Introduction
Despite advances in perioperative care, a
significant percentage of elderly patients experience postoperative
cognitive dysfunction (POCD), which is a postoperative disorder of
cognitive functions including memory, attention, speech and
abstract thinking (1). As POCD has
been associated with increased mortality in elderly patients, the
development of potential preventive or therapeutic agents for POCD
is of increasing significance (2).
Volatile anesthetics such as isoflurane may interact
with neurodegenerative disease-associated proteins and induce
processes that may be associated with Alzheimer disease
neuropathology (3). Furthermore,
clinical studies have highlighted the risk factors for POCD,
including age of patients, type of surgery and the type of
anesthesia (4). In addition,
neuroinflammation has been demonstrated to be an important
etiological factor for POCD. Lin et al have suggested that
isoflurane induces neuroinflammation and causes cell injury in the
hippocampus, which may contribute to cognitive impairment in aged
rats (5). Besides, isoflurane can
increase neuronal cell death vulnerability and decrease levels of
acetylcholine in the brain tissues, which contributes to the
development of cognitive dysfunction (6,7).
Therefore, effective prevention of isoflurane-induced
neuroinflammation shows promises for the treatment of POCD.
Tumor necrosis factor (TNF)-α, primarily secreted by
macrophages, is a member of the TNF super-family (8–10). TNF-α
plays an important role in the regulation of a number of biological
processes including cell proliferation, apoptosis, differentiation,
lipid metabolism and coagulation through binding to its receptors
including TNFR1 and TNFBR (8–10).
Furthermore, TNF-α is also involved in a variety of diseases, such
as autoimmune diseases, insulin resistance and cancer (8–10). In
addition, through activation of inflammatory-related signaling
pathways such as nuclear factor-kappa B (NF-κB) and c-jun
N-terminal kinase (c-JNK), TNF-α is able to induce
neuroinflammation, and isoflurane anesthesia significantly
increases the expression levels of TNF-α in the brain tissues
(11,12). Accordingly, TNF-α may be used as a
potential target for the treatment of isoflurane-induced cognitive
dysfunction.
The aim of the present study was to clarify whether
TNF-α signaling is involved in the isoflurane-induced cognitive
impairment in aged rats, and whether TNF-α receptor antagonist can
attenuate isoflurane-induced cognitive impairment in aged rats. A
population of 20-month-old rats were treated with isoflurane to
model cognitive impairment following anesthesia in old
patients.
Materials and methods
Animals and groups
This study was approved by the Ethics Committee of
Wenzhou Medical University (Wenzhou, China). Male 20-month-old
Sprague-Dawley rats (n=50) were purchased from Laboratory Animal
Centre of Life Science Institute (Shanghai, China). All rats were
housed separately under controlled temperature conditions (22±1°C)
with a 12 h light/dark cycle, and allowed free access to standard
rat chow and sterile water. A total of 25 rats were used for the
experiments and separated into 5 groups (n=5 per group). For the
first experiment, the rats were separated into a control group and
an isoflurane group. For the following experiments, 3 groups of
rats were used: A control group (n=5), an isoflurane + saline group
(n=5) and an isoflurane + R-7050 group.
Exposure to anesthetic
Rats were exposed to 1.3% isoflurane (Shanghai Yuyan
Instruments Co., Ltd., Shanghai, China) in a humidified atmosphere
with 30% oxygen carrier gas for 4 h. In the control group, rats
were exposed to humidified 30% oxygen for 4 h. The 1.3%
concentration, which is commonly performed in clinical practice, is
the minimum alveolar concentration of isoflurane used in rats.
Intracisternal administration of TNF-α
receptor antagonist
To determine whether TNF-α signaling is involved in
the isoflurane-induced cognitive impairment in aged rats, rats
received an administration of TNF-α receptor antagonist R-7050 (EMD
Millipore, Billerica, CA, USA) prior to receiving anesthesia.
Briefly, after the dorsal aspect of the skull was shaved and
cleaned, a 27-gauge needle (Rysstech, Shanghai, China) attached via
PE50 tubing (Rysstech) to a 25-µl Hamilton syringe (Rysstech) was
inserted into the cisterna magna. After confirming that the needle
had entered the cisterna magna, 3 µl R-7050 was administered. In
the control group, rats received intracisternal administration of 3
µl sterile physiological saline.
Morris water maze (MWM) assay
Each rat was placed on the platform in the center of
the MWM (Shanghai Xinruan Information Technology Co., Ltd.,
Shanghai, China) for 30 sec, then released into water from an
assigned release point. The rat was allowed to swim for 60 sec or
until it landed on the platform. If the rat did failed to reach the
platform within 60 sec, it was placed on the platform for a further
30 sec. Swimming distance and time to the platform were recorded
using video tracking (Shanghai Xinruan Information Technology Co.,
Ltd.) and analyzed by MWM software (Shanghai Xinruan Information
Technology Co., Ltd.).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol®
reagent (Thermo Fisher Scientific, Inc., Waltham, CA, USA) from the
hippocampus tissue of three rats randomly selected from each group.
