Introduction
Patients with ulcerative colitis (UC) have a
2.4-fold-increased overall colorectal cancer (CRC) risk. Irritable
bowel syndrome (IBD)-related CRC accounts for 1–2% of all CRC cases
in the general population, and CRC accounts for 15% of all
mortality in patients with IBD (1).
It has been generally accepted that the risk of developing CRC is
associated with the extent of inflammation in the colon, as well as
the duration of disease (2).
Phosphoinositide-specific phospholipase C (PLC) represents a
large gene family characterized by the ability to catalyze the
hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into two
vital secondary messengers: Diacylglycerol (DAG) and inositol
1,4,5-trisphosphate (IP3). The family is composed of six isoforms,
β, γ, δ, ε, ζ and η; among these isoforms, PLCε has been reported
to be a key downstream effecter of Ras family small GTPases
(3,4).
The role of PLCε1 in tumorigenesis and
inflammation has recently become a research focus. PLCε1
expression has been found to be positively associated with human
cancer, such as bladder and skin cancer, as well as the severity of
the inflammation. Furthermore, the downregulation of PLCε in
vitro and in vivo can suppress bladder tumor
proliferation (5) In
PLCε-knockout (PLCε−/−) mice, a
substantial resistance to tumor formation and to
12-O-tetradecanoylphorbol-13-acetate-induced skin
inflammation has been observed (6).
Another study, which used an adenomatous polyposis coli mouse
model, has demonstrated that PLCε plays essential roles in
spontaneous intestinal tumorigenesis, with an
angiogenesis-promoting effect, and inflammation (7) Furthermore, PLCε was found to be
necessary for activating cytokine production in skin cells in a
wide spectrum of inflammatory reactions, and this role of
PLCε was further confirmed in PLCε transgenic mice.
PLCε was additionally shown to be required for tumor
necrosis factor-α (TNFα)-induced chemokine (C-C motif) ligand 2
expression in human keratinocytes, due to its involvement in the
nuclear factor κB (NF-κB) pathway. Such functions of PLCε in
inflammation are quite unique among the PLC isozymes.
To further explore the role of PLCε in the
inflammation of UC and its conversion to malignancy, the aim of the
present study was to construct a virus vector expressing short
hairpin RNA (shRNA) targeting PLCε and investigate the
effect of the downregulation of PLCε on the extent of
inflammation and tumorigenesis.
Materials and methods
Equipment and reagents
Glycine and sodium dodecyl sulfate (SDS) were
purchased from Sigma (St. Louis, MO, USA). All restriction enzymes
and ligases were obtained from New England Biolabs (Ipswich, MA,
USA). Plasmid Miniprep kits and transfection kits were purchased
from Tiangen (Beijing, China). All other materials were domestic or
imported analytical reagents. The equipment used in this study
included a high-speed, refrigerated centrifuge (Universal 16R;
Hettich, Tuttlingen, Germany), an ultraviolet spectrophotometer
(UVl601; Shimadzu Corp., Kyoto, Japan), a GeneGenius gel image
analysis system (Synoptics Ltd., Cambridge, UK), a
microelectrophoresis and transfer system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Animal model
Swiss Webster mice (weighing 25–30 g, 8 weeks old)
were obtained from the Beijing Laboratory Animal Research Center
(Beijing, China). The mice were fed a standard diet and treated
with appropriate medicine or sacrificed when in pain or distress.
This animal protocol was approved by the local Ethics Committee
(8). The animal model of colitis was
established by the ad libitum feeding of the mice with 5%
dextran sulfate sodium (DSS; ICN Pharmaceuticals, Inc., Costa Mesa,
CA, USA), as described by Cooper et al (6). Briefly, the mice were exposed to four
cycles of DSS with a basic cycle composed of 7 days of DSS followed
by 14 days of tap water. Animals were sacrificed by an overdose of
sodium phenobarbital at the end of the four cycles, i.e. 84 days.
