Introduction
Hepatocellular carcinoma (HCC) has become a global
health problem, as it is the third most common cause of
cancer-associated mortalities worldwide, with >800,000
mortalities per year (1,2); however, systemic treatment options for
HCC are limited. Surgery remains the most efficacious and
mainstream solution for the eradication of cancer nodules; however,
relapse and distant organ invasion are common following tumor
dissection (3). Anti-tumor agents
have demonstrated strong cytotoxicity against cancer cells and oral
administration of chemotherapeutics is recommended for the
clearance of tumor cells that survive tumor dissection (4). Therefore, choosing appropriate
anti-tumor agents and planning their administration is an
indispensable part of systemic treatment HCC.
Natural products have been widely used in the
development of various anticancer drugs. Paclitaxel, which is
extracted from bark of the Pacific yew (Taxus brevifolia),
has been successfully used to treat breast, lung and ovarian cancer
(5). Chrysin, a natural flavonoid
that is commonly found in honey and bee propolis (Fig. 1), is known for its various biological
activities (6,7). Previous studies have suggested that
chrysin possesses antioxidant (8),
antihypertensive (9),
antidiabetogenic (10) and
anxiolytic properties (11). In
addition, chrysin exerts a strong anticancer effect in multiple
types of human cancer, since it induces cell cycle arrest and cell
apoptosis (12); however, whether
chrysin may be used as a novel antitumor drug against HCC has not
been extensively investigated.
Therefore, the aim of the present study was to
examine the anticancer effect of chrysin on HCC cells, and
determine the underlying mechanism of chrysin-induced apoptosis in
HepG2 and QGY7701 human HCC cells.
Materials and methods
Cell culture
HepG2 and QGY7701 cells were provided by The Cell
Bank of Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in RPMI 1640 supplemented
with 10% heat-inactivated fetal bovine serum plus 100 U/ml
penicillin and 100 µg/ml streptomycin (all Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C, in a humidified
atmosphere of 5% CO2. Chrysin was diluted to various
concentrations with cell culture media. After the cells had reached
60% confluence, various concentrations of chrysin were added (0,
10, 15, 20, 25, 30, 40 and 50 µg/ml).
MTT assay
Cells were seeded in 96-well plates at a density of
5,000–10,000 cells/well. Different concentrations of chrysin (0,
10, 15, 20, 25, 30, 40 and 50 µg/ml; 5 replicates per concentration
group) were added to the cell cultures for 24 h, followed by 20 µl
MTT solution (5 mg/ml; Beyotime Institute of Biotechnology,
Shanghai, China). After incubation for 4 h at 37°C in an atmosphere
of 5% CO2, supernatants were removed and 150 µl dimethyl
sulfoxide (Beyotime Institute of Biotechnology) was added. The
plates were then placed on an orbital shaker for 10 min.
Subsequently, the absorbance at 490 nm was measured using a
spectrophotometer (EnSpire 2300 Multilabel reader; PerkinElmer
Inc., Waltham, MA, USA). The inhibitory rate was calculated
according to the following formula:
(A490control-A490treated)/(A490control-A490blank)
×100%.
Apoptosis assay
Apoptotic cells were detected using an Annexin
V-FITC/PI kit (BioVision Inc., Milpitas, CA, USA). HepG2 and
QGY7701 cells were seeded in 6-well plates at a density of
1×105 cells/well. According to the results of the MTT
assay, the half maximal inhibitory concentration (IC50)
concentration of chrysin was acquired for each cell line using
GraFit-Erithacus IC50 software (Erithacus Software Ltd.,
Horley, UK). Lower (20 µg/ml) and higher dosages (40 µg/ml) were
selected for the cell apoptosis assay. Cells were incubated in
different concentrations of chrysin (0, 20 and 40 µg/ml) for 24 h.