The rats were sacrificed by cervical dislocation under anesthesia
with 10% chloral hydrate (0.3 ml/100 g; Dalian Meilun Biotech Co.,
Ltd., Dalian, China). The RNA was then reverse transcribed into a
cDNA template using a PrimeScript™ reverse transcription reagent
kit (cat. no. 6110A; Takara Bio, Inc., Otsu, Japan). DNA polymerase
was contained within the kit. The cDNA was amplified using a SYBR
green qPCR assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
for qPCR analysis using the ABI 7500 PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The reaction mixture
contained 1 µl cDNA, 10 µl SYBR green PCR master mix, 2 µl primer
and 7 µl H2O in a final reaction volume of 20 µl. The
PCR thermal cycling conditions were as follows: 95°C for 5 min, 40
cycles of denaturation at 95°C for 15 sec and annealing/elongation
at 60°C for 30 sec. The reaction was repeated 3 times and a
negative control (no cDNA) and RT control (no RT) were used. The
expression of target mRNA relative to glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) mRNA was calculated using crossing point (Cp)
values and scaled relative to control samples set at a value of 1.
The relative expression was calculated using the 2−ΔΔCq
method (8) The primer sequences used
were as follows: TNF-α forward, 5′-CATGATCCGAGATGTGGAACTGGC-3′ and
reverse, 5′-CTGGCTCAGCCACTCCAGC-3′; interleukin (IL)-1β forward,
5′-CATGATCCGAGATGTGGAACTGGC-3′ and reverse,
5′-CTGGCTCAGCCACTCCAGC-3′; IL-6 forward,
5′-ACTCACCTCTTCAGAACGAATTG-3′ and reverse,
5′-CCATCTTTGGAAGGTTCAGGTTG-3′; IL-8 forward,
5′-TTTTGCCAAGGAGTGCTAAAGA-3′ and reverse,
5′-AACCCTCTGCACCCAGTTTTC-3′.
Western blot analysis
Protein (100 µg) was extracted using a Nuclear and
Cytoplasmic Protein Extraction Kit (Pierce; Thermo Fisher
Scientific, Inc.). Protein concentration was determined using a
Bradford DC protein assay (Bio-Rad Laboratories, Inc.).
Subsequently, proteins were separated in 10% SDS-PAGE (Beyotime
Institute of Biotechnology, Inc., Shanghai, China) and transferred
onto polyvinylidene difluoride (PVDF) membranes (Thermo Fisher
Scientific, Inc.), which was then incubated with phosphate-buffered
saline (PBS) containing 50 g/l skimmed milk at room temperature for
4 h. Next, the PVDF membrane was incubated with mouse anti-rat P65
NF-κB polyclonal antibody (1:100; cat. no. ab13594), mouse anti-rat
p-c-JNK polyclonal antibody (1:100; cat. no. ab192200), rabbit
anti-rat total-c-JNK monoclonal antibody (1:100; cat. no.
ab179461), rabbit anti-rat p-p38 mitogen-activated protein kinase
(MAPK) polyclonal antibody (1:200; cat. no. ab47363), mouse
anti-rat total-p38 MAPK monoclonal antibody (1:200; cat. no.
ab31828), rabbit anti-rat TNF-α polyclonal antibody (1:100; cat.
no. ab6671), rabbit anti-rat IL-1β polyclonal antibody (1:200; cat.
no. ab9722), mouse anti-rat IL-6 monoclonal antibody (1:200; cat.
no. ab9324) and rabbit anti-rat IL-8 monoclonal antibody (1:100;
cat.no. ab7747) (all Abcam, Cambridge, UK), at 37°C for 1 h. After
washing with PBS three times, the PVDF membrane was incubated with
the goat anti-mouse (1:10,000; cat. no. ab97023) or goat
anti-rabbit peroxidase-conjugated secondary antibody (1:10,000;
cat. no. ab6721; both Abcam) at room temperature for 1 h.
Chemiluminescence detection was performed using an ECL Western
Blotting kit (cat. no. 32109; Pierce; Thermo Fisher Scientific,
Inc.).
Statistical analysis
Data was expressed as the mean ± standard deviation.
