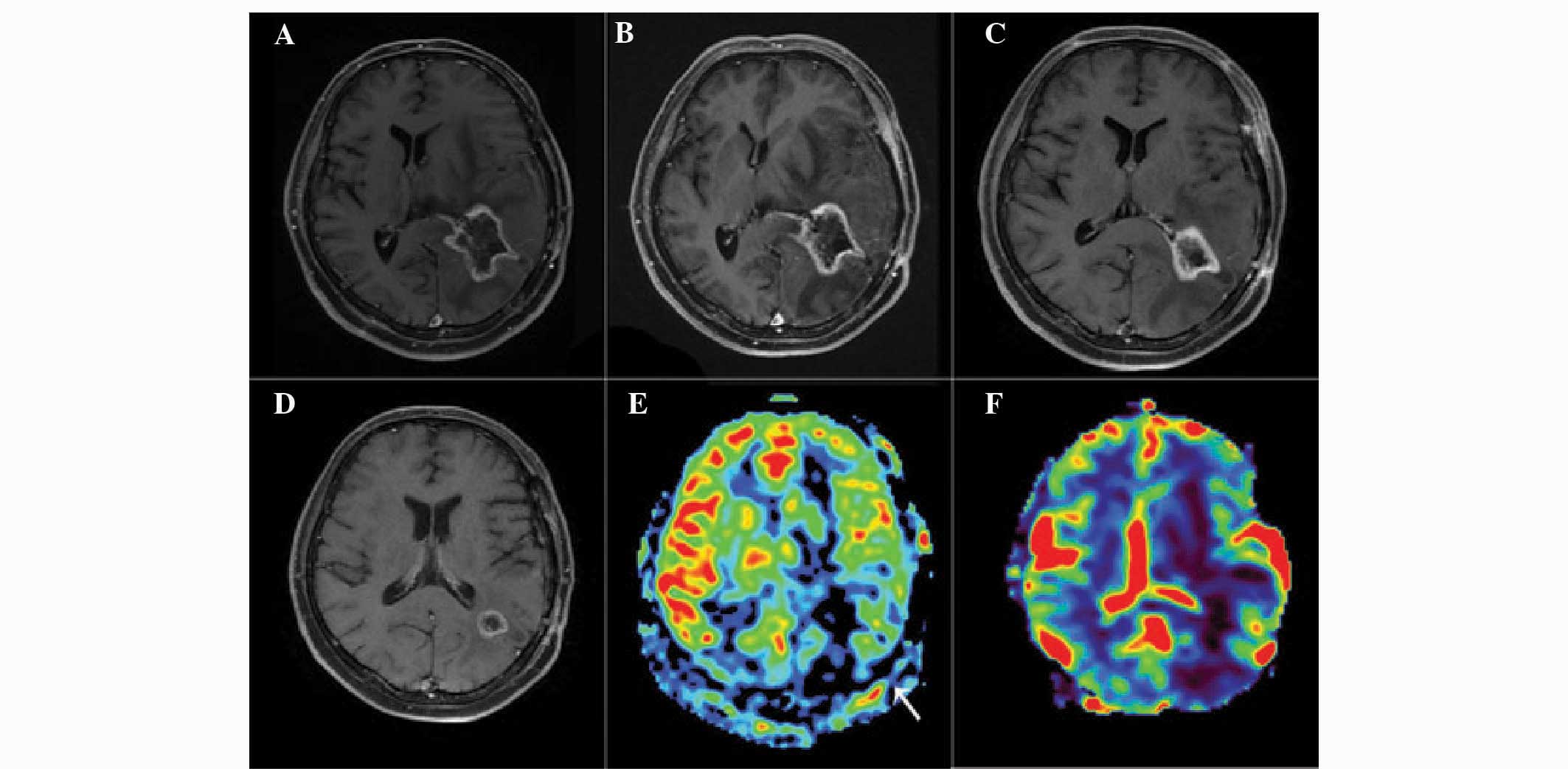
Representative images of patients with glioma
recurrence and radiation injury are presented in Figs. 1 and 2. Analysis of the images revealed that
glioma recurrence exhibited a higher normalized ASL-CBF ratio
(4.45±2.72) compared with that of radiation injury (1.22±0.61)
(P<0.01). In addition, the normalized DSC-rCBV ratio in glioma
recurrence (3.38±2.08) was significantly higher than that in
radiation injury (1.09±0.55) (P<0.05).
