Introduction
Isatin is a type of marine active drug exerting
anti-cancer effects, with a cancer-prevention function, and is an
endogenous substance in human bodies, which possesses
pharmacological activities (1–3), such as
nerve protection, antibacterial and antivirus activities. Through
the synthesis of a large number of materials, we aimed to identify
a new drug and conducted relevant investigations (4,5), funded
by the National Major Projects for New Drugs Innovation.
To design an improved drug dose and administration
regimen, based on earlier studies of the lavage and dose of rats,
pharmacokinetic studies were conducted following oral and
intravenous injection of Isatin given to Beagles.
Materials and methods
Drugs and reagents
Isatin was purchased from Shanghai Xin Sheng Yuan
Biological Pharmaceutical Co., Ltd. (Shanghai, China) and served as
a control. Internal standard, quetiapine, was purchased from Maddie
Xipuya Medical Technology Co., Ltd. (Shanghai, China). Methanol of
HPLC grade was purchased from Burdick and Jackson (Morristown, NJ,
USA). Formic acid of HPLC grade and ultrapure water were purchased
Acros Organics (Geel, Belgium).
Instruments and equipment
Liquid chromatography (LC) instrument (Agilent 1200)
and mass spectrometer (6410B) were purchased from Agilent
Technologies, Inc. (Santa Clara, CA, USA), and the electrospray ion
source and tandem quadrupole mass analyzer were purchased from
Zhejiang Haochuang Biotechnology Co. (Hangzhou, China). The data
processing system was MassHunter software (Agilent Technologies,
Inc.). The following instruments were also purchased: Vortex-Genie
2, a vortex generator (Scientific Industries, Inc., Bohemia, NY,
USA); a small desktop high-speed refrigerated centrifuge (5417R;
Eppendorf, Hamburg, Germany); a trace analytical balance [XP26;
Mettler-Toledo Instrument (Shanghai) Co., Ltd., Shanghai, China];
and an ultrapure water machine (Millipore Corp., Billerica, MA,
USA). The chromatographic column used was Venusil XBP PH, 5 µm,
100×2.0 mm.
Experimental animals
Nine male and nine female Beagles, weighing
7.80–9.60 kg were purchased from Beijing Thomas Biotechnology Co.
Ltd. (Beijing, China), license no.: SCXK (Beijing) 2010-0003.
Experimental methods
Solution preparation
A suitable amount of Isatin (10 g) was weighed and
the required concentration was compounded according to the dose and
dose volume. West astragalus gum solution (1.25%) was compounded
for the gavage, as well as 5% DMSO and 40% polyethylene glycol
(both from Haian Petrochemical Co., Nantong, China), and 55%
physiological saline (Shanghai Chemical Reagent Co., Shanghai,
China) was compounded for intravenous injections.
Drug delivery and sample collection
Intravenous drug delivery was carried out in the
Beagles. Briefly, 3 male and 3 female healthy beagles were injected
with 15 mg/kg of Isatin through the saphenous vein at a dosing
volume of 1.5 ml/kg. Blood (1 ml) was taken from the jugular vein
prior to adminstration of test substances (0 h) and after 0.083,
0.25, 0.5, 1, 2, 4, 6, 8 and 24 h, in the K2EDTA tube and kept on
ice. Blood samples were centrifuged at 1,900 × g for 10 min at 4°C.
Plasma was collected and stored at −80°C until analysis.
Intragastric adminstration of Beagles was
subsequently carried out. Briefly, 3 male and 3 female dogs in each
dose group, were fasted for 14–18 h, albeit drinking water was
provided ad libitum prior to drug delivery. The animals were
lavaged with Isatin at doses of 15, 30 and 60 mg/kg, respectively
at a dosing volume of 2 ml/kg. Blood (1 ml) was taken from the
jugular vein prior to adminstration of test substances (0 h) and
after 0.083, 0.25, 0.5, 1, 2, 4, 6, 8 and 24 h in the K2EDTA tube
and kept on ice Blood samples were centrifuged at 1,900 × g for 10
min at 4°C, and plasma was separated and stored at −80°C prior to
analysis.
Measurement methods of plasma samples
The LC-mass spectrometry (MS)/MS method was used to
measure the concentration of Isatin in the plasma at different time
points after drug injection. The required conditions for the
measurement of Isatin in the plasma were similar to those reported
in rats earlier (6), and were used
in blank dog plasma to validate the methodology. The specificity,
sensitivity, linearity, rate of extraction recovery, precision in
the day or between days, stability, and the matrix effect of the
analytical method was confirmed to the relevant provisions of the
biological sample analysis worldwide (7–9).
