Introduction
Radiation damage may occur by exposure to various
types of technology which employ nuclear energy, including devices
used in power generation, geological exploration, industrial
inspection, medical exposure and radiation sterilization (1). The limitations of radiotherapy, in
particular radiation injury to the gastrointestinal tract, have
produced an urgent requirement for novel and effective treatments
for radiation damage (2). Previous
studies have shown that mesenchymal stem cells (MSCs) are able to
promote the repair of intestinal structures and functions,
indicating their potential for the treatment of intestinal
radiation injury (3–5).
Francois et al (6) reported the transplantation of bone
marrow-derived MSCs (BMSCs) into intestinal tissues subjected to
radiation injury. Furthermore, Okamoto et al (7) detected donor-derived epithelial cells
in the intestinal epithelium of BMSC-transplanted recipient rats,
confirming that BMSCs are able to differentiate into intestinal
epithelial cells. Linard et al (3) demonstrated that BMSCs are able to
proliferate in the intestinal tract and promote the repair of the
intestinal tissues damaged by radiation. Another study reported
that, although MSCs have been observed in the gut, the intestinal
transplantation rate was low (8).
MSC-induced repair has been reported in intestinal tract tissues
following radiation-induced damage (9); however, the optimum cell type, dose,
treatment course and the mechanisms underlying MSC-mediated damage
repair remain unclear (10).
In the present study, a rat model of
radiation-induced acute intestinal injury was established using
linear accelerators in order to investigate the ability of BMSCs to
repair radiation-induced acute intestinal damage. In addition, the
potential repair mechanisms involved were preliminarily studied by
monitoring the expression of a number of cytokines, including
interleukin (IL)-2, prostaglandin E2 (PGE2) and stromal
cell-derived factor 1 (SDF-1).
Materials and methods
Isolation and culturing of BMSCs
A total of 40 male Sprague-Dawley (SD) rats (age,
4–6 weeks) were provided by the Shanghai SLAC Laboratory Animal
Co., Ltd. (Shanghai, China), and were sacrificed by neck
dislocation, while anesthetized with 2% pentobarbital sodium
(Sigma-Aldrich, St. Louis, MO, USA). The femur and tibia were
separated under sterile conditions to expose the bone marrow
cavity, which was rinsed with saline. The bone marrow filtrate was
collected and centrifuged at 225 × g for 5 min. The supernatant was
discarded and the cells were resuspended in HyClone low-glucose
(LG)-Dulbecco's modified Eagle's medium (DMEM; GE Healthcare Life
Sciences, Logan, UT, USA) at 1×106 cells per 100 µl. The
cell suspension was gradually added to a rat lymphocyte separation
medium (Sigma-Aldrich) at a ratio of 1:1 and centrifuged at 978 × g
for 20 min. A milky turbid mononuclear cell layer (the separation
between the supernatant liquid) was collected and the cells were
resuspended in LG-DMEM medium without fetal bovine serum (FBS) at
1×106 cells per 100 µl, then centrifuged at 225 × g for
5 min and the pelleted cells were collected. The cells were
resuspended in LG-DMEM complete medium containing 10% FBS in 5%
CO2 saturated humidity at 37°C. The culture medium was
changed every 3 days, and was subcultured at a ratio of 1:3 when
the cell confluence reached 80–90%. This study was conducted in
strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health (1996, 7th ed.). The animal use protocol has been reviewed
and approved by the Institutional Animal Care and Use Committee of
Fuzhou General Hospital (Fuzhou, China). Written informed consent
was obtained from all participants.
Detection of surface antigen molecular
expression
Third passage rat BMSCs showing good growth were
rinsed twice with phosphate-buffered saline (PBS) and digested with
0.25 g/l trypsin containing ethylenediaminetetraacetic acid
(HyClone; GE Healthcare Life Sciences). The cell suspension was
collected and centrifuged at 225 × g for 5 min. The pelleted cells
were resuspended in PBS to achieve 1×106 cell density.
The cells were incubated with phycoerythrin (PE) or fluorescein
isothiocyanate (FITC)-labeled mouse anti-rat CD34 (1:200; 11-0341),
CD45 (1:100; 11-0451), CD29 (1:200; 12-0291) and CD90 (1:200;
17-0900) monoclonal antibodies (eBioscience, Inc., San Diego, CA,
USA) at 37°C in the dark for 30 min and tested using an EPICS XL
flow cytometer (Beckman Coulter, Inc., Brea, CA, USA).
Induced differentiation
Third passage rat BMSCs showing good growth were
seeded in a coverslipped preset 6-well plate with 1×105
cells/well and cultured in a 5% CO2 humidified incubator
at 37°C. When the cell infusion rate reached 90%, the following
osteogenic agents were added: LG-DMEM, 10% FBS (HyClone; GE
Healthcare Life Sciences), 10−7 mol dexamethasone, 10
mmol β-glycerophosphate, 50 µmol/l vitamin C (Sigma-Aldrich), 100
U/ml sodium penicillin and 100 µg/ml streptomycin (pH 7.4; CSPC
Pharmaceutical Group Ltd., Shijiazhuang, China). The medium was
changed every 3 days, and after 3 weeks of incubation Von Kossa
staining was performed to detect calcified nodules. The coverslip
was fixed with 4% paraformaldehyde (Sigma-Aldrich) at 37°C for 30
min after being washed three times with PBS. Then 2%
AgNO3 (Sigma-Aldrich) was added in the dark for 30 min.