The samples were then washed twice with cold D-Hank's buffer
solution and resuspended in binding buffer (1×106
cells/ml). Subsequently, 100 µl cell supernatants were transferred
to a tube with 5 µl Annexin V-FITC (BD Biosciences, Franklin Lakes,
NJ, USA) and 5 µl PI (Beyotime Institute of Biotechnology).
Following incubation for 15 min at room temperature in the dark,
the apoptotic cells were detected using flow cytometry (FACS Canto
II; BD Biosciences), and analyzed using Modfit and CellQuest 5.1
software (BD Biosciences). Cells located in the Q4 zone were deemed
to be in the early apoptosis stage, whereas cells in the Q2 zone
were in the late apoptosis stage.
Western blot analysis
Drug-induced cell apoptosis is due to mitochondrial
dysfunction and apoptotic signaling pathway activation (13). p53, B-cell lymphoma (Bcl)-2,
Bcl-2-associated death promoter (Bad), Bcl-2-associated X (Bax),
Bcl-2 homologous antagonist/killer (Bak), caspase-9 and caspase-3
are associated with apoptosis-induced mitochondrial damage
(14); therefore the expression
levels of these proteins were investigated. Cultured cells were
collected and total protein was extracted using
radioimmunoprecipitation assay lysis buffer [50 mmol/l Tris (pH
7.4), 150 mmol/l NaCl, 1% Triton X-100, 1% sodium deoxycholate,
0.1% sodium dodecyl sulfate, 1 mM sodium orthovanadate, 1 mM sodium
fluoride, 1 mM EDTA and 40 µg.ml leupeptin], containing 1 mM
phenylmethylsulfonyl fluoride (both Beyotime Institute of
Biotechnology). After laying the extraction on ice for 30 min, cell
lysate was centrifuged for 12,000 × g for 10 min at 4°C and the
supernatants were collected. The total protein concentration in
each supernatant sample was measured using a BCA protein assay kit
(P0012; Beyotime Institute of Biotechnology). Subsequently,
proteins (20 µg/lane) were separated by 8% SDS-PAGE and transferred
to a polyvinylidene difluoride membrane. Following blocking with 5%
non-fat milk, the membrane was incubated with specific primary
antibodies at a dilution of 1:1,000 for 2 h at room temperature.
The following primary antibodies were used: p53 (2527), Bcl-2
(2870), Bax (5023), Bak (6947), Bad (9239), caspase-3 (9665),
caspase-9 (9505) and GAPDH (2118; all Cell Signaling Technology,
Beverly, CA, USA). The membrane was then washed three times in
Tris-buffered saline-Tween 20 (TBST) for 5 min, and incubated with
a goat anti-rabbit horseradish peroxidase-labeled secondary
antibody (E030120-01; EarthOx Life Sciences, Millbrae, CA, USA) at
a dilution of 1:5,000 for 1 h at room temperature. Following three
washes in TBST (10 min each), the membranes were exposed to an
enhanced chemiluminescence buffer (EMD Millipore, Billerica, MA,
USA) and measured on an Sally Sue western blot imaging system
(ProteinSimple, San Jose, CA, USA). Gel images were then analyzed
using ImageJ (National Institutes of Health, Bethesda, MA, USA) to
calculate the gray value of each band.
Statistical analysis
Experiments were repeated ≥3 times and all data were
analyzed using Graphpad Prism 5.0 software. Between-group
differences were analyzed using Student's t-test. Data are
presented as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference
(*P<0.05; **P<0.01; ***P<0.001).