Differences between two groups were determined using Student's
t-test. Statistical analysis was performed using SPSS software,
version 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Aged rats show cognitive dysfunction
following exposure to isoflurane
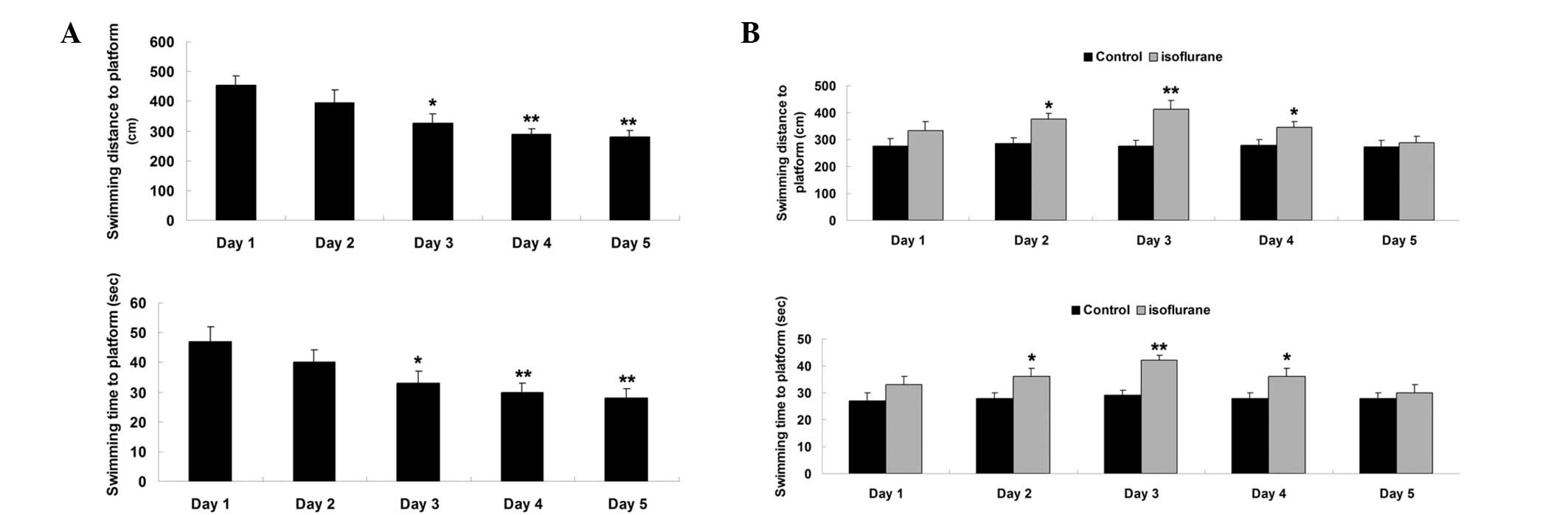
All aged rats were trained in MWM for 5 days prior
to exposure to isoflurane. Swimming distance and time spent in the
MWM were used to evaluate their spatial memory function. The data
showed that swimming distance and time to the platform in MWM were
notably reduced during the 5 days of training (Fig. 1A). These results suggest that the rat
spatial memory is gradually developed.
Then, the MWM assay was performed on days 1–5 after
exposure to isoflurane. As shown in Fig.
1B, the swimming distance and time to the platform were
increased on days 1–3 after exposure to isoflurane when compared
with the control group, suggesting that the spatial memory was
impaired after exposure to isoflurane. From day 4 after exposure to
isoflurane, the swimming distance and time to the platform were
gradually reduced. On day 5 after exposure to isoflurane, the
swimming distance and time to the platform showed no significant
difference when compared with the control group (Fig. 1B). These findings suggest that their
impaired spatial memory was gradually recovered.
TNF-α receptor antagonist attenuates
isoflurane-induced cognitive dysfunction in aged rats
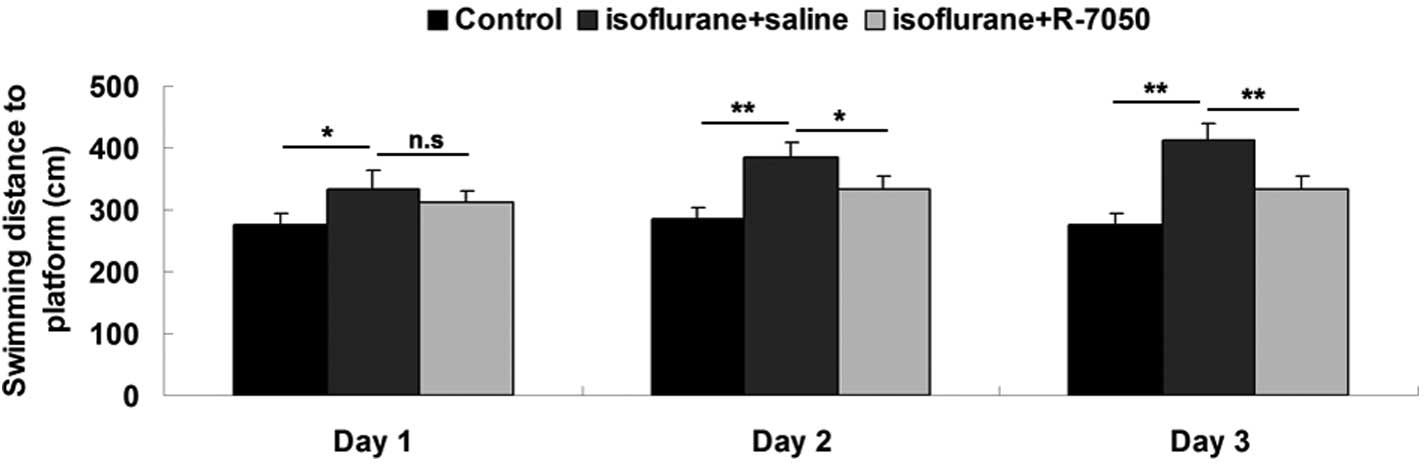
To reveal the role of TNF-α in isoflurane-induced
cognitive dysfunction in aged rats, intracisternal administration
of TNF-α receptor antagonist R-7050 was performed prior to rat
exposure to isoflurane. The swimming distance and time to the
platform on days 1–3 after exposure to isoflurane. Following the
intracisternal administration of R-7050, the swimming distance and
time to the platform were notably reduced, when compared with the
rats in the isoflurane + saline group, although they remained
higher than the control group in which the rats did not receive
isoflurane anesthesia (Fig. 2).
These data show that the intracisternal administration of TNF-α
receptor antagonist R-7050 notably attenuated isoflurane-induced
cognitive dysfunction in aged rats.