Once the mice had been sacrificed, the entire bowel was sampled and
the diagnosis of colitis was confirmed by at least two experienced
pathologists.
Cells and vector construction
The viruses were prepared using HEK293 cells derived
from a human embryonic kidney, obtained from the Cell Resource
Center (Institute of Basic Medical Sciences; Beijing, China). We
designed an shRNA targeting the 3′-untranslated region of
PLCε (NM016341), as well as a scramble control with the
restriction sites BamHI and HindIII at the end of the
oligos (PLCε shRNA sequence,
5′-GATCCGCAATACTGTCAGACGAACTGTTCAAGACGACTTCGTCTGACAGTATGTGCTTTTTTGTCGACA-3′;
scramble control shRNA sequence,
5′-GATCCACTACCGTTGTTATAGGTGTTCAAGACGCACCTATAACAACGGTAGTTTTTTGTCGACA-3′).
The plasmid pGenesil-PLCε was subsequently constructed by
inserting the annealed oligo into the corresponding double-digested
vector. Viruses containing PLCε were generated in HEK293
cells. HEK293 cells were transfected with the empty plasmid
(pGenesil-NC) or the target gene expression vector
(pGenesil-PLCε) using Lipofectamine® 2000 (Life
Technologies, Carlsbad, CA, USA) and incubated at 37°C with 5% CO2
for 72 h until the log phase was reached. The cells were then
harvested, total RNA and protein were extracted and the expression
of PLCε was determined using a reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blotting for the
protein. The titer of the standard virus was determined using the
copy number of serially diluted plasmid DNA. Mice were divided into
two groups (n=5 per group), which were treated with either the
pGenesil-PLCε or the negative control (pGenesil-NC).
RT-qPCR
The mRNA levels of PLCε and other candidate genes
were quantified using the TaqMan® Real-Time PCR
Detection kit (Applied Biosystems, Foster City, CA, USA), according
to the manufacturer's instructions. The total RNA of sampled cells
was extracted, and the expression level of target RNA and U6
(correction standard) was quantified via RT-qPCR using an ABI
Prism® 7000 Sequence Detection system (Applied
Biosystems). The ratio of the target RNA to U6 was then calculated.
Primer sequences were synthesized by Takara Biotechnology Co., Ltd.
(Dalian, China). The qPCR reaction was performed according to the
manufacturer's protocol: 50°C for 2 min and 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min
(Applied Biosystems).
Western blot analysis
Fresh tissue samples were ground to powder in liquid
nitrogen and then lysed in sampling buffer [62.5 mmol/l TrisHCl (pH
6.8), 2% SDS, 10% glycerol and 5% 2-mercaptoethanol]. The Bradford
assay (Bio-Rad Laboratories, Inc.) was used to determine the total
protein concentration. Briefly, protein samples were loaded onto
10% SDS polyacrylamide gels, separated by electrophoresis and then
transferred onto polyvinylidene fluoride membranes (GE Healthcare
Life Sciences, Little Chalfont, UK). The membrane was incubated
with rabbit polyclonal againt PLCε (ab121859), K-ras (ab84573),
NF-κB (ab16502), Fas (ab15285), β-actin (ab59381) rabbit monoclonal
Bcl-2 (ab32124) and mouse monoclonal P53 (ab1101) primary
antibodies (all 1:5,000; Abcam, Cambridge, UK) overnight at 4°C.
Next, the membranes were incubated with corresponding horseradish
peroxidase-conjugated goat (ab6721) or mouse (ab6789) anti-rabbit
IgG (1:1,000; Abcam) for 2 h at 37°C according to the
manufacturer's instructions. β-actin (ab59381; Abcam) was used as
the loading control.
ELISA
Samples were analyzed in a blinded manner. The serum
levels of the cytokines IL-1 and TNF-α were determined using
commercially available ELISA kits (Mybiosource LLC, San Diego, CA,
USA), and the assays were performed according to the manufacturer's
instruction.