Results
Chrysin inhibits the viability of HCC
cells
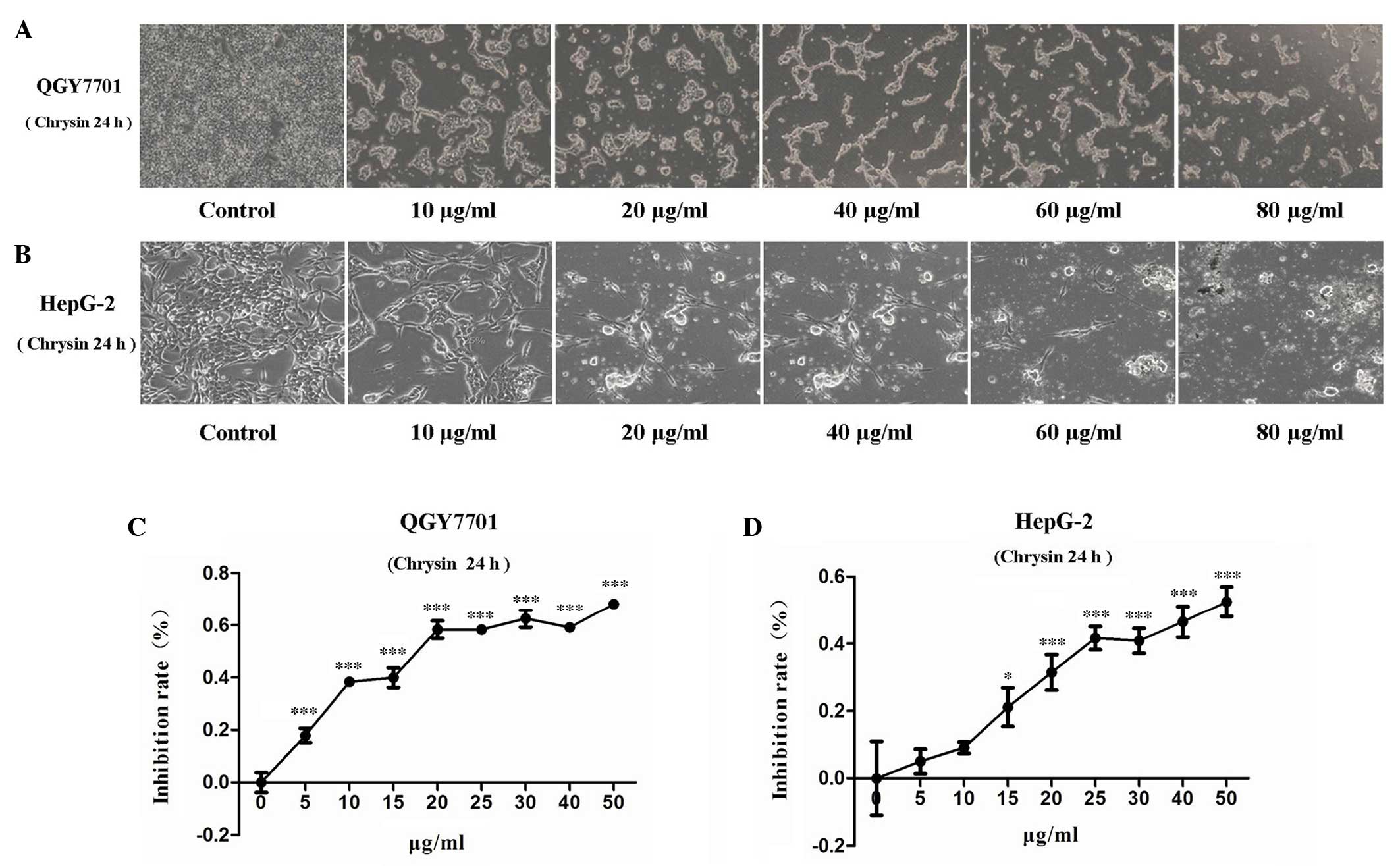
Following treatment of the QGY7701 and HepG2 cells
with chrysin, the cells were found to slow growing, distorted,
round in shape and detached from the bottom of the plate.
Furthermore, the numbers of detached cells increased with
increasing drug concentration (Figs. 2A
and B). MTT assay was used to evaluate the viability of QGY7701
and HepG2 cells treated with chrysin. The present results revealed
that, after 24 h of treatment, chrysin significantly inhibited cell
viability in the HCC cell lines in a dose-dependent manner
(P<0.001; Figs. 2C and D).