After washing three times with distilled water, the coverslip was
placed in UV light for 1 h and stained with hematoxylin
(Sigma-Aldrich). Then the coverslip was detected using a phase
contrast microscope (CKX41; Olympus Corporation, Tokyo, Japan).
Third passage rat BMSCs showing good growth were
seeded in a coverslip preset 6-well plate seeded at
1×105 cells/well and cultured in 5% CO2
humidified incubator at 37°C. When the cell infusion rate reached
90%, the following adipogenic induction agents were added: LG-DMEM,
10% FBS, 10−6 M dexamethasone, 0.5 mmol IBMX solution,
10 µg/ml insulin, 200 µmol 100 U/ml indomethacin, 100 µg/ml chain
ADM (all Sigma-Aldrich) and sodium penicillin (pH 7.4; CSPC
Pharmaceutical Group Ltd.). The medium was changed every 3 days,
and after 9 days of incubation Oil Red O staining (Xiamen Tagene
Biotechnology Co., Ltd., Xiamen, China) was performed.
Preparation of model and cell
therapy
Rats were anesthetized with pentobarbital sodium (40
mg/kg) by intraperitoneal injection. A WDVE-6/100 medical linear
accelerator (Philips Electronics United States Ltd.) was used to
perform X-ray irradiation of the whole abdominal area from the
xiphoid sternum to the pubic symphysis (radiation field, 5×7 cm;
examined area length, 100 cm), including the head, chest and limbs.
The dose rate was 427 cGY/min and the total radiation dose was 12
Gy. A total of 40 male SD rats were randomly divided into two
groups (n=20 per group). The control group was infused with 1 ml
saline via the tail vein immediately after irradiation, while the
BMSC-treated group was infused with 1 ml rat BMSC suspension
(2×106 cells/ml) via the tail vein immediately following
irradiation. The diet of rats (standard rodent chow provided ad
libitum) was monitored, and their body weights were
recorded.
Plasma citrulline content
measurement
Six rats from each group were selected on days 3, 7,
and 14 after irradiation and punctured in their right ventricle for
the collection of anticoagulant, from which fresh plasma was
obtained. The plasma citrulline content was detected using a
citrulline enzyme-linked immunosorbent assay (ELISA) kit
(CSB-E13414r; Cusabio Biotech Co., Ltd., Wuhan, China), according
to the manufacturers instructions.
Hematoxylin and eosin (HE) staining
and radiation injury score
Six rats from each group were selected on days 3, 7
and 14 after irradiation and sacrificed. Then, the ileum tissues
were collected from a 20-cm distance to the ileocecal section,
washed with precooled 0.9% saline and fixed with 10% formalin. The
tissues were paraffin-embedded and sliced into 4-µm sections.
Routine HE staining was performed, and the changes in intestinal
structure were observed by light microscopy (CX21; Olympus
Corporation). The villus height and intestinal gland depth were
measured with VIDS semi-automatic image analyzer (Alenia Marconi
Systems, Rome, Italy). The radiation injury scoring was performed
according to the score standard from related references.
Immunohistochemical analysis
SDF-1 levels were measured using an
immunohistochemical detection kit (SA1055; Wuhan Boster Biological
Technology Ltd., Wuhan, China) according to the manufacturer's
instructions. Dewaxed and hydrated paraffin sections were treated
with 50 µl 3% hydrogen peroxide and incubated at room temperature
for 10 min. The sections were incubated with 50 µl non-immune goat
serum at room temperature for 10 min. Next, the sections were
incubated with 50 µl primary rabbit anti-mouse IL-2, PGE2 or SDF-1
antibody (Wuhan Boster Biological Technology Ltd.) at room
temperature for 60 min. Finally, the sections were incubated with
50 µl secondary goat anti-rabbit horse radish-peroxidase quick
IgG-type polymer antibody at room temperature for 15 min. The
sections were then stained with 3,3′-diaminobenzidine and observed
under a light microscope. Randomly, 10 fields were selected for
counting under the microscope at ×400 magnification, and the number
of positive cells was averaged.
Statistical analysis
SPSS software, version 13.0 (SPSS, Inc., Chicago,
IL, USA) was used for the statistical analysis, the results of the
experimental data was described using the mean ± standard deviation
and independent sample t-test. P<0.01 was considered to indicate
a statistically significant difference.