Statistical analysis
All statistical analyses were carried out using the
SPSS 17.0 software package (SPSS, Inc., Chicago, IL, USA). Analysis
of variance or the Student's t-test was used to analyze the
data from the RT-qPCR, western blotting and ELISA. Each experiment
was performed independently in triplicate, and data are presented
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
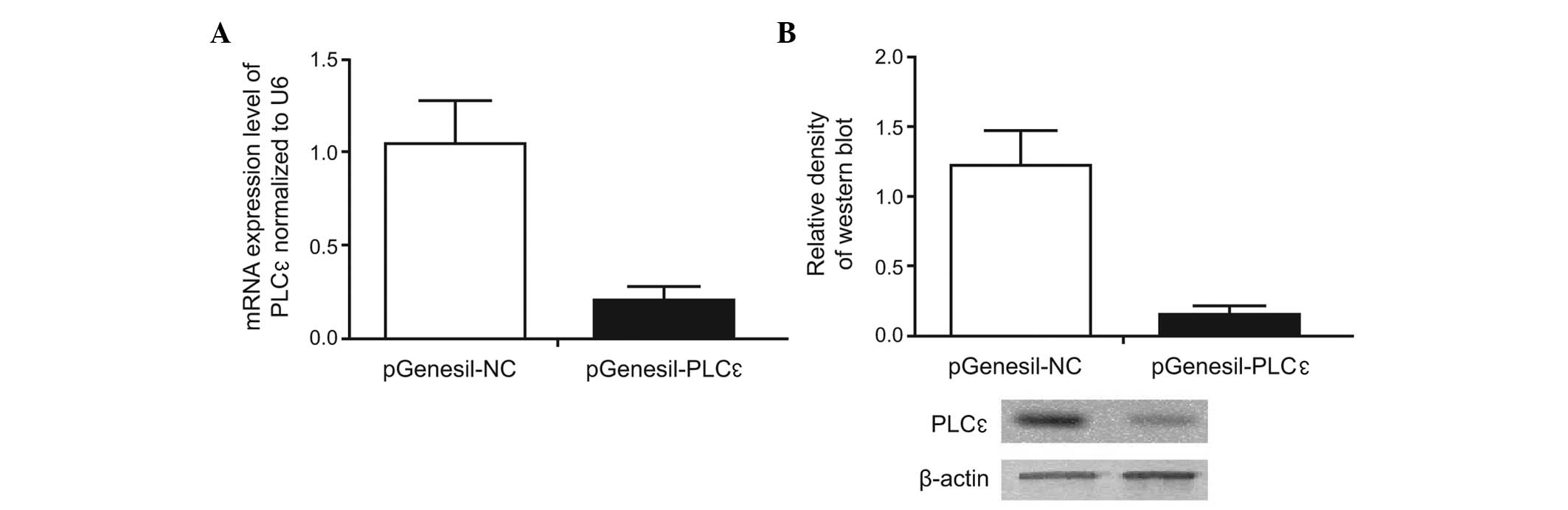
Transfection efficiency of recombinant
expression vector
HEK293 cells were transfected with the empty plasmid
(pGenesil-NC) or the target gene expression vector
(pGenesil-PLCε), and the expression of PLCε was
determined using an RT-qPCR. In addition, western blotting was used
to determine the PLCε protein expression. The results showed
that, compared with the pGenesil-NC group, the mRNA and protein
levels of PLCε were significantly decreased in the
pGenesil-PLCε group (Fig. 1),
suggesting that the recombinant expression vector
pGenesil-PLCε effectively inhibited the expression of
PLCε.