IC50 is an index for the evaluation of drug sensitivity.
The IC50 values of chrysin in QGY7701 and HepG2 cells
were measured using GraFit-Erithacus IC50 software and
were found to be 18 and 25 µg/ml, respectively.
Chrysin promotes HCC cell
apoptosis
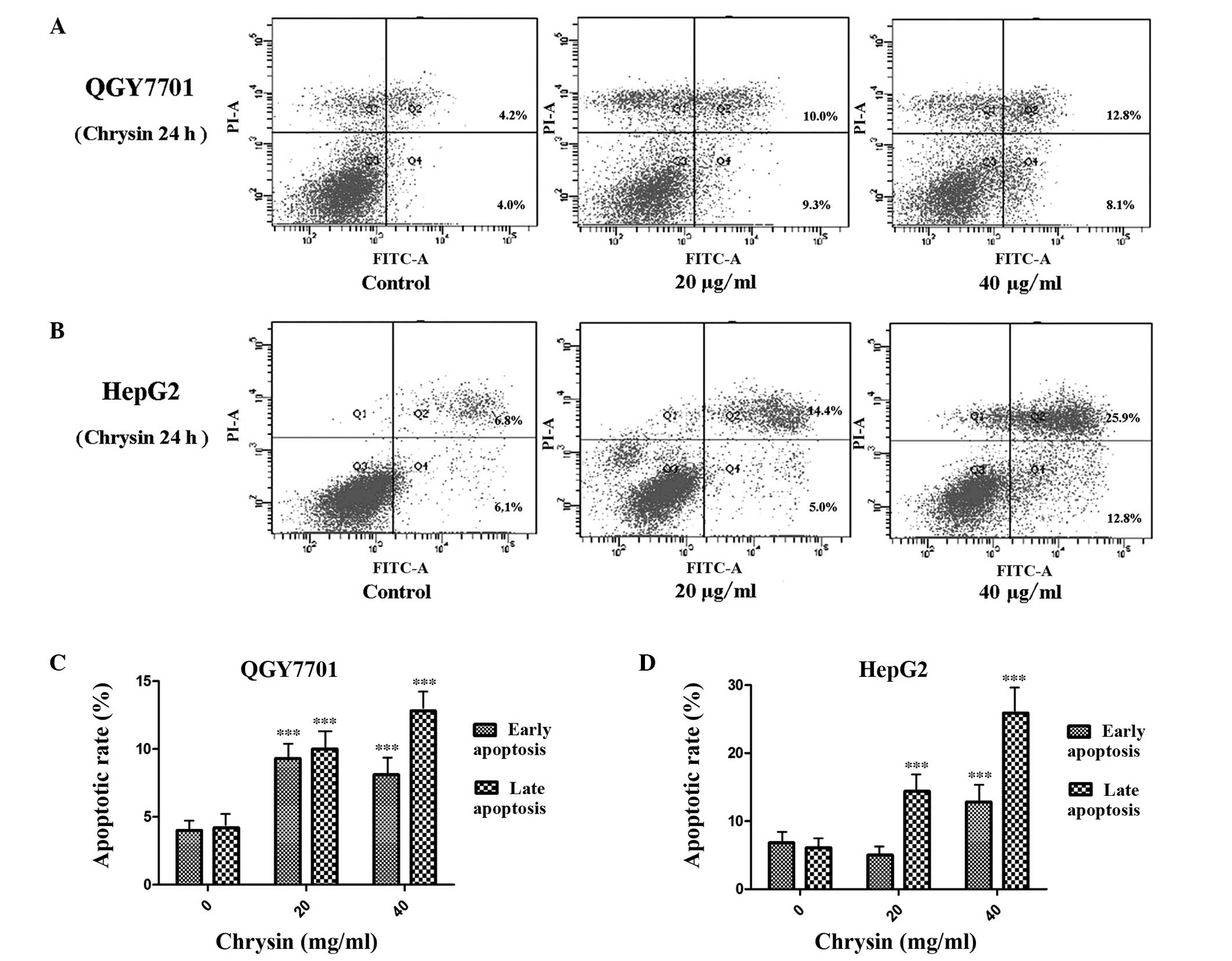
The apoptosis of the QGY7701 and HepG2 cells was
detected using Annexin V/PI double staining and flow cytometric
analysis. The results indicated that chrysin induced HCC cell
apoptosis. Chrysin induced-apoptosis in HepG2 and QGY7701 cells was
found to significantly increase in a concentration-dependent manner
(P<0.001; Fig. 3). QGY7701 cells
were found to be more sensitive to chrysin treatment, with higher
levels of apoptosis observed compared with the HepG2 cells.