Data analysis
The pharmacokinetic parameters of Isatin were
analyzed and processed by the atrioventricular model of
WinNonlin5.2 software (Pharsight Corporation, Mountain View, CA,
USA). The experimental data were presented as mean ± standard
deviation).
Results
Blood concentration as well as
concentration and time curve
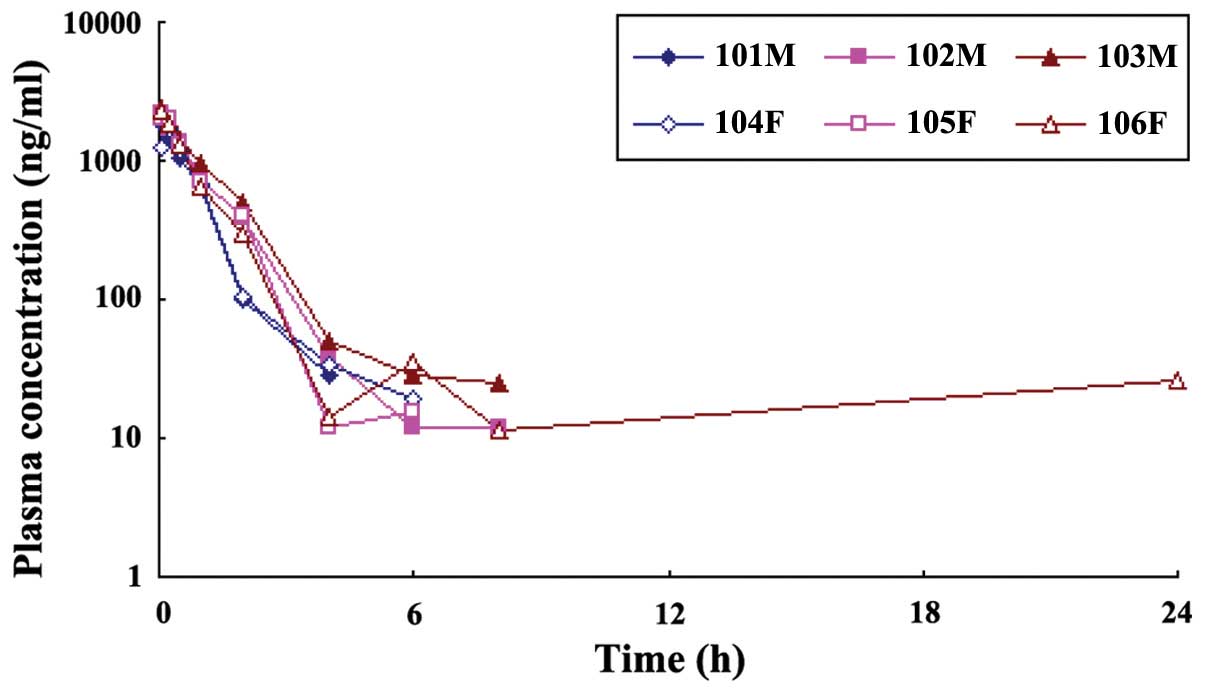
After 15 mg/kg Isatin (n=6) was injected into the
Beagles' vein, the association between blood concentration of
Isatin and time were measured (Fig.
1). The drug concentration in the plasma decreased rapidly
after the intravenous injection of Isatin. After 8 h, the prototype
drugs could not be tested in the plasma and only trace amounts of
drugs were tested in one dog, which was considered an endogenous
drug. Elimination of the drug in the body had no obvious gender
difference. WinNonlin pharmacokinetic software was used to process
the plasma concentration of Isatin using an atrioventricular model
following drug administration in dogs and the pharmacokinetic
parameters after fitting (Fig.
1).