TNF-α receptor antagonist suppresses
the isoflurane-induced expression of proinflammatory cytokines in
the hippocampus tissue of aged rats
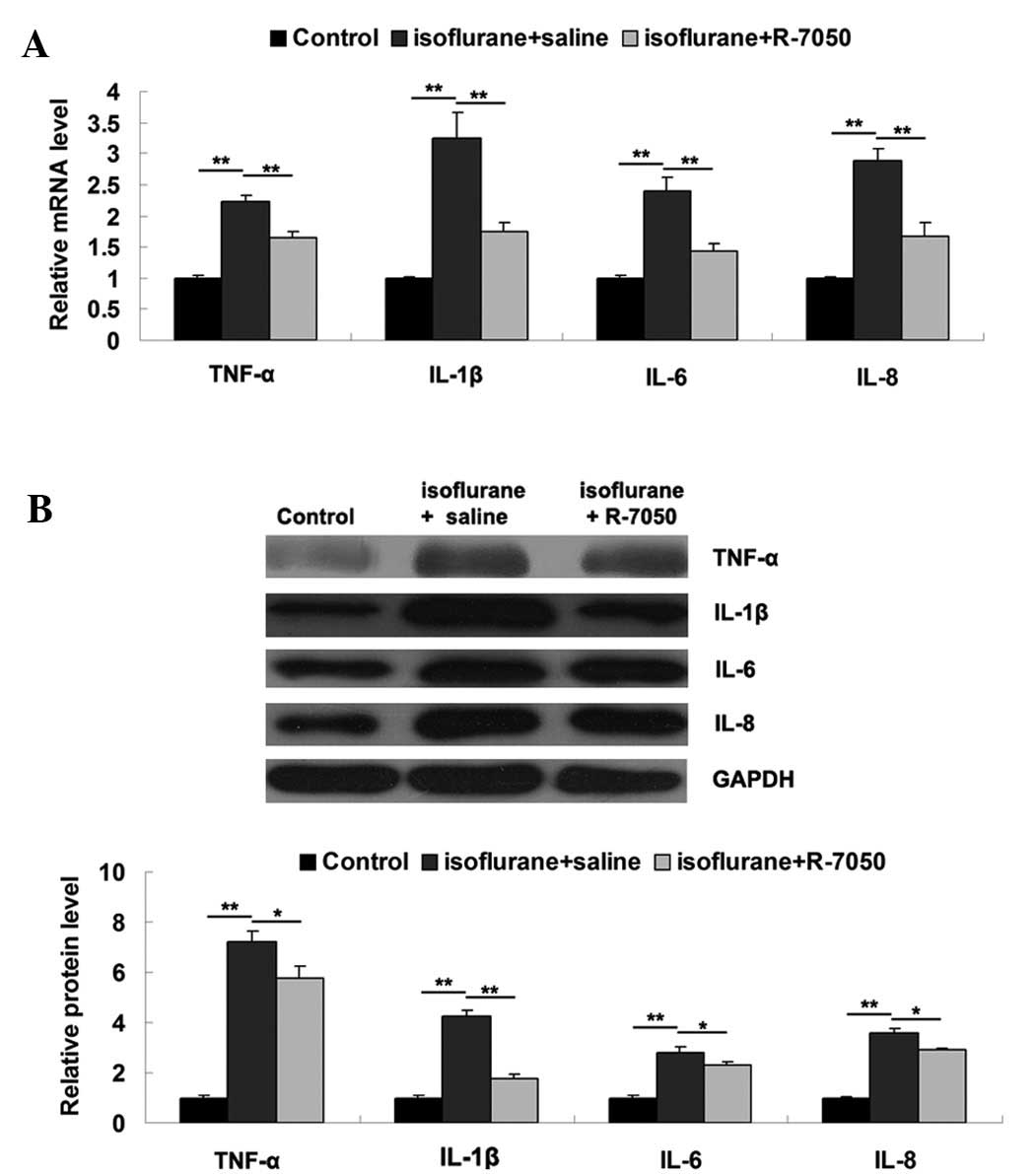
It has been demonstrated that proinflammatory
cytokines, including TNF-α, IL-1β, IL-6 and IL-8, play key roles in
POCD, and their expression levels may be notably upregulated after
surgery and anesthesia (5). To
further reveal the underlying mechanisms, we evaluated the
expression levels of these proinflammatory cytokines in the
hippocampus tissue of aged rats in each group by performing RT-qPCR
and western blot analyses. As shown in Fig. 3A and B, the mRNA and protein levels
of these proinflammatory cytokines were significantly increased on
day 3 after exposure to isoflurane, when compared to the control
group. However, intracisternal administration of R-7050
significantly attenuated the isoflurane-induced upregulation of
these proinflammatory cytokines, suggesting that inhibition of
TNF-α effectively inhibits the isoflurane-induced expression of
proinflammatory cytokines in the hippocampus tissue of aged
rats.