Results
Morphological observation
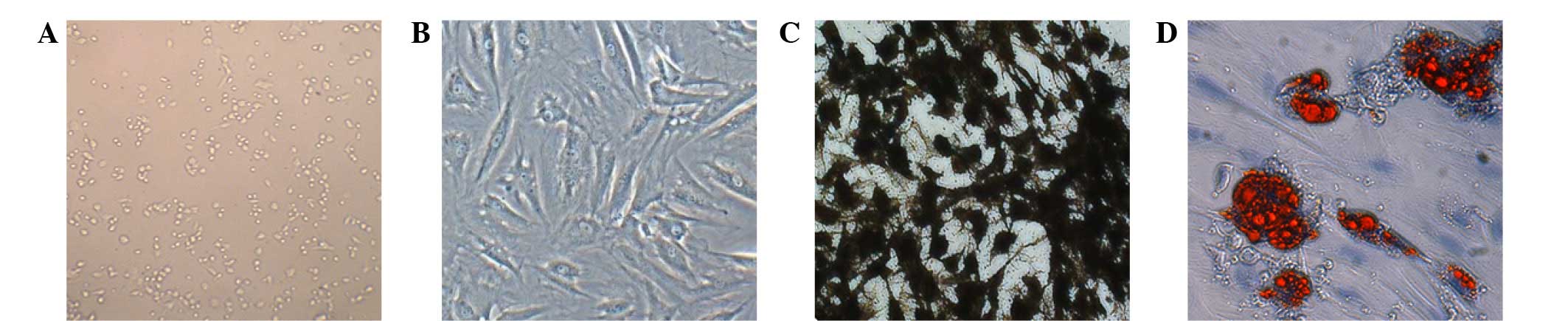
The observation of the primary cells from the
culture flasks revealed spherical, large and small cells, with
portions of cells adherent to the flask after 24 h. The density of
the adherent cells increased after 48–72 h culturing, and these
cells were primarily fusiform, star and round shaped, with various
lengths and uneven pseudopodia thickness. The number of cells
increased after 3–5 days of culturing, and a large number of cell
colonies showing a uniform morphology and long spindle-like
arrangement were observed (Fig. 1A).
Following the third passage, the basic cell forms appeared to be
relatively longer fusiform in shape, and were arranged uniformly
(Fig. 1B).
Induced differentiation
At 5 days after the third passage, treated rat BMSCs
were used as an osteogenic inducer, as a result of which the
cellular structure gradually changed, with the enlargement of the
cytoplasm, change in the shape of the cells from long spindle to
polygonal or irregular, and the development of multiple
pseudopodia. After 2 weeks of induction, ~50% of the cells
transformed into a polygonal spindle, which grew in multiple layers
and formed weak translucent cell nodules, with the initial
characteristics of bone cells; the osteoblasts were slightly
basophilic, with large and spherical nuclei and prominent Golgi
apparatus that appear histologically as a clear zone adjacent to
the nucleus. After 3 weeks, the number of nodules significantly
increased. Von Kossa staining indicated that calcium nodules had
been formed by bone mineralization (Fig.
1C).
At 3 days after third passage, the rat BMSCs were
used as an adipogenic inducer, which resulted in the formation of
small cytoplasmic lipid droplets in an irregular arrangement.
Between days 7 and 14, the number of cells containing lipid
droplets gradually increased, the cellular morphology converted
from the original long spindle to oval or irregular shape, and the
number of fat droplets increased in number and fused gradually.
Staining by Oil Red O revealed red-stained fat cells (Fig. 1D).
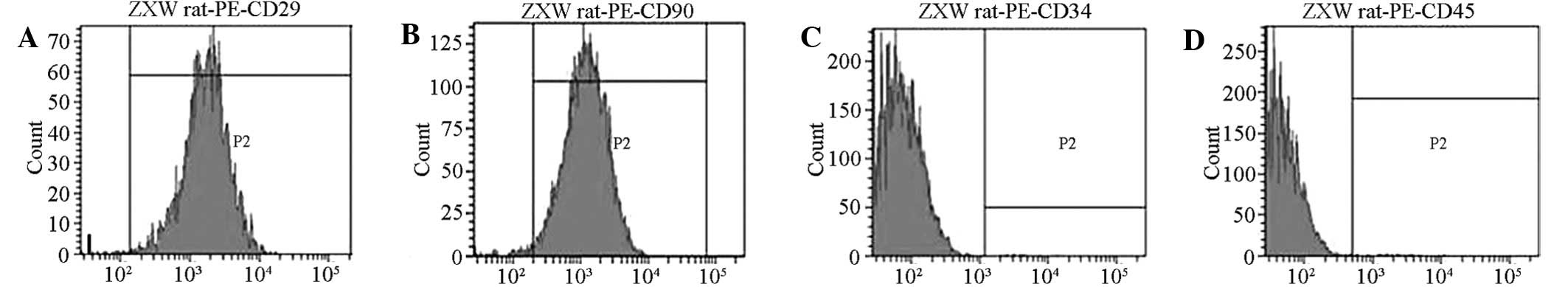
Surface antigen
Flow cytometry analysis detected the positive
expression of the surface antigen CD29 on the cells. By contrast,
CD90 cells of the cultured rat BMSCs did not express the antigens
CD34 and CD45. The proportions of CD29+,
CD90+, CD34+ and CD45+ cells were
99.25, 98.37, 1.12 and 1.03%, respectively (Fig. 2).