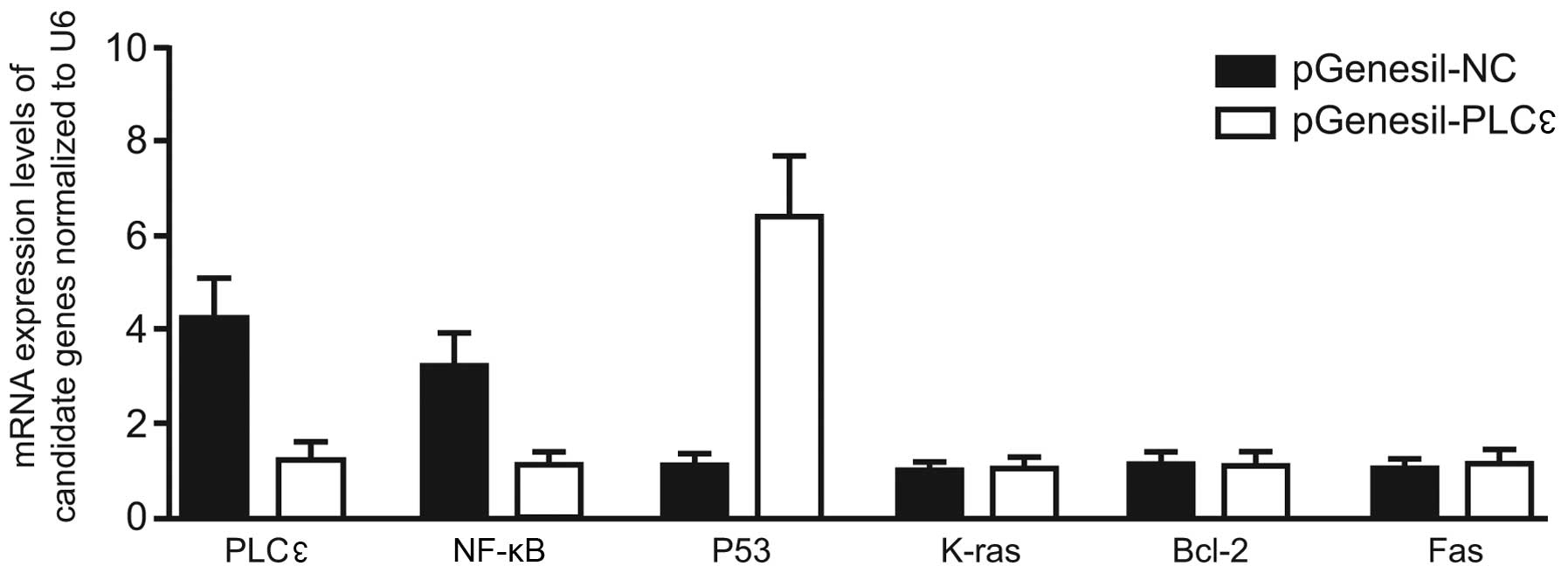
Comparison of the mRNA levels of
PLCε-related genes
The mRNA levels of K-ras, NF-κB, Bcl-2
and P53 were also detected using RT-qPCR, and the results
demonstrated that the introduction of pGenesil-PLCε
significantly reduced the mRNA levels of K-ras,
NF-κB, Fas and Bcl-2, while notably enhancing
the mRNA level of P53 (Fig.
2). This indicated that PLCε RNA silencing could inhibit
the expression of K-ras, NF-κB, Fas and
Bcl-2 and promote the expression of P53.