p53/Bcl-2/caspase-9 signaling pathway
activation following treatment with chrysin in HCC cells
HCC cells were treated with various concentrations
of chrysin for 24 h. Apoptosis protein expression levels of cleaved
caspase-3 and caspase-9 were found to significantly increase in a
concentration-dependent manner following chrysin treatment in the
QGY7701 and HepG2 cell lines (P<0.001; Fig. 4). These results indicated that the
caspase-9/caspase-3-associated apoptosis pathway was activated. In
addition, the possible activation of the p53/Bcl-2 signaling
pathway was also investigated based on the protein expression
levels of p53, Bcl-2, Bax, Bad and Bak. The results demonstrated
that this intrinsic apoptosis pathway was also activated following
chrysin treatment in the two HCC cell lines (P<0.001; Fig. 4).
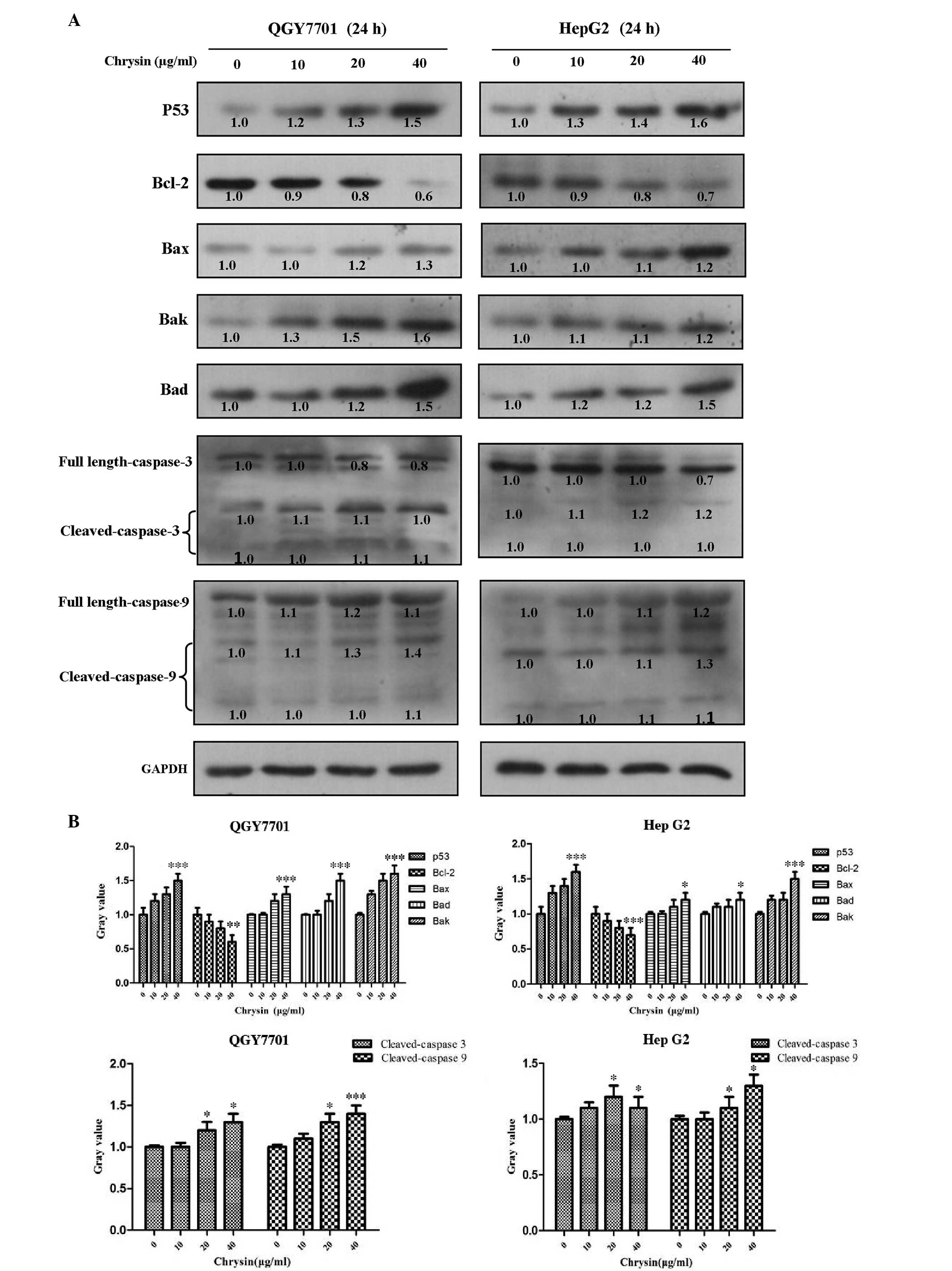 | Figure 4.Chrysin regulates the
p53/Bcl-2/caspase-9 signaling pathway. Chrysin regulates the
content of apoptotic-related proteins in HepG2 and QGY7701 cells
following various concentrations of chrysin treatment for 24 h. (A)
Western blotting was performed in order to analyze the protein
content of p53, Bcl-2, Bax, Bak, Bad, caspases-3 and −9, and
cleaved-caspases-3 and −9 (B) and this data was subsequently
quantified. *P<0.05, **P<0.01 and ***P<0.001 vs. 0 µg/ml
chrysin. Bcl-2, B-cell lymphoma-2; Bax, Bcl-2-associated X; Bad,
Bcl-2-associated death promoter; Bak, Bcl-2 homologous
antagonist/killer. |
Discussion
HCC is a highly malignant tumor that affects
individuals worldwide. Efficacious chemotherapeutic is critical for
the eradication of tumors that cannot be completely removed by
surgery. Although there are several therapeutic agents for the