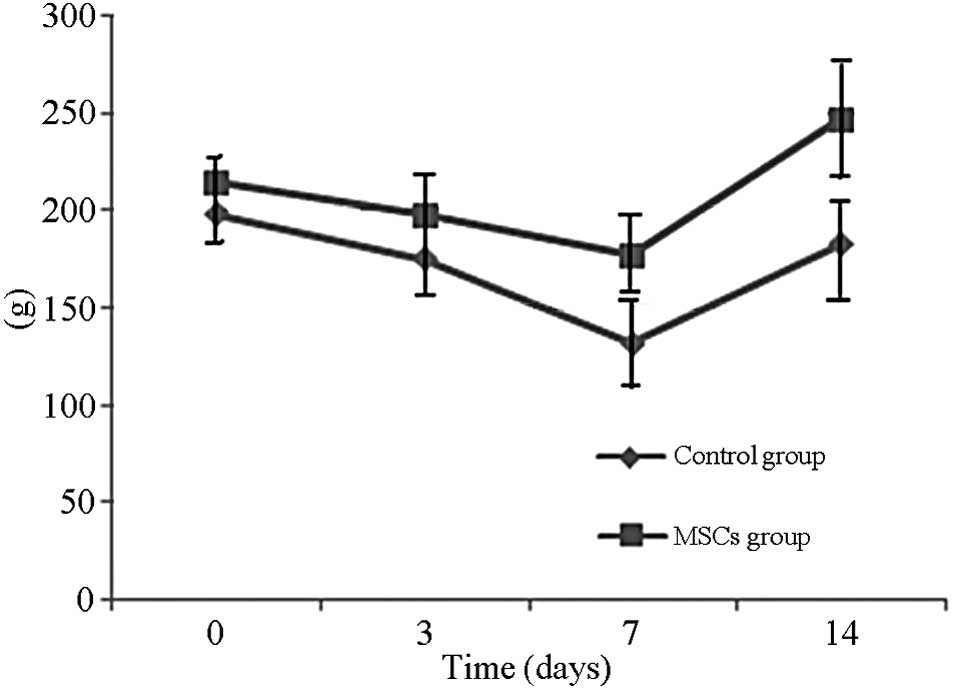
Rat weight and general condition
The body weight of the rats declined and reached the
lowest point at day 7. The body weights of the BMSC-treated rats
normalized by day 14, whereas the weights of the control group rats
were improved, but were not fully normalized to their pre-treatment
weights. A significant differences was noted in body weight between
the two groups (P<0.01) (Fig. 3).
In addition, the rat mental state was poor following irradiation,
with poor response to stimulation and reduced physical activity and
food intake. After 2 days of irradiation, the rats in the control
and BMSC-treated groups excreted thin feces containing mucus-like
substance, indicating diarrhea. Two rats in the control group died
on days 4 and 5 after irradiation and one rat in the BMSC-treated
group died on day 5 after irradiation.
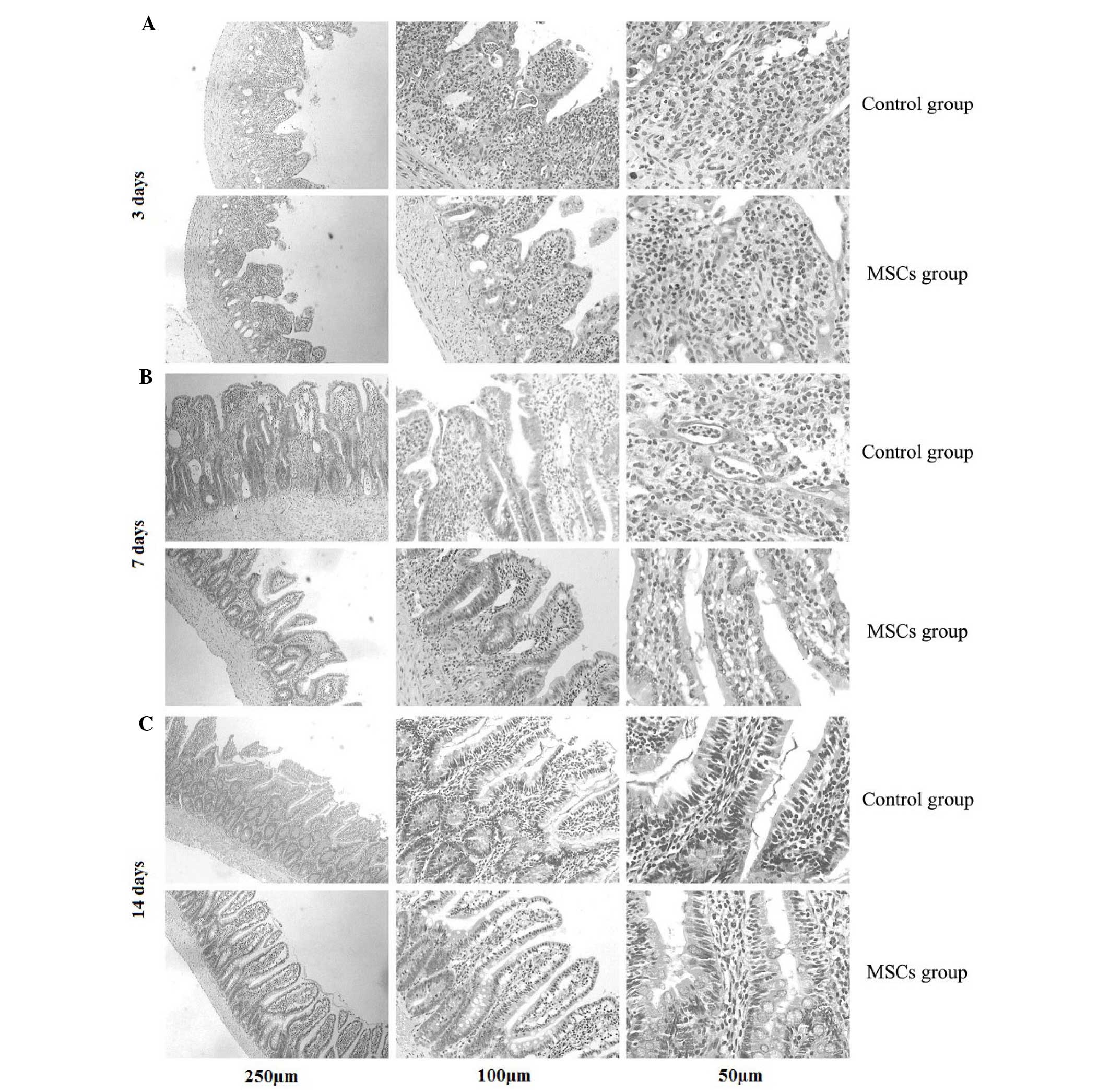
Morphology and radiation injury
score
At day 3 after irradiation, the following
observations were observed (Fig. 4):
Disordered structure of the rat ileum, necrosis of a large number
of epithelial cells to form necrotic villi with infiltration of a
large numbers of inflammatory cells, and a significant reduction in
the numbers of villi and glands. The ileum structure persisted, the
numbers of mucosal epithelial necrosis cells and inflammatory cells
were reduced, and the numbers of villi and glands were larger in
the BMSC-treated group compared with the control group. At day 14
after irradiation, the ileum structure was clearly visible, the
villi showed marked growth and the glands were formed more closely
and were better structurally organized in the BMSC-treated group
compared with the control group (Fig.
4).
The most severe effects of irradiation observed on
day 3 included necrosis of the ileum glands and thinning of the
mucosal layer. The intestinal tissues exhibited repair on day 7