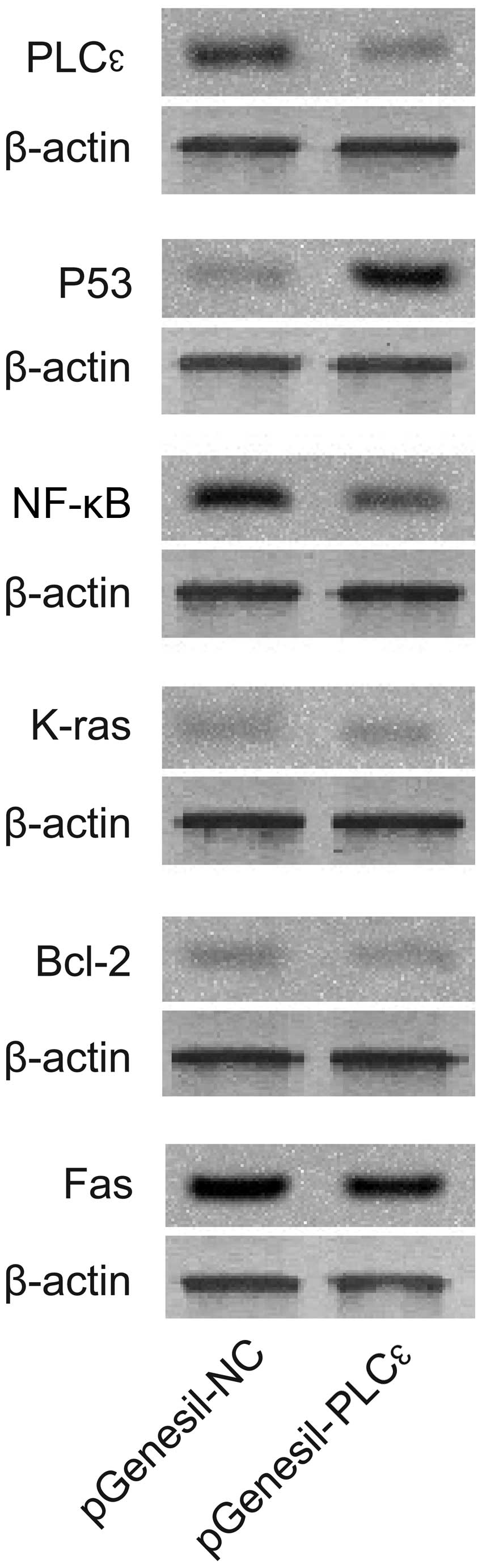
Comparison of protein levels of
PLCε-related genes
Western blot analysis showed that, compared with the
control group, the protein levels of K-ras, NF-κB, Fas and Bcl-2 in
the pGenesil-PLCε-treated group were significantly reduced,
while the level of P53 protein was substantially increased
(Fig. 3). These data suggested that
the downregulation of PLCε expression was associated with the
inhibition of tumor-related proteins and enhanced P53 protein
expression.
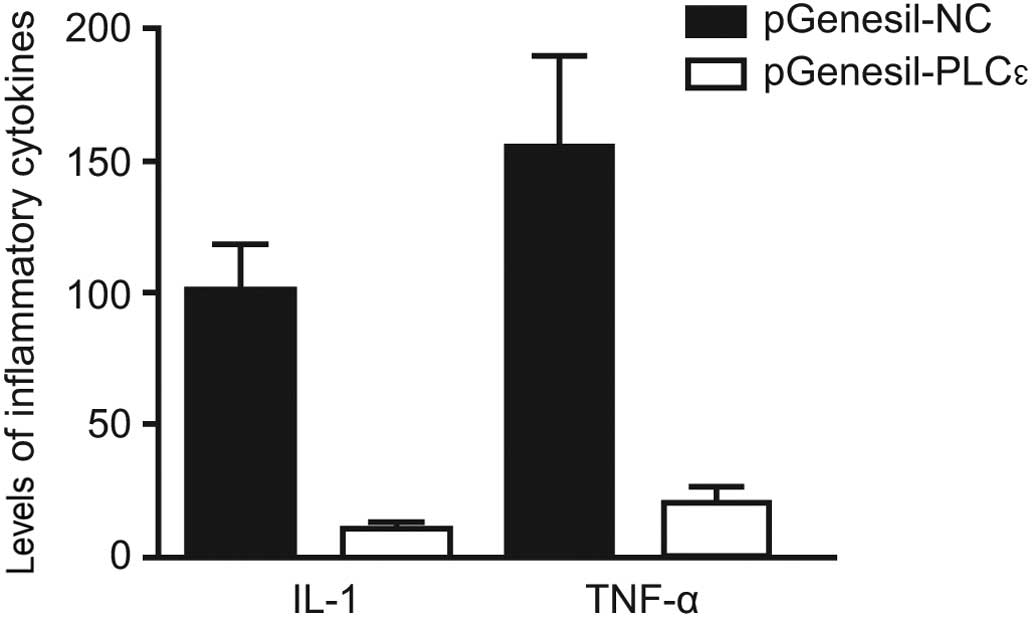
Changes in the levels of serum IL-1
and TNF-α in PLCε gene-silenced mice
The PLCε gene-silenced mouse model was
generated, and changes in the levels of serum IL-1 and TNF-α in the
mice were detected using ELISA. The serum IL-1 and TNF-α levels in
the PLCε gene-silenced mice were significantly reduced compared
with those in the control mice (Fig.