management of HCC, drug resistance and adverse effects remain
pivotal issues (3). Natural products
are a large source of novel therapeutic agents and natural products
have been widely investigated as anti-cancer drugs, however the
exact mechanism of their anti-cancer action requires further
elucidation. In the present study, chrysin was demonstrated to
significantly inhibit the viability of two HCC cell lines,
suggesting that chrysin may comprise a novel candidate agent for
the treatment of HCC.
Cell apoptosis is a complicated biological process
that is associated with complex signaling pathway responses. The
activation of cysteine proteases, in particular caspases, is a key
intracellular regulator of cell apoptosis (15,16).
Caspase-3 is an important mediator of apoptosis (17) that is activated by a variety of
activators classified into two main signaling pathways: The death
receptor-mediated pathway, involving caspase-8 and caspase-10, and
the mitochondria-mediated pathway, involving caspase-9 (18,19).
Caspase-3 is an executioner caspase that is activated by death
ligands and mitochondrial dysfunction-induced cell apoptosis
(20). In the present study, chrysin
treatment was not found to have a significant effect on the
expression of caspase-8, but promoted the accumulation of cleaved
caspase-9, suggesting that chrysin selectively induces apoptosis in
HCC cells via the mitochondria-mediated apoptosis pathway. Due to
the role of caspase-3 as an executioner of cell apoptosis,
caspase-3 expression levels were also detected to confirm cell
apoptosis. The results demonstrated that caspase-3 was cleaved
significantly, indicating that the cells were undergoing apoptosis.