after exposure. Furthermore, the height of the ileum villus and
intestinal gland depth began to increase, whereas the radiation
injury score decreased. The villus height and intestinal gland
depth of the BMSC-treated group were significantly increased
compared with the control group. The radiation injury scores were
significantly reduced compared with the control group (P<0.01).
At day 14, the villus height and intestinal gland depth of the
BMSC-treated group were similar to the normal intestinal tissue,
and no significant improvement was observed on day 7 in comparison
with the control group (Table
I).
 | Table I.Histological detection of ileal
tissues following irradiation (n=6). |
Table I.
Histological detection of ileal
tissues following irradiation (n=6).
| Time after
irradiation | Villus height
(µm) | Intestinal gland
depth (µm) | Radiation injury
score | Plasma Citrulline
(µg/ml) |
|---|
| Day 3 |
|
|
|
|
|
Control |
211.46±11.52 |
112.72±8.96 |
15.50±1.68 |
51.15±4.56 |
|
BMSC-treated |
245.54±12.75a |
148.82±10.12a |
11.97±1.22a |
64.53±6.42a |
| Day 7 |
|
|
|
|
|
Control |
296.30±16.65 |
182.50±12.23 |
10.65±1.37 |
6.41±1.10 |
|
BMSC-treated |
332.13±19.21a |
204.25±13.58a |
5.05±1.15a |
17.12±2.39a |
| Day 14 |
|
|
|
|
|
Control |
339.14±19.21 |
207.92±16.41 |
8.26±1.03 |
72.23±7.08 |
|
BMSC-treated |
386.45±22.36a |
242.67±19.28a |
3.68±0.45a |
91.99±9.87a |
Plasma citrulline content
Following irradiation, the rat plasma citrulline
content decreased significantly, reaching the lowest point on the
day 7 (P<0.01). We speculated that the repair mechanism was
initiated in the intestinal tract, as a result of which the plasma
citrulline content began to increase. The recovery speed of the
plasma citrulline content in the BMSC-treated group was
significantly faster compared with the control group (P<0.01).
The plasma citrulline content in the BMSC-treated group basically
recovered to the near normal levels on day 14 (Table I).