4).
Discussion
UC is a common clinical disease that severely
threatens human health and is associated with an annually
increasing incidence in Germany (9).
In addition to the progress in medical technology, numerous drugs
have been developed and used for the treatment of UC, achieving a
continually improving prognosis. In the absence of early treatment,
however, UC can undergo a transformation into colon cancer
(10). A previous study showed that,
for patients with colon cancer, the 5-year survival rate was 65%,
which increased to 90% for patients in the early stages of the
disease. Patients whose cancers had metastasized had a 5-year
survival rate of <10%; therefore, the early detection of the
cancerous transformation of UC is crucial (11).
It is well known that the cancerous transformation
of UC is associated with hereditary and environmental factors, and
the pathogenic process involves a complex regulatory network
comprising multiple genes, steps and stages, which leads to cell
proliferation disorders, apoptosis inhibition and diffusion through
different signal transduction pathways. Finally, normal cells
undergo a series of carcinogenic changes and metastasis. Overall,
the cancerous transformation of UC is closely associated with the
expression imbalance of multiple genes and proteins (12).
PLC is a key enzyme in the phosphoinositide
signaling pathway and can be activated by a number of molecules,
including hormones, neurotransmitters and growth factors,
catalyzing the hydrolysis of PIP2 into DAG and IP3, which activate
protein kinase C (PKC) and induce intracellular calcium release,
respectively, thereby triggering a downstream cascade reaction
(13). PLCε, an isoform of PLC, is
an effector molecule of Ras protein that is regulated by
GTP-dependent Ras. PLCε can affect inflammation and
tumorigenesis (14,15) and is an important signal transducer.
It has been suggested that the concentration of PLCε is
significantly increased in cancer cells, indicating a link between
PLCε and cancer. In vivo and in vitro studies
have found that the overexpression of PLCε promotes cell
transformation and increases the invasion of cancer cells (16). By contrast, blocking the expression
of PLCε reverses the phenotypic characteristics of malignant
cells and reduces the invasion of cancer cells (17).
In the present study, a PLCε expression
inhibition system was constructed through shRNA technology, and the
effect of PLCε gene silencing on the inflammation and
cancerous transformation of UC was investigated. The data showed
that, compared with the pGenesil-NC group, the mRNA and protein
levels of PLCε were significantly decreased in the
pGenesil-PLCε group, suggesting that the recombinant
expression vector pGenesil-PLCε effectively inhibited the
expression of PLCε. A previous study demonstrated that
certain isoforms of PLC, depending on their phospholipase activity,
could promote cell mitosis by transmitting mitogenic signals
(18). Consistently, it is possible
that PLCε upregulates the PKC signal transduction pathway through
its phospholipase activity, acting as a mitosis promoting factor.