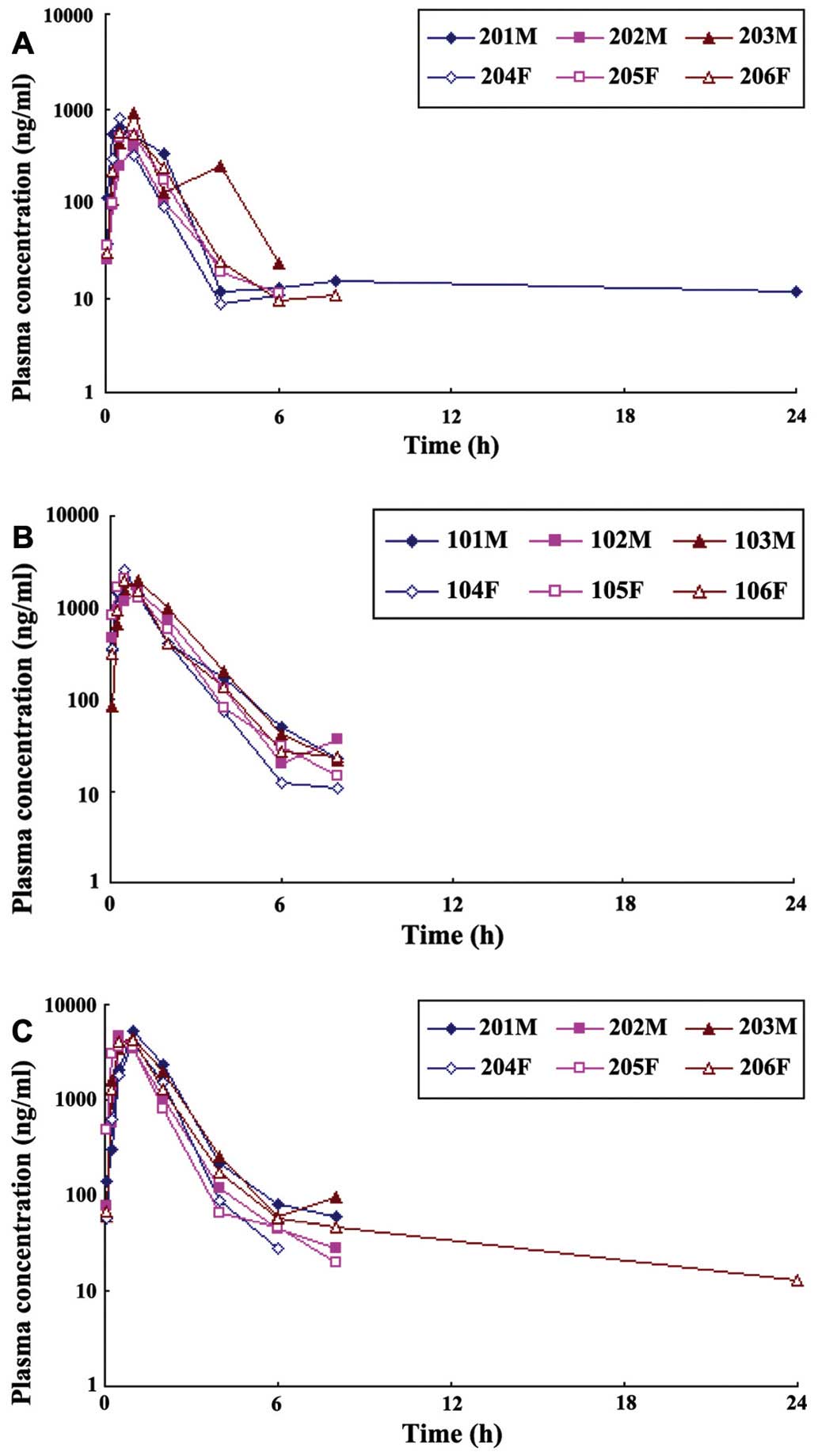
The curve of blood Isatin concentration and time are
shown in Fig. 2 after three
different doses of Isatin were respectively lavaged into the
Beagles. The blood Isatin concentration peaked within 1 h, and then
decreased rapidly. After 8 h, the prototype drugs could not be
tested in the plasma.
Pharmacokinetic parameters
An atrioventricular model was used to calculate the
pharmacokinetic parameters (Table
II). Indole quinone was rapidly absorbed following lavage into
Beagles and peaked in <1 h, while the drug concentration in the
plasma decreased rapidly. After 8 h, the prototype drugs could not
be tested in the plasma. Elimination of the drugs in the body had
no evident gender differences.
 | Table II.The pharmacokinetic parameters after
different dosages of Isatin are lavaged into Beagles. |
Table II.
The pharmacokinetic parameters after
different dosages of Isatin are lavaged into Beagles.