Cytokine expression
IL-2 and PGE2 appeared to serve a crucial function
in the inflammatory reaction. Under microscopic observation, IL-2
and PGE2 were primarily detected in the fibroblasts, inflammatory
cells and intestinal epithelial cells. At day 3 after irradiation,
the express levels of IL-2 and PGE2 in the control group were
significantly increased compared with the BMSC-treated group
(P<0.01).
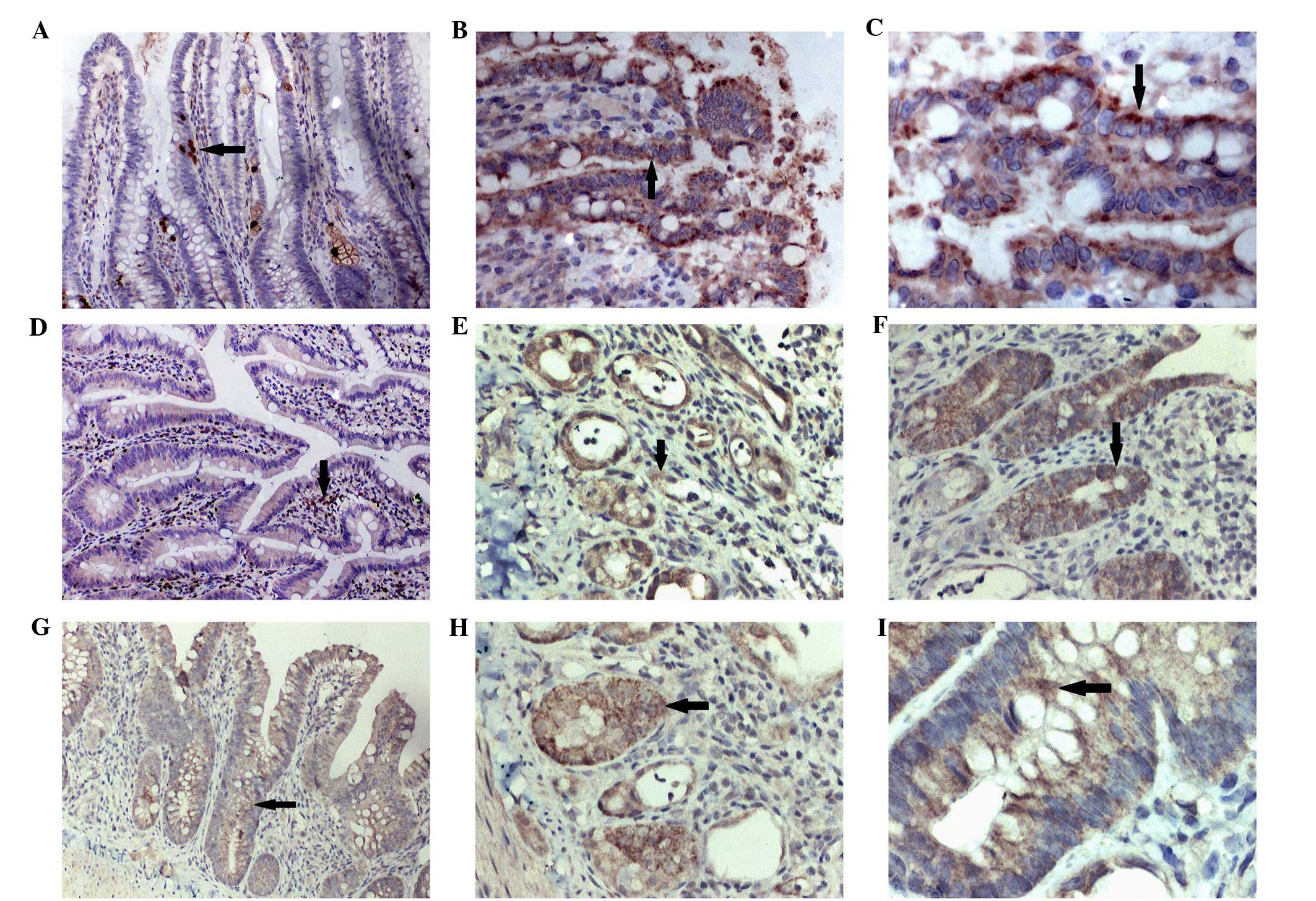
SDF-1 is one of the major chemokines in vivo.
SDF-1 was predominantly expressed in the hair follicles around the
wound margins, newborn glandular cells, fibroblasts and capillary
endothelial cells. Following irradiation, the positive expression
of SDF-1 in the BMSC-treated group appeared significantly increased
compared with the control group (P<0.01) (Fig. 5).
Discussion
Intestinal tissues are highly sensitive to radiation
and are among the most common sites of clinical radiation damage
(11). Radiation-induced acute
intestinal injury has been frequently reported in individuals
exposed to nuclear accidents and radiation therapies for abdominal
tumors, and there are currently no effective treatments for this
damage (2). In the present study, a
rat model of radiation-induced acute intestinal injury was
established, and the results indicated that BMSCs exert a
protective effect in damaged intestinal tissues. BMSCs are able to
promote the structural and functional repair of radiation-induced
acute damage of intestinal tissues. Furthermore, BMSCs may inhibit
inflammation and induce the secretion of cytokines to modify the
local microenvironment in order to promote intestinal tissue
reconstruction.