On the other hand, PLCε may inhibit mitosis caused by the abnormal
expression of PLCε and Ras. It has also been suggested that
PLC is associated with the Ras/raf/mitogen-activated protein kinase
kinase/mitogen-activated protein kinase pathway through its
activation of PKC, enhancing cell proliferation via its SH3 domain
(19). The present findings showed
that the mRNA levels of K-ras, NF-κB, Fas and
Bcl-2 were significantly reduced in the presence of
pGenesil-PLCε; however, there was a marked increase in the level of
P53 mRNA, which indicated that PLCε RNA silencing
could inhibit the expression of K-ras, NF-B,
Fas and Bcl-2, while enhancing the expression of
P53. The present data indicated that the suppression of
tumor-related proteins could promote the expression of P53 protein,
effectively preventing the development of cancer.
Previous in vitro studies have found that
PLC-γ1 promotes cell transformation and tumorigenesis, and
the overexpression of PLC-γ1 in mouse fibroblasts induced malignant
transformation. In addition, tumorigenesis has been shown to occur
following the implantation of PLC-γ1 into nude mice
(20). These data indicate that
PLC-γ1 has tumorigenic ability (21). The present results showed that
PLCε RNA silencing effectively suppressed the expression of
IL-1 and TNF-α in vivo, contributing to the anti-tumor and
anti-inflammatory effects; however, its specific mechanisms,
including acting elements and binding proteins, remain to be
further studied.
In conclusion, PLCε RNA silencing can
effectively inhibit the cancerous transformation of UC by
regulating the CRC-related cell proliferation and cell cycle in
vivo. In addition, PLCε RNA silencing can suppress the
expression of inflammatory factors in vitro.
Acknowledgements
This study was funded by the Science Foundation of
the Science and Technology Department of Sichuan Province, China
(grant no. 2012JY0087). The authors would like to acknowledge the
reviewers for their helpful comments on this paper.
References
|
1
|
Barro-Soria R, Stindl J, Muller C,
Foeckler R, Todorov V, Castrop H and Strauß O:
Angiotensin-2-mediated Ca2+ signaling in the retinal pigment
epithelium: Role of angiotensin-receptor-associated-protein and
TRPV2 channel. PLoS One. 7:e496242012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Windischhofer W, Huber E, Rossmann C,
Semlitsch M, Kitz K, Rauh A, Devaney T, Leis HJ and Malle E:
LPA-induced suppression of periostin in human osteosarcoma cells is
mediated by the LPA (1)/Egr-1 axis. Biochimie. 94:1997–2005. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rodríguez RA, Gundy PM, Rijal GK and Gerba
CP: The impact of combined sewage overflows on the viral
contamination of receiving waters. Food Environ Virol. 4:34–40.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Badheka D, Borbiro I and Rohacs T:
Transient receptor potential melastatin 3 is a
phosphoinositide-dependent ion channel. J Gen Physiol. 146:65–77.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sato M, Matsuda Y, Wakai T, Kubota M,
Osawa M, Fujimaki S, Sanpei A, Takamura M, Yamagiwa S and Aoyagi Y:
P21-activated kinase-2 is a critical mediator of transforming
growth factor-β-induced hepatoma cell migration. J Gastroenterol
Hepatol. 28:1047–1055. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cooper KF, Mallory MJ and Strich R:
Oxidative stress-induced destruction of the yeast C-type cyclin
Ume3p requires phosphatidylinositol-specific phospholipase C and
the 26S proteasome. Mol Cell Biol. 19:3338–3348. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barreto RA, Walker FR, Dunkley PR, Day TA
and Smith DW: Fluoxetine prevents development of an early
stress-related molecular signature in the rat infralimbic medial
prefrontal cortex. Implications for depression? BMC Neurosci.