The tumor suppressor protein, p53, is a positive regulator of the
Bax, Bad and Bak proapoptotic proteins to prevent Bcl-2 capture.
Free Bax, Bad and Bak subsequently bind to the mitochondrial
membrane to induce mitochondrial damage and cell apoptosis
(21–23). Previous studies have demonstrated
that p53 promotes the transcription of Bax and Bak, which regulate
the release of cytochrome c from the mitochondria, and
result in cell apoptosis by activating the cleaving of caspase-3
and caspase-9 (22,24). In the present study, p53, Bax, Bad
and Bak were found to be significantly upregulated, whereas Bcl-2
was found to be downregulated in the HepG2 and QGY7701 cells
following chrysin treatment. These results suggested that chrysin
upregulates p53 and activates its downstream target gene, which
induces mitochondrial dysfunction by releasing cytochrome c
and inducing the apoptosis of cancer cells. Cell apoptosis is a
process of programmed cell death which includes early membrane
extroversion in the early stage and cell membrane disruption and
cell death during late apoptosis. In the present study, it was
demonstrated that the early and late stages of apoptosis were
increased in QGY7701 cells; whereas only late apoptosis was
increased in HepG2 cells. Notably, significantly more HepG2 cells
were killed, as compared with QGY7701 cells, which suggests that
chrysin may have a stronger effect on HepG2 cells by promptly
inducing late cell apoptosis. These results demonstrated that the
anti-cancer effect of chrysin anticancer effect is associated with
the genetic background of the cancer cells rather than a general
cytotoxic effect.
In conclusion, the results of the present study
suggested that chrysin effectively inhibits cell viability and
induces cell apoptosis in HCC cells. It was confirmed that chrysin
promoted HCC cell apoptosis via the activation of the
p53/Bcl2/caspase-9 apoptotic signaling pathway. Based on the
aforementioned findings, the present study suggests that chrysin
may be a candidate agent for the treatment of human HCC.
Acknowledgements
The present study was supported by grants from the
Special Funds from Education Department of Guangdong Province
(grant no. JB1212), the Chinese NSFC grants (grant no. 31370824),
the Yangfan Plan of Talents Recruitment Grant, Guangdong, China
(grant nos. YueRenCaiBan[2014]1 and YueRenCaiBan[2016]1), the
University Talents Recruitment Grant of Guangdong, China (grant no.
YueCaiJiao[2012]328), The Excellent Postgraduate Essay Development
Project of Guangdong Medical College (grant no. 2014-18) and the
Key Laboratory of Zhanjiang project (grant no. 2013A402-4).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Au JS and Frenette CT: Management of
hepatocellular carcinoma: Current status and future directions. Gut
Liver. 9:437–448. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fitzmorris P, Shoreibah M, Anand BS and
Singal AK: Management of hepatocellular carcinoma. J Cancer Res
Clin Oncol. 141:861–876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Manfredi JJ and Horwitz SB: Taxol: An
antimitotic agent with a new mechanism of action. Pharmacol Ther.
25:83–125. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pichichero E, Cicconi R, Mattei M, Muzi MG
and Canini A: Acacia honey and chrysin reduce proliferation of
melanoma cells through alterations in cell cycle progression. Int J
Oncol. 37:973–981. 2010.PubMed/NCBI
|
|
7
|
Barbarić M, Mišković K, Bojić M, Lončar
MB, Smolčić-Bubalo A, Debeljak Z and Medić-Šarić M: Chemical
composition of the ethanolic propolis extracts and its effect on
HeLa cells. J Ethnopharmacol. 135:772–778. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khan R, Khan AQ, Qamar W, Lateef A, Tahir
M, Rehman MU, Ali F and Sultana S: Chrysin protects against
cisplatin-induced colon toxicity via amelioration of oxidative
stress and apoptosis: Probable role of p38MAPK and p53. Toxicol
Appl Pharmacol. 258:315–329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cherkaoui-Tangi K, Lachkar M, Wibo M,
Morel N, Gilani AH and Lyoussi B: Pharmacological studies on
hypotensive, diuretic and vasodilator activities of chrysin
glucoside from Calycotome villosa in rats. Phytother Res.