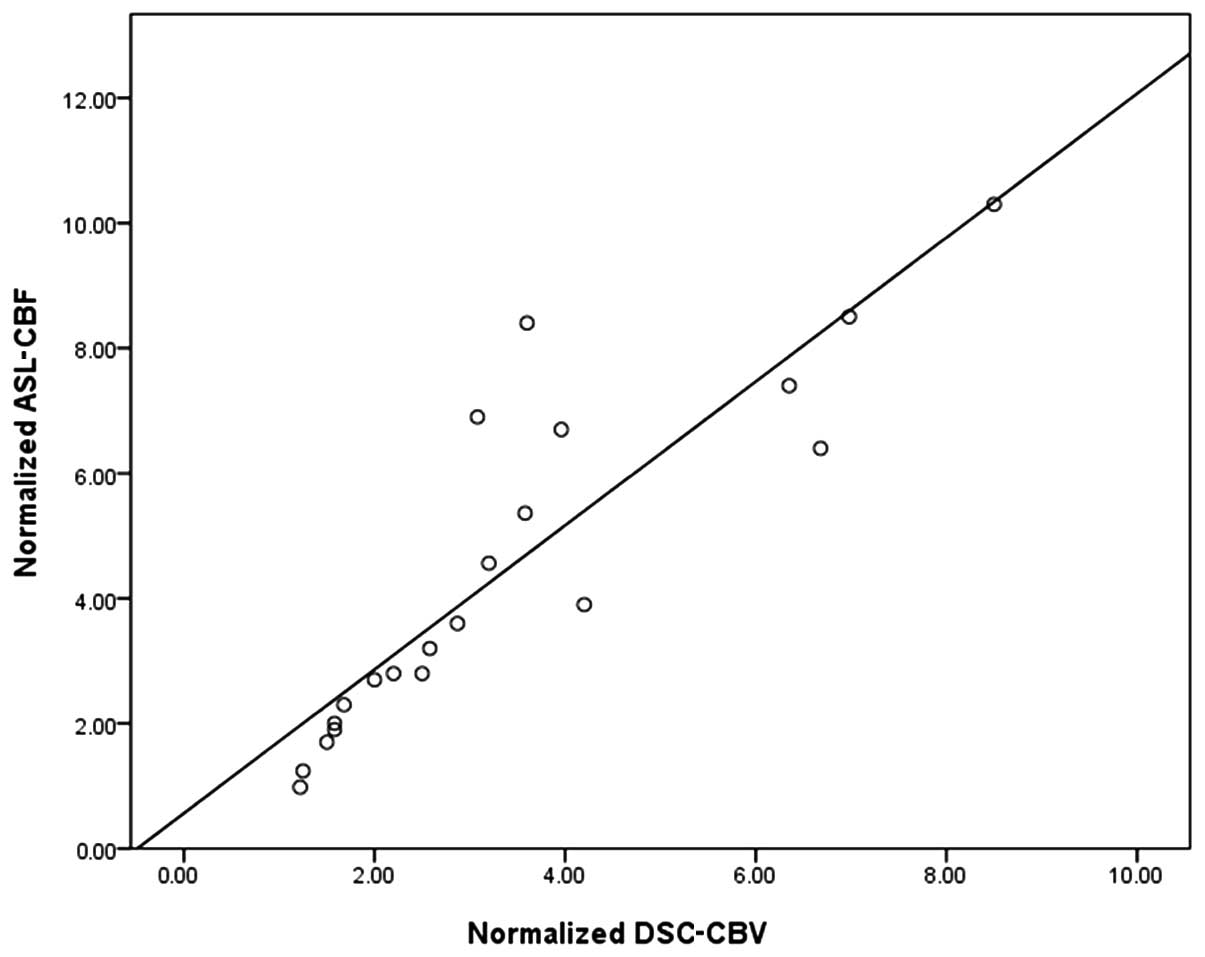
Linear regression analysis
As demonstrated in Fig.