Chapel et al (12) used labeled MSCs to treat primates
exposed to radiation and found that the labeled cells were
undetectable in the damaged intestinal tissues following a number
of months of transplantation. Linard et al (3) established a porcine model of colorectal
radiation injury and performed multiple intravenous infusions of
autologous BMSCs, which appeared to reduce local inflammatory
cytokine expression and increase the expression of IL-10. The
radiation-induced fibrosis was suppressed by reducing collagen
deposition, transforming growth factor-β expression and regulating
the balance between matrix metalloproteinase and tissue inhibitors
of metalloproteinases. In addition, Chang et al (4) injected human fat source MSCs (hAd-MSCs)
into rats following complete irradiation of the rat stomach. The
hAd-MSCs exerted a therapeutic effect, in addition to
anti-inflammatory and pro-angiogenic effects. Gao et al
(5) found that the intravenous
injection of human umbilical cord-derived MSCs into BALB/C male
mice following abdominal irradiation (10 Gy) improved the survival
rate of rats and reduced the incidence of diarrhea.
In the present study, SD rats were exposed to a
total dose of 12 Gy whole abdominal irradiation using a single
linear medical accelerator. The results showed that the mental
state of the rats was worsened following radiation exposure, with
poor response to stimulation and reduced physical activity and food
intake. Under microscopic observation, obvious mesenteric
congestion, intestinal epithelial cell necrosis, destroyed glands,
the formation of ‘pseudomembranous’-like structure and a large
number of inflammatory cell infiltration were visible. The
intestinal villus height and the intestinal gland depth in the
BMSC-treated cats were significantly increased compared with the
control group after irradiation. The reduction in the intestinal
radiation injury score indicated the protective effects of BMSCs
against radiation injury.
Plasma citrulline is a functional parameter of
intestinal epithelial cells, which indicates the total intestinal
metabolism of the intestinal tract (13). Evaluation of plasma citrulline is
simpler and more easily repeated compared with traditional
detection methods for intestinal function, such as nitrogen balance
(14), fecal fatty acid
determination (15) and the D-xylose
absorption test (16). The results
of the present study showed that the recovery speed of the plasma
citrulline in the BMSC-treated rats was significantly increased
compared with the control group, suggesting that BMSCs are able to
promote the recovery of intestinal function.
The aforementioned results suggest that MSC
transplantation exerts a reparative effect in damaged organs, which
may be achieved via the following mechanisms: MSCs differentiate
into the target organ tissue type through horizontal
differentiation or dedifferentiation, thus serving complementary
and repair functions (17), and in
addition, MSCs secrete various cytokines (18). The inflammatory response has been
shown to be among the primary mechanisms underlying radiation
damage (19). The results of the
present study were consistent with these previous findings, as the
expression levels of the inflammatory factors PGE2 and IL-2 in the
intestinal tissues of the BMSC-treated group were significantly
reduced compared with the control group. This result supported our
hypothesis that MSCs are able to inhibit inflammation and regulate
the local microenvironment in order to promote the repair of
intestinal tissues damaged by radiation. SDF-1 and its receptor
CXCR4 serve a crucial function in the stem cell-homing process
(20). SDF-1 expression in the
intestinal tissue of BMSC-treated tissues has been reported to be
significantly increased (20). We
hypothesized that BMSCs migration to the intestinal tract and their
involvement in the repair of radiation-induced intestinal injury
are closely associated with the SDF-1/CXCR4 axis. This may indicate
the positive feedback mechanism of the BMSCs implanted intestinal
tissues is mediated via the paracrine-stimulated secretion of SDF-1
by intestinal lamina propria stromal cells, which attracts
increasing numbers of MSCs to accelerate the repair of intestinal
tissues.
In summary, the present results suggest that BMSCs
are able to inhibit the local inflammatory response, enhance the
secretion of SDF-1 to promote the movement of BMSCs to
radiation-damaged intestinal tissues and promote the repair of
damaged intestinal structure and function. The application of MSCs
in the treatment of radiation injury remains preliminary. The
precise underlying mechanism of damage repair, and the optimum
treatment dose and route remain unclear. Therefore, further studies
are required to provide novel insights into the treatment of acute
diseases caused by radiation exposure.
Acknowledgements
This study was supported by the Army ‘Twelfth
Five-Year’ Science and Technology Key Project (grant no.
BWS11J004), Nanjing Military Science and Technology Key Project
(grant no. 10z031) and the Fujian Provincial Science and Technology
Innovation Platform Project (grant no. 2010Y2006).
References
|
1
|
Akita S: Treatment of radiation injury.
Adv Wound Care (New Rochelle). 3:1–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shadad AK, Sullivan FJ, Martin JD and Egan
LJ: Gastrointestinal radiation injury: Prevention and treatment.