22:356–361. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song MY, Jeong GS, Kwon KB, Ka SO, Jang
HY, Park JW, Kim YC and Park BH: Sulfuretin protects against
cytokine-induced beta-cell damage and prevents
streptozotocin-induced diabetes. Exp Mol Med. 42:628–638. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tahir M and Sultana S: Chrysin modulates
ethanol metabolism in Wistar rats: A promising role against organ
toxicities. Alcohol Alcohol. 46:383–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang F, Jin H, Pi J, Jiang JH, Liu L, Bai
HH, Yang PH and Cai JY: Anti-tumor activity evaluation of novel
chrysin-organogermanium (IV) complex in MCF-7 cell. Bioorg Med Chem
Lett. 23:5544–5551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wen S, Zhu D and Huang P: Targeting cancer
cell mitochondria as a therapeutic approach. Future Med Chem.
5:53–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mu R, Lu N, Wang J, Yin Y, Ding Y, Zhang
X, Gui H, Sun Q, et al: An oxidative analogue of gambogic
acid-induced apoptosis of human hepatocellular carcinoma cell line
HepG2 is involved in its anticancer activity in vitro. Eur J
Cancer Prev. 19:61–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alenzi FQ, Alenazi BQ, Al-Anazy FH,
Mubaraki AM, Salem ML, Al-Jabri AA, Lotfy M, Bamaga MS, Alrabia MW
and Wyse RK: The role of caspase activation and mitochondrial
depolarisation in cultured human apoptotic eosinophils. Saudi J
Biol Sci. 17:29–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolf BB, Schuler M, Echeverri F and Green
DR: Caspase-3 is the primary activator of apoptotic DNA
fragmentation via DNA fragmentation factor-45/inhibitor of
caspase-activated DNase inactivation. J Biol Chem. 274:30651–30656.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ling Y, Lu N, Gao Y, Chen Y, Wang S, Yang
Y and Guo Q: Endostar induces apoptotic effects in HUVECs through
activation of caspase-3 and decrease of Bcl-2. Anticancer Res.
29:411–417. 2009.PubMed/NCBI
|
|
20
|
Slee EA, Adrain C and Martin SJ:
Executioner caspase-3, −6, and −7 perform distinct, non-redundant
roles during the demolition phase of apoptosis. J Biol Chem.
276:7320–7326. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng EH, Wei MC, Weiler S, Flavell RA,
Mak TW, Lindsten T and Korsmeyer SJ: Bcl-2, Bcl-X(L) sequester BH3
domain-only molecules preventing BAX and BAK-mediated mitochondrial
apoptosis. Mol Cell. 8:705–711. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Degenhardt K, Chen G, Lindsten T and White
E: BAX and BAK mediate p53 independent suppression of
tumorigenesis. Cancer Cell. 2:193–203. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu J, Shu Y, Zhang Q, Liu B, Xia J, Qiu
M, Miao H, Li M and Zhu R: Dihydromyricetin induces apoptosis and
inhibits proliferation in hepatocellular carcinoma cells. Oncol
Lett. 8:1645–1651. 2014.PubMed/NCBI
|
|
24
|
Henry H, Thomas A, Shen Y and White E:
Regulation of the mitochondrial checkpoint in p53 mediated
apoptosis confers resistance to cell death. Oncogene. 21:748–760.
2002. View Article : Google Scholar : PubMed/NCBI
|