|
|
| Beagles' drug dosage
of lavage (mg/kg) |
|---|
|
|
|
|
|---|
| Pharmacokinetic
parameters | Animals | 15 | 30 | 60 |
|---|
| Tmax,
h | Male |
0.67±0.29 |
0.83±0.29 |
0.83±0.29 |
|
| Female |
0.67±0.29 |
0.50±0.00 |
1.00±0.00 |
|
| Average |
0.67±0.26 |
0.67±0.26 |
0.92±0.20 |
| Cmax,
µg/l | Male |
634±253 |
1,902±357 |
4,812±412 |
|
| Female |
619±152 |
2,213±347 |
3,891±284 |
|
| Average |
631±187 |
2,057±358 |
4,352±596 |
| AUC0-t,
µg*h/l | Male |
1,012±466 |
3,578±553 |
8,071±1464 |
|
| Female |
922±161 |
3,184±128 |
6,748±927 |
|
| Average |
967±316 |
3,381±419 |
7,409±1313 |
| AUC0-∞,
µg*h/l | Male |
1,031±459 |
3,624±541 |
8,164±1509 |
|
| Female |
937±163 |
3,212±128 |
6,793±923 |
|
| Average |
984±313 |
3,418±418 |
7,479±1348 |
| T1/2,
h | Male |
0.71±0.10 |
1.19±0.19 |
1.04±0.09 |
|
| Female |
0.95±0.15 |
1.20±0.36 |
1.70±0.89 |
|
| Average |
0.83±0.18 |
1.19±0.26 |
1.37±0.67 |
| MRT, h | Male |
2.06±1.23 |
1.69±0.07 |
1.58±0.2 |
|
| Female |
1.40±0.23 |
1.37±0.17 |
1.61±0.47 |
|
| Average |
1.73±0.87 |
1.53±0.21 |
1.60±0.32 |
Discussion
As an endogenous component, indole quinone exists
widely in human and animal bodies (2). The current results showed that the
plasma concentration of indole quinone in the majority of the
Beagles was relatively high but extremely low in certain Beagles,
and could not be tested. The plasma of the Beagles was initially
tested, followed by methodology validation and pharmacokinetic
examination to select the Beagles whose blood concentration was
lower than the minimum quantitative limit 1/10. After 8 h of
intravenous injection or intragastric administration, the prototype
indole quinones could not be tested in the plasma of most of the
Beagles, with the exception of some dogs, and this was considered
an endogenous drug.
The clearance of Isatin in dogs was 6.64±1.54
l/h/kg, which is 6.64-fold that of the canine liver plasma flow
(approximately 1.0 l/h/kg) (10). To
calculate the average value of AUC0-∞, the absolute
bioavailability of 15 mg/kg Isatin given to lavage Beagles was
41.77%, which was lower than the bioavailability (57.75%) of rats,
and could therefore be used as oral medicine (6). However, additional experiments are
required to determine whether the drugs were likely to have renal
excretion or liver metabolism.
Acknowledgements
This study was supported by the Natural Science
Foundation of Shandong Province (no. ZR2013HM065) and the Science
and Technology Bureau of Qingdao (no. 11-2-4-2-(4)-jch).
References
|
1
|
Hamaue N, Minami M, Terado M, Hirafuji M,
Endo T, Machida M, Hiroshige T, Ogata A, Tashiro K, Saito H, et al:
Comparative study of the effects of isatin, an endogenous
MAO-inhibitor, and selegiline on bradykinesia and dopamine levels
in a rat model of Parkinson's disease induced by the Japanese
encephalitis virus. Neurotoxicology. 25:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Igosheva N, Lorz C, O'Conner E, Glover V
and Mehmet H: Isatin, an endogenous monoamine oxidase inhibitor,
triggers a dose- and time-dependent switch from apoptosis to
necrosis in human neuroblastoma cells. Neurochem Int. 47:216–224.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Medvedev AE, Clow A, Sandler M and Glover
V: Isatin: a link between natriuretic peptides and monoamines?
Biochem Pharmacol. 52:385–391. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yue W, Wang L, Liu Z, Zhang F and Zhou M:
A preparation method of marine anti-cancer active drugs. Appl.
20041002387 1.1 (In Chinese).
|
|
5
|
Wang Y, Liu Z, Lei W, Zhong W, Yang Z and
Xu M: The application of indole-2,3-diketone in the preparation of
drugs on antivirus or immune-enhancer. Patent ZL10105691. Filed
September 30, 2005; issued. April 19–2006.
|
|
6
|
Zhang Y and Yue W: The pharmacokinetic
study of 2,3-indole quinones in rats. Chin Practic Med. 6:28–31.
2011.
|
|
7
|
Shah VP, Midha KK, Findlay JW, Hill HM,
Hulse JD, McGilveray IJ, McKay G, Miller KJ, Patnaik RN, Powell ML,
et al: Bioanalytical method validation - a revisit with a decade of
progress. Pharm Res. 17:1551–1557. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karnes HT and March C: Precision,
accuracy, and data acceptance criteria in biopharmaceutical
analysis. Pharm Res. 10:1420–1426. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen X and Chen J: Certification of
biological pharmaceutical analysis method. Chin Pharm J.
24:425–426. 1993.
|
|
10
|
Davies B and Morris T: Physiological
parameters in laboratory animals and humans. Pharm Res.
10:1093–1095. 1993. View Article : Google Scholar : PubMed/NCBI
|