World J Gastroenterol. 19:199–208. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Linard C, Busson E, Holler V, Strup-Perrot
C, Lacave-Lapalun JV, Lhomme B, Prat M, Devauchelle P, Sabourin JC,
Simon JM, et al: Repeated autologous bone marrow-derived
mesenchymal stem cell injections improve radiation-induced
proctitis in pigs. Stem Cells Transl Med. 2:916–927. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang P, Qu Y, Liu Y, Cui S, Zhu D, Wang H
and Jin X: Multi-therapeutic effects of human adipose-derived
mesenchymal stem cells on radiation-induced intestinal injury. Cell
Death Dis. 4:e6852013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao Z, Zhang Q, Han Y, Cheng X, Lu Y, Fan
L and Wu Z: Mesenchymal stromal cell-conditioned medium prevents
radiation-induced small intestine injury in mice. Cytotherapy.
14:267–273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Francois S, Bensidhoum M, Mouiseddine M,
Mazurier C, Allenet B, Semont A, Frick J, Saché A, Bouchet S,
Thierry D, et al: Local irradiation not only induces homing of
human mesenchymal stem cells at exposed sites but promotes their
widespread engraftment to multiple organs: A study of their
quantitative distribution after irradiation damage. Stem Cells.
24:1020–1029. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okamoto R, Yajima T, Yamazaki M, Kanai T,
Mukai M, Okamoto S, Ikeda Y, Hibi T, Inazawa J and Watanabe M:
Damaged epithelia regenerated by bone marrow derived cells in the
human gastrointestinal tract. Nat Med. 8:1011–1017. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Herzog EL, Chai L and Krause DS:
Plasticity of marrow-derived stem cells. Blood. 102:3483–3493.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sémont A, François S, Mouiseddine M,
François A, Saché A, Frick J, Thierry D and Chapel A: Mesenchymal
stem cells increase self-renewal of small intestinal epithelium and
accelerate structural recovery after radiation injury. Adv Exp Med
Biol. 585:19–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawakatsu M, Urata Y, Goto S, Ono Y and Li
TS: Placental extract protects bone marrow-derived stem/progenitor
cells against radiation injury through anti-inflammatory activity.
J Radiat Res. 54:268–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JS, Yang M, Lee CG, Kim SD, Kim JK and
Yang K: In vitro and in vivo protective effects of granulocyte
colony-stimulating factor against radiation-induced intestinal
injury. Arch Pharm Res. 36:1252–1261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chapel A, Bertho JM, Bensidhoum M,
Fouillard L, Young RG, Frick J, Demarquay C, Cuvelier F, Mathieu E,
Trompier F, et al: Mesenchymal stem cells home to injured tissues
when co-infused with hematopoietic cells to treat a
radiation-induced multi-organ failure syndrome. J Gene Med.
5:1028–1038. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lutgens L and Lambin P: Biomarkers for
radiation-induced small bowel epithelial damage: An emerging role
for plasma Citrulline. World J Gastroenterol. 13:3033–3042.
2007.PubMed/NCBI
|
|
14
|
Boutry C, Matsumoto H, Bos C, Moinard C,
Cynober L, Yin Y, Tomé D and Blachier F: Decreased glutamate,
glutamine and citrulline concentrations in plasma and muscle in
endotoxemia cannot be reversed by glutamate or glutamine
supplementation: A primary intestinal defect? Amino Acids.
43:1485–1498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walton C, Fowler DP, Turner C, Jia W,
Whitehead RN, Griffiths L, Dawson C, Waring RH, Ramsden DB, Cole
JA, et al: Analysis of volatile organic compounds of bacterial
origin in chronic gastrointestinal diseases. Inflamm Bowel Dis.
19:2069–2078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spallek A, Recknagel S, Breuer J, Koeller
G and Schusser GF: Influence of laxatives on gastric emptying in
healthy Warmblood horses evaluated with the D-xylose absorption
test. Berl Munch Tierarztl Wochenschr. 126:245–250. 2013.PubMed/NCBI
|
|
17
|
Das M, Sundell IB and Koka PS: Adult
mesenchymal stem cells and their potency in the cell-based therapy.
J Stem Cells. 8:1–16. 2013.PubMed/NCBI
|
|
18
|
Fernández Vallone VB, Romaniuk MA, Choi H,
Labovsky V, Otaegui J and Chasseing NA: Mesenchymal stem cells and
their use in therapy: What has been achieved? Differentiation.
85:1–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anuranjani and Bala M: Concerted action of
Nrf2-ARE pathway, MRN complex, HMGB1 and inflammatory
cytokines-implication in modification of radiation damage. Redox
Biol. 2:832–846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marquez-Curtis LA and Janowska-Wieczorek
A: Enhancing the migration ability of mesenchymal stromal cells by
targeting the SDF-1/CXCR4 axis. Biomed Res Int. 2013:5610982013.
View Article : Google Scholar : PubMed/NCBI
|