TNF-α receptor antagonist suppresses
isoflurane-induced activation of p38 MAPK signaling in the
hippocampus tissue of aged rats
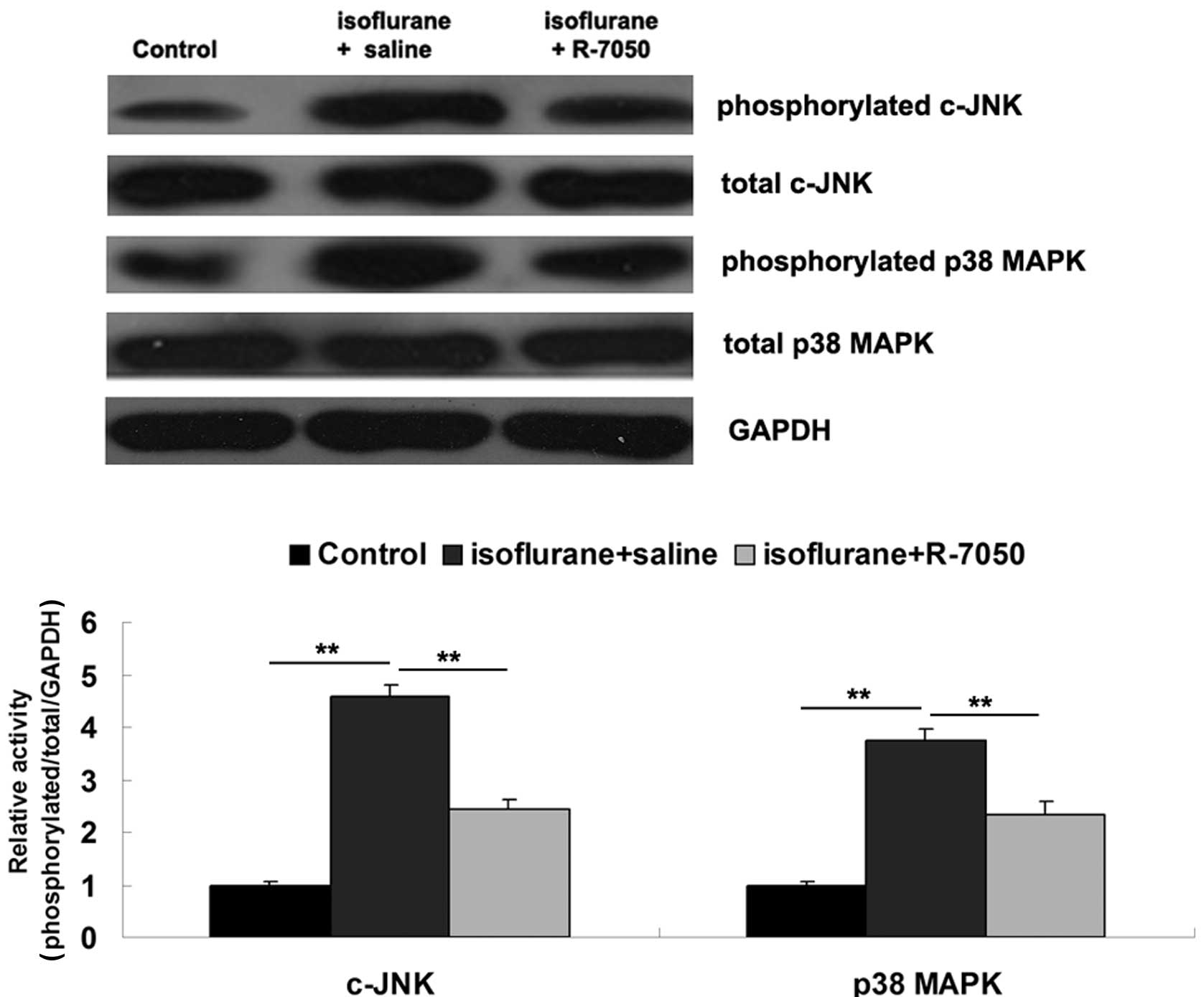
MAPKs signaling pathways have been demonstrated to
be involved in TNF-α-mediated inflammatory responses (9). Therefore, we further determined the
involvement of c-JNK and p38 MAPK signaling pathways in hippocampus
tissue of aged rats on day 3 after exposure to isoflurane. As
demonstrated in Fig. 4, the
phosphorylated protein levels of c-JNK and p38 MAPK were
significantly increased on day 3 after exposure to isoflurane when
compared to the control group, suggesting that the c-JNK and p38
MAPK signaling pathways in hippocampus tissue were activated by
isoflurane. However, intracisternal administration of R-7050
significantly attenuated the isoflurane-induced upregulation of
phosphorylated protein levels of c-JNK and p38 MAPK in hippocampus
tissue, suggesting that inhibition of TNF-α suppressed
isoflurane-induced activation of MAPKs signaling in the hippocampus
tissue of aged rats.