3, the linear regression analysis revealed that there was a
close correlation between normalized ASL-CBF and normalized
DSC-CBV, with R=0.85 and an equation of y=0.56+1.15× for the
regression line, which was statistically different from identity at
P<0.05.
Artifact scores
Artifacts in the ASL-CBF and DSC-CBV images were
scored as follows (Table I): Motion
artifact, 4.75±0.44 and 4.60±0.50, respectively (P=0.508);
susceptibility artifact, 4.85±0.37 and 4.15±0.75, respectively
(P<0.01). No case scored ≤3 due to motion artifacts in either of
the two imaging techniques or due to susceptibility artifacts in
the measurement of ASL-CBF. With regard to the estimation of
DSC-rCBV, 5 cases scored 3, due to susceptibility artifacts.
 | Table I.Artifact scores in ASL and DSC
imaging. |
Table I.
Artifact scores in ASL and DSC
imaging.
|
| Artifact score (mean
± SD) |
|---|
|
|
|
|---|
| Imaging | Motion artifact | Susceptibility
artifact |
|---|
| ASL-CBF | 4.75±0.44 | 4.85±0.37 |
| DSC-CBV | 4.60±0.50 | 4.15±0.75 |
| P-value | 0.508 | 0.0004 |
Discussion
In the present study, the ability of the ASL
technique to differentiate between glioma recurrence and radiation
necrosis was evaluated. The results demonstrated that there is a
close correlation between the ASL and DSC methods with regard to
distinguishing between the two conditions.
Perfusion imaging of brain tumors, which mainly
includes DSC-MR perfusion techniques, has been used for tumor
grading, guiding tumor biopsy and assessing the response to
treatment (21). However, the use of
an exogenous contrast agent is a major limitation in the routine
clinical application of this method, since contrast agent
extravasation and high sensitivity to susceptibility can result in
a decreased rCBV for high-grade tumors (9). ASL imaging constitutes another MR
perfusion method, which is used for the assessment of brain tumor
vascularity (22). In this
technique, the contrast agent used is labeled arterial blood water
proximal to the brain. Since 90% of the labeled water passes
through the capillary bed on the first pass and T1 decay is
considerably shorter than the capillary transit time, contrast
agent extravasation and dispersion do not interfere with ASL signal
intensity (19,23). In addition, since no exogenous
contrast agent is required, ASL imaging could be ideal for the
long-term follow-up of gliomas following radiation, including those
with renal dysfunction.
Previous studies have shown that ASL imaging could
potentially differentiate between glioma recurrence and radiation
injury. The study conducted by Ozsunar et al (24) demonstrated that ASL imaging could
accurately distinguish predominant recurrent high-grade glioma from
radiation necrosis; however, the results were based on a
single-slice method with a scanning time of 4–8 min, dependent on
lesion size, which limited the wide range of clinical applications.
Choi et al (25) showed the
diagnostic superiority of combined ASL and DSC perfusion compared
with DSC imaging alone in the differentiation of pseudoprogression
from early tumor progression, with ASL having a lower sensitivity
than DSC perfusion. There are, however, differences between the
study of Choi et al (25) and
the present study. ASL imaging in the previous study was based on
an gradient-echo sequence, which is more vulnerable to magnetic
susceptibility artifacts. Furthermore, Choi et al only
assessed the diagnostic performance of DCS perfusion alone or
combined with ASL imaging, while the present study aimed to assess
the diagnostic performance of ASL imaging. The results of the
present study suggest that there is a close correlation between ASL
perfusion imaging and DSC-MRI (Fig.
3) and that ASL-CBF could effectively distinguish glioma
recurrence from radiation injury.
DSC-MRI was shown to exhibit a high sensitivity to
susceptibility, due to magnetic susceptibility artifacts, which
could lead to an underestimation of tumor perfusion when the ROI is
close to surgically treated regions of the brain or areas affected
by bleeding (26,27). In the present study, however, the ASL
imaging was conducted utilizing the 3D FSE technique, which
features high spatial resolution and reduced magnetic
susceptibility. No evident magnetic susceptibility artifacts were
found to influence the image quality in the present study,
indicating that this ASL imaging method is suitable for the
follow-up of glioma after surgical excision.
The study did, however, have several limitations.