13:1252012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng L, Liang P, Li J, Huang XB, Liu SC,
Zhao HZ, Han KQ and Wang Z: ShRNA-targeted COMMD7 suppresses
hepatocellular carcinoma growth. PLoS One. 7:e454122012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bala K, Bosco R, Gramolelli S, Haas DA,
Kati S, Pietrek M, Hävemeier A, Yakushko Y, Singh VV,
Dittrich-Breiholz O, et al: Kaposi's sarcoma herpesvirus K15
protein contributes to virus-induced angiogenesis by recruiting
PLCγ1 and activating NFAT1-dependent RCAN1 expression. PLoS Pathog.
8:e10029272012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kunii N, Zhao Y, Jiang S, Liu X, Scholler
J, Balagopalan L, Samelson LE, Milone MC and June CH: Enhanced
function of redirected human T cells expressing linker for
activation of T cells that is resistant to ubiquitylation. Hum Gene
Ther. 24:27–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee MH, Hammad SM, Semler AJ, Luttrell LM,
Lopes-Virella MF and Klein RL: HDL3, but not HDL2, stimulates
plasminogen activator inhibitor-1 release from adipocytes: the role
of sphingosine-1-phosphate. J Lipid Res. 51:2619–2628. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brkić L, Riederer M, Graier WF, Malli R
and Frank S: Acyl chain-dependent effect of lysophosphatidylcholine
on cyclooxygenase (COX)-2 expression in endothelial cells.
Atherosclerosis. 224:348–354. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Obba S, Hizir Z, Boyer L, Selimoglu-Buet
D, Pfeifer A, Michel G, Hamouda MA, Gonçalvès D, Cerezo M,
Marchetti S, et al: The PRKAA1/AMPKα1 pathway triggers autophagy
during CSF1- induced human monocyte differentiation and is a
potential target in CMML. Autophagy. 11:1114–1129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim HS, Hwang YC, Koo SH, Park KS, Lee MS,
Kim KW and Lee MK: PPAR-γ activation increases insulin secretion
through the up-regulation of the free fatty acid receptor GPR40 in
pancreatic β-cells. PLoS One. 8:e501282013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Baloucoune GA, Chun L, Zhang W, Xu C,
Huang S, Sun Q, Wang Y, Tu H and Liu J: GABAB receptor subunit GB1
at the cell surface independently activates ERK1/2 through IGF-1R
transactivation. PLoS One. 7:e396982012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsuda K, Fujishima Y, Maeda N, Mori T,
Hirata A, Sekimoto R, Tsushima Y, Masuda S, Yamaoka M, Inoue K, et
al: Endocrinology. 156:934–946. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stavik B, Tinholt M, Sletten M, Skretting
G, Sandset PM and Iversen N: TFPIα and TFPIβ are expressed at the
surface of breast cancer cells and inhibit TF-FVIIa activity. J
Hematol Oncol. 6:52013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kohga K, Takehara T, Tatsumi T, Ishida H,
Miyagi T, Hosui A and Hayashi N: Sorafenib inhibits the shedding of
major histocompatibility complex class I-related chain A on
hepatocellular carcinoma cells by down-regulating a disintegrin and
metalloproteinase 9. Hepatology. 51:1264–1273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gaboardi GC, Ramazzotti G, Bavelloni A,
Piazzi M, Fiume R, Billi AM, Matteucci A, Faenza I and Cocco L: A
role for PKCepsilon during C2C12 myogenic differentiation. Cell
Signal. 22:629–635. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun Q, Weber CR, Sohail A, Bernardo MM,
Toth M, Zhao H, Turner JR and Fridman R: MMP25 (MT6-MMP) is highly
expressed in human colon cancer, promotes tumor growth, and
exhibits unique biochemical properties. J Biol Chem.
282:21998–2010. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spadaro F, Cecchetti S, Purificato C,
Sabbatucci M, Podo F, Ramoni C, Gessani S and Fantuzzi L: Nuclear
phosphoinositide-specific phospholipase C β1 controls cytoplasmic
CCL2 mRNA levels in HIV-1 gp120-stimulated primary human
macrophages. PLoS One. 8:e597052013. View Article : Google Scholar : PubMed/NCBI
|