TNF-α receptor antagonist suppresses
the isoflurane-induced activation of NF-κB signaling in the
hippocampus tissue of aged rats
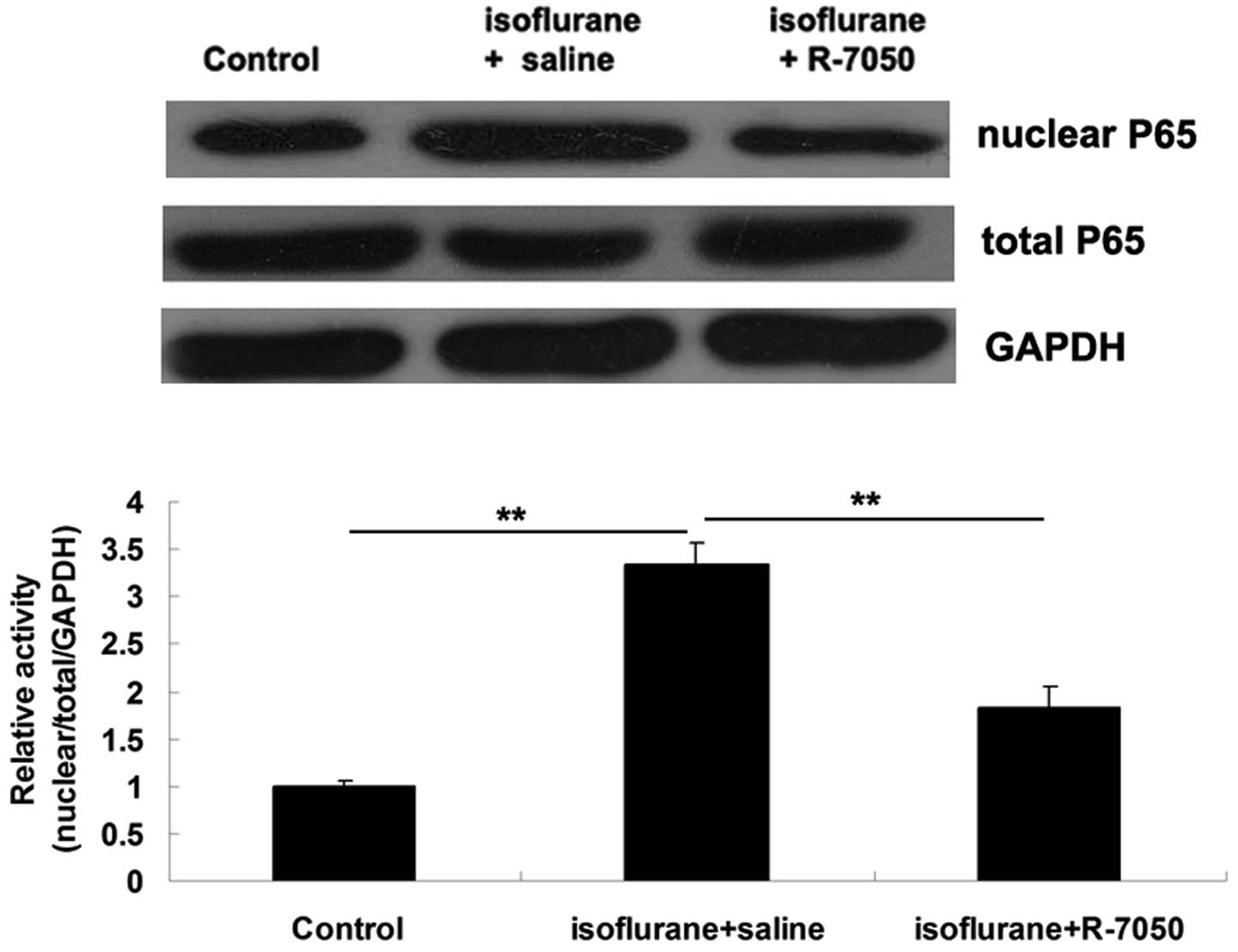
NF-κB is known to function as a downstream signaling
molecule of TNF-α (9). Accordingly,
we further examined the activity of NF-κB signaling in the
hippocampus tissue of aged rats in each group. The protein
expression levels of NF-κB P65 in nucleus was significantly
upregulated on day 3 after exposure to isoflurane in the
hippocampal tissue, when compared with the control group,
indicating that NF-κB signaling was activated (Fig. 5). However, intracisternal
administration of the TNF-α receptor antagonist R-7050
significantly attenuated the isoflurane-induced activation of NF-κB
signaling in the hippocampus tissue of aged rats (Fig. 5).
Discussion
Neuroinflammation is known to play a key role in the
development of POCD as well as other diseases in the central
nervous system. Among the risk factors of POCD, anesthesia has been
well studied recently (13). For
example, Callaway et al showed that desflurane anesthesia
induced memory impairment in rats, which was age- and
dose-dependent (14). Liu et
al investigated the effects of different concentrations and
duration times of isoflurane administration on acute and long-term
neurocognitive function in young mice, and suggested that
isoflurane may cause neurotoxicity by inducing caspase activation
and apoptosis with increased anesthetic concentration and prolonged
duration (15). A single injection
of emulsified isoflurane causes reversible learning and memory
dysfunction in adult rats, and the downregulation of brain-derived
neurotrophic factor expression may be involved in the
isoflurane-induced cognitive impairment (16). Furthermore, in streptozotocin-induced
diabetic rats, isoflurane anesthesia also induces cognitive
dysfunction (11). The present
results suggest that isoflurane caused impaired cognitive function,
as demonstrated by increased swimming distance and time to the
platform in an MWM assay. Yang et al compared the roles of
propofol and isoflurane in the neurodegeneration and cognitive
impairment in neonatal mice, and found that both caused significant
apoptosis in the developing brain, with isoflurane being more
potent (17). In addition,
isoflurane significantly increased the levels of the plasma
neurodegenerative biomarker, S100β (18).