First, the sample size was rather small, particularly that of
patients with radiation injury, which does not allow for
generalization of the present findings; further research using a
larger population is required. Secondly, only the most clearly
enhanced regions were analyzed; however, edema in a portion of the
glioma may represent tumor infiltration, the identification of
which may contribute to an improved evaluation of the tumor.
Finally, in the ASL imaging, only a single delay time between
labeling and imaging was used, which may result in an inaccurate
estimation of the CBF, due to differences in the cerebral
circulation among individuals.
In conclusion, the aforementioned findings
demonstrate the potential of CBF as determined by ASL perfusion
imaging in the differentiation of glioma recurrence from radiation
injury. ASL imaging could potentially be used to determine the
perfusion patterns in patients with surgically treated primary
gliomas, and could also prove useful in the selection of the
appropriate treatment option.
Acknowledgements
This study was supported by The National Natural
Science Fund of China (grant no. 81371377).
References
|
1
|
Tsuruda JS, Kortman KE, Bradley WG,
Wheeler DC, Van Dalsem W and Bradley TP: Radiation effects on
cerebral white matter: MR evaluation. AJR Am J Roentgenol.
149:165–171. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Remler MP, Marcussen WH and Tiller-Borsich
J: The late effects of radiation on the blood brain barrier. Int J
Radiat Oncol Biol Phys. 12:1965–1969. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leon SP, Folkerth RD and Black PM:
Microvessel density is a prognostic indicator for patients with
astroglial brain tumors. Cancer. 77:362–372. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang AP, Tsai JC, Kuo LT, Lee CW, Lai HS,
Tsai LK, Huang SJ, Chen CM, Chen YS, Chuang HY and Wintermark M:
Clinical application of perfusion computed tomography in
neurosurgery. J Neurosurg. 120:473–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mullins ME, Barest GD, Schaefer PW,
Hochberg FH, Gonzalez RG and Lev MH: Radiation necrosis versus
glioma recurrence: Conventional MR imaging clues to diagnosis. AJNR
Am J Neuroradiol. 26:1967–1972. 2005.PubMed/NCBI
|
|
6
|
Brandes AA, Tosoni A, Spagnolli F, Frezza
G, Leonardi M, Calbucci F and Franceschi E: Disease progression or
pseudoprogression after concomitant radiochemotherapy treatment:
Pitfalls in neurooncology. Neuro Oncol. 10:361–367. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kumar AJ, Leeds NE, Fuller GN, Van Tassel
P, Maor MH, Sawaya RE and Levin VA: Malignant gliomas: MR imaging
spectrum of radiation therapy- and chemotherapy-induced necrosis of
the brain after treatment. Radiology. 217:377–384. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cha S, Knopp EA, Johnson G, Wetzel SG,
Litt AW and Zagzag D: Intracranial mass lesions: Dynamic
contrast-enhanced susceptibility-weighted echo-planar perfusion MR
imaging. Radiology. 223:11–29. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paulson ES and Schmainda KM: Comparison of
dynamic susceptibility-weighted contrast-enhanced MR methods:
Recommendations for measuring relative cerebral blood volume in
brain tumors. Radiology. 249:601–613. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thomsen H, Steffensen E and Larsson EM:
Perfusion MRI (dynamic susceptibility contrast imaging) with
different measurement approaches for the evaluation of blood flow
and blood volume in human gliomas. Acta Radiol. 53:95–101. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barajas RF Jr, Chang JS, Segal MR, Parsa
AT, McDermott MW, Berger MS and Cha S: Differentiation of recurrent
glioblastoma multiforme from radiation necrosis after external beam
radiation therapy with dynamic susceptibility-weighted
contrast-enhanced perfusion MR imaging. Radiology. 253:486–496.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumura T, Hayakawa M, Shimada F, Yabuki
M, Dohanish S, Palkowitsch P and Yoshikawa K: Safety of
gadopentetate dimeglumine after 120 million administrations over 25