Neuroinflammation has been demonstrated to be a key
factor for the progression of cognitive dysfunction after surgery
and/or anesthesia (19). During the
development of POCD, the expression levels of various
proinflammatory cytokines has been reported to be significantly
increased, including TNF-α, IL-1β, IL-6 and IL-8 (18). For example, Yu et al (18) showed that splenectomy induced a
transient cognitive deficiency in elderly rats, accompanied by
notable upregulation of TNF-α, IL-1β, IL-6 and IL-8 expression in
the in the hippocampal tissues. Moreover, it has been reported that
these proinflammatory cytokines further led to the activation of
microglial cells and caused neuroinflammation responses and brain
injury (20,21). The present data show that exposure to
isoflurane led to increased productions of these proinflammatory
cytokines in the hippocampus tissue of aged rats, which is
consistent with previous studies. For instance, Li et al
found that isoflurane anesthesia increased the hippocampal levels
of IL-1β and TNF-α (22).
Furthermore, Zhang et al found that isoflurane-induced
neuroinflammation by promoting the expression of IL-6 (23).
TNF-α mediates various biological processes by
binding to its receptors (24,25). Two
TNF-α-mediated signaling pathways have been found to be involved in
inflammation, including MAPKs and NF-κB (24,25). As
a member of MAPKs family, c-JNK has been found to be function as a
downstream effecter of TNF-α (24,25).
Kassardjian et al found that c-JNK mediated the effect of
caspases and NF-κB in the TNF-α-induced inhibition of
Na+/K+ ATPase in HepG2 cells (26). Furthermore, TNF-α reduces
Na+/K+ ATPase activity by activating JNK in
LLC-PK1 cells (27). In addition,
the activation of the p38 MAPK is involved in the TNF-α-induced
upregulation of inflammation responses (28). In the present study, the inhibition
of TNF-α suppressed the isoflurane-induced activation of the c-JNK
and p38 MAPK signaling pathways in the hippocampus tissue of aged
rats. In addition, TNF-α has been found to lead to the activation
of NF-κB signaling, which may result in the abundant expression of
cytokines involved in inflammation (29,30). Zhu
et al have found that NF-κB signaling is involved in
bisphenol A-induced TNF-α expression in microglial cells (31). The present findings showed that the
inhibition of TNF-α suppressed the activation of the NF-κB
signaling pathway, accompanied by the downregulation of
proinflammatory cytokines in the hippocampus tissue of aged rats
after exposure to isoflurane, suggesting that the inhibitory effect
of TNF-α antagonist on isoflurane-induced cognitive decline may
occur via the suppression of NF-κB signaling in aged rats.
In conclusion, the present study showed that
exposure to isoflurane led to cognitive decline and
neuroinflammation in aged rats. Further investigation suggested
that inhibition of TNF-α significantly attenuated
isoflurane-induced cognitive dysfunctions, which may occur be via
the mediation of the MAPK and NF-κB signaling pathways. Therefore,
TNF-α may become a potential molecular target for the prevention of
POCD.
References
|
1
|
Bekker AY and Weeks EJ: Cognitive function
after anaesthesia in the elderly. Best Pract Res Clin Anaesthesiol.
17:259–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deiner S and Silverstein JH: Postoperative
delirium and cognitive dysfunction. Br J Anaesth 103 Suppl.
1:i41–46. 2009. View Article : Google Scholar
|
|
3
|
Kalenka A, Gross B, Maurer MH, Thierse HJ
and Feldmann RE Jr: Isoflurane anesthesia elicits protein pattern
changes in rat hippocampus. J Neurosurg Anesthesiol. 22:144–154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Postoperative cognitive dysfunction in
elderly patients. Anesteziol Reanimatol. 13–19. 2012.PubMed/NCBI
|
|
5
|
Lin D and Zuo Z: Isoflurane induces
hippocampal cell injury and cognitive impairments in adult rats.
Neuropharmacology. 61:1354–1359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan H, Xu T, Zhao H, Lee KC, Wang HY and
Zhang Y: Isoflurane increases neuronal cell death vulnerability by
downregulating miR-214. PLoS One. 8:e552762013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Xu Z, Feng C, Wang Y, Jia X, Wu A
and Yue Y: Changes of learning and memory in aged rats after
isoflurane inhalational anaesthesia correlated with hippocampal
acetylcholine level. Ann Fr Anesth Reanim. 31:e61–e66. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cortez M, Carmo LS, Rogero MM, Borelli P
and Fock RA: A high-fat diet increases IL-1, IL-6, and TNF-α
production by increasing NF-κB and attenuating PPAR-γ expression in
bone marrow mesenchymal stem cells. Inflammation. 36:379–386. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin L, Wessely O, Marcusson EG, Ivan C,
Calin GA and Alahari SK: Pro-oncogenic factors miR-23b and miR-27b
are regulated by Her2/Neu, EGF, and TNF-α in breast cancer. Cancer
Res. 73:2884–2896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Riches DW, Chan ED and Winston BW:
TNF-alpha-induced regulation and signalling in macrophages.
Immunobiology. 195:477–490. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang C, Zhu B, Ding J and Wang ZG:
Isoflurane anesthesia aggravates cognitive impairment in
streptozotocin-induced diabetic rats. Int J Clin Exp Med.
7:903–910. 2014.PubMed/NCBI
|
|
12
|
Song HY, Regnier CH, Kirschning CJ,
Goeddel DV and Rothe M: Tumor necrosis factor (TNF)-mediated kinase
cascades: Bifurcation of nuclear factor-kappaB and c-jun N-terminal
kinase (JNK/SAPK) pathways at TNF receptor-associated factor 2.