years of clinical use. Magn Reson Med Sci. 12:297–304. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang L, Krefting I, Gorovets A, Marzella
L, Kaiser J, Boucher R and Rieves D: Nephrogenic systemic fibrosis
and class labeling of gadolinium-based contrast agents by the food
and drug administration. Radiology. 265:248–253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bjornerud A and Emblem KE: A fully
automated method for quantitative cerebral hemodynamic analysis
using DSC-MRI. J Cereb Blood Flow Metab. 30:1066–1078. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carlsson A, Starck G, Ljungberg M, Ekholm
S and Forssell-Aronsson E: Accurate and sensitive measurements of
magnetic susceptibility using echo planar imaging. Magn Reson
Imaging. 24:1179–1185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Detre JA, Rao H, Wang DJ, Chen YF and Wang
Z: Applications of arterial spin labeled MRI in the brain. J Magn
Reson Imaging. 35:1026–1037. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Detre JA, Wang J, Wang Z and Rao H:
Arterial spin-labeled perfusion MRI in basic and clinical
neuroscience. Curr Opin Neuro. 22:348–355. 2009. View Article : Google Scholar
|
|
18
|
Chawla S, Wang S, Wolf RL, Woo JH, Wang J,
O'Rourke DM, Judy KD, Grady MS, Melhem ER and Poptani H: Arterial
spin-labeling and MR spectroscopy in the differentiation of
gliomas. AJNR Am J Neuroradiol. 28:1683–1689. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Macdonald DR, Cascino TL, Schold SC Jr and
Cairncross JG: Response criteria for phase II studies of
supratentorial malignant glioma. J Clin Oncol. 8:1277–1280.
1990.PubMed/NCBI
|
|
20
|
Tan H, Chen L, Guan Y and Lin X:
Comparison of MRI, F-18 FDG and 11C-choline PET/CT for their
potentials in differentiating brain tumor recurrence from brain
tumor necrosis following radiotherapy. Clin Nucl Med. 36:978–981.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sugahara T, Korogi Y, Tomiguchi S,
Shigematsu Y, Ikushima I, Kira T, Liang L, Ushio Y and Takahashi M:
Posttherapeutic intraaxial brain tumor: The value of
perfusion-sensitive contrast-enhanced MR imaging for
differentiating tumor recurrence from nonneoplastic
contrast-enhancing tissue. AJNR Am J Neuroradiol. 21:901–909.
2000.PubMed/NCBI
|
|
22
|
Warmuth C, Gunther M and Zimmer C:
Quantification of blood flow in brain tumors: Comparison of
arterial spin labeling and dynamic susceptibility-weighted
contrast-enhanced MR imaging. Radiology. 228:523–532. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
White CM, Pope WB, Zaw T, Qiao J, Naeini
KM, Lai A, Nghiemphu PL, Wang JJ, Cloughesy TF and Ellingson BM:
Regional and voxel-wise comparisons of blood flow measurements
between dynamic susceptibility contrast magnetic resonance imaging
(DSC-MRI) and arterial spin labeling (ASL) in brain tumors. J
Neuroimaging. 24:23–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozsunar Y, Mullins ME, Kwong K, Hochberg
FH, Ament C, Schaefer PW, Gonzalez RG and Lev MH: Glioma recurrence
versus radiation necrosis? A pilot comparison of arterial
spin-labeled, dynamic susceptibility contrast enhanced MRI and
FDG-PET imaging. Acad Radiol. 17:282–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choi YJ, Kim HS, Jahng GH, Kim SJ and Suh
DC: Pseudoprogression in patients with glioblastoma: Added value of
arterial spin labeling to dynamic susceptibility contrast perfusion
MR imaging. Acta Radiol. 54:448–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu LS, Baxter LC, Smith KA, Feuerstein BG,
Karis JP, Eschbacher JM, Coons SW, Nakaji P, Yeh RF, Debbins J and
Heiserman JE: Relative cerebral blood volume values to
differentiate high-grade glioma recurrence from posttreatment
radiation effect: Direct correlation between image-guided tissue
histopathology and localized dynamic susceptibility-weighted
contrast-enhanced perfusion MR imaging measurements. AJNR Am J
Neuroradiol. 30:552–558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Larsen VA, Simonsen HJ, Law I, Larsson HB
and Hansen AE: Evaluation of dynamic contrast-enhanced T1-weighted
perfusion MRI in the differentiation of tumor recurrence from
radiation necrosis. Neuroradiology. 55:361–369. 2013. View Article : Google Scholar : PubMed/NCBI
|