Proc Natl Acad Sci USA. 94:9792–9796. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao XZ, Ma H, Wang JK, Liu F, Wu BY, Tian
AY, Wang LL and Tan WF: Postoperative cognitive deficits and
neuroinflammation in the hippocampus triggered by surgical trauma
are exacerbated in aged rats. Prog Neuropsychopharmacol Biol
Psychiatry. 34:1426–1432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Callaway JK, Jones NC, Royse AG and Royse
CF: Memory impairment in rats after desflurane anesthesia is age
and dose dependent. J Alzheimers Dis. 44:995–1005. 2014.
|
|
15
|
Liu J, Wang P, Zhang X, Zhang W and Gu G:
Effects of different concentration and duration time of isoflurane
on acute and long-term neurocognitve function of young adult
C57BL/6 mouse. Int J Clin Exp Pathol. 7:5828–5836. 2014.PubMed/NCBI
|
|
16
|
Zhang F, Zhu ZQ, Liu DX, Zhang C, Gong QH
and Zhu YH: Emulsified isoflurane anesthesia decreases
brain-derived neurotrophic factor expression and induces cognitive
dysfunction in adult rats. Exp Ther Med. 8:471–477. 2014.PubMed/NCBI
|
|
17
|
Yang B, Liang G, Khojasteh S, Wu Z, Yang
W, Joseph D and Wei H: Comparison of neurodegeneration and
cognitive impairment in neonatal mice exposed to propofol or
isoflurane. PLoS One. 9:e991712014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu L, Sun L and Chen S: Protective effect
of senegenin on splenectomy-induced postoperative cognitive
dysfunction in elderly rats. Exp Ther Med. 7:821–826.
2014.PubMed/NCBI
|
|
19
|
Johnson T, Monk T, Rasmussen LS,
Abildstrom H, Houx P, Korttila K, Kuipers HM, Hanning CD, Siersma
VD, Kristensen D, et al: Postoperative cognitive dysfunction in
middle-aged patients. Anesthesiology. 96:1351–1357. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong X, Luo M, Huang G, Zhang J, Tong F,
Cheng Y, Cai Q, Dong J, Wu G and Cheng J: Relationship between
irradiation-induced neuro-inflammatory environments and impaired
cognitive function in the developing brain of mice. Int J Radiat
Biol. 91:224–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng KW, Zhang T, Fu H, Liu GX and Wang
XM: Schisandrin B exerts anti-neuroinflammatory activity by
inhibiting the Toll-like receptor 4-dependent MyD88/IKK/NF-kappaB
signaling pathway in lipopolysaccharide-induced microglia. Eur J
Pharmacol. 692:29–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li XM, Zhou MT, Wang XM, Ji MH, Zhou ZQ
and Yang JJ: Resveratrol pretreatment attenuates the
isoflurane-induced cognitive impairment through its
anti-inflammation and -apoptosis actions in aged mice. J Mol
Neurosci. 52:286–293. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Zhang J, Yang L, Dong Y, Zhang Y
and Xie Z: Isoflurane and sevoflurane increase interleukin-6 levels
through the nuclear factor-kappa B pathway in neuroglioma cells. Br
J Anaesth. 110(Suppl 1): i82–i91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen WL, Sheu JR, Hsiao CJ, Hsiao SH,
Chung CL and Hsiao G: Histone deacetylase inhibitor impairs
plasminogen activator inhibitor-1 expression via inhibiting
TNF-α-activated MAPK/AP-1 signaling cascade. Biomed Res Int.
2014:2310122014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kassardjian A and Kreydiyyeh SI: JNK
modulates the effect of caspases and NF-kappaB in the
TNF-alpha-induced down-regulation of Na+/K+ATPase in HepG2 cells. J
Cell Physiol. 216:615–620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kassardjian A and Kreydiyyeh SI: JNK
modulates the effect of caspases and NF-kappaB in the
TNF-alpha-induced down-regulation of Na+/K+ATPase in HepG2 cells. J
Cell Physiol. 216:615–620. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramia N and Kreydiyyeh SI: TNF-alpha
reduces the Na+/K+ ATPase activity in LLC-PK1 cells by activating
caspases and JNK and inhibiting NF-kappaB. Cell Biol Int.
34:607–613. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gudes S, Barkai O, Caspi Y, Katz B, Lev S
and Binshtok AM: The role of slow and persistent TTX-resistant
sodium currents in acute tumor necrosis factor-α-mediated increase
in nociceptors excitability. J Neurophysiol. 113:601–619. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Urbano PC, Soccol VT and Azevedo VF:
Apoptosis and the FLIP and NF-kappa B proteins as pharmacodynamic
criteria for biosimilar TNF-alpha antagonists. Biologics.
8:211–220. 2014.PubMed/NCBI
|
|
30
|
Li W, Wang C, Peng J, Liang J, Jin Y, Liu
Q, Meng Q, Liu K and Sun H: Naringin inhibits TNF-α induced
oxidative stress and inflammatory response in HUVECs via Nox4/NF-κ
B and PI3 K/Akt pathways. Curr Pharm Biotechnol. 15:1173–1182.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu J, Jiang L, Liu Y, Qian W, Liu J, Zhou
J, Gao R, Xiao H and Wang J: MAPK and NF-κB Pathways Are Involved
in Bisphenol A-Induced TNF-α and IL-6 Production in BV2 Microglial
Cells. Inflammation. 38:637–648. 2015. View Article : Google Scholar : PubMed/NCBI
|