Introduction
The levels of cysteinyl leukotrienes(LTs) in nasal
secretions are elevated following allergen challenge or during
natural exposure to allergens in allergic rhinitis (AR) patients
(1,2). LTs play a vital role in the
pathogenesis of AR and asthma (3,4), and as
asthma patients frequently exhibit AR, patients that suffered from
asthma and AR were particular good candidates for anti-LT therapy
(5).
Anti-LT therapy has been indicated to be effective
in relieving the symptoms of AR or asthma in large-scale
randomized, double-blind, placebo-controlled clinical trials
(6,7). Nasal congestion and rhinorrhea in AR
were reduced via the administration of montelukast, a LT receptor
antagonist (LTRA), to a degree similar to that of antihistamines
(8,9); however, the results of other trials
were inconsistent with this (6,7,10). It remains unclear which population
group may benefit most since the efficacy of the LTRA varied among
asthmatic patients. Since it is difficult to predict responsiveness
to anti-LT therapy in an individual patient, it is critical to
develop a simplified method to identify the treatment response.
LTC and LTD can induce an increase in nasal mucosal
blood flow and nasal airway resistance (NAR); however, inhalation
of LTD4 is associated with higher potency (11) and slow deactivation in vivo
(12) as compared with LTC4 and
LTE4. It has been reported that LTC4 is ~10 times (13), while LTD4 was ~5,000 times, as potent
as histamine in achieving a 150% increase in NAR (14), as measured by rhinomanometry.
Furthermore, LTD4 has been suggested to be an effective bronchial
provocation agent in our previous studies (15,16).
Consequently, LTD4 is speculated to be a potentially useful
provocation agent (15). To the best
of our knowledge, few prior studies have investigated the nasal
physiological response to LTs.
The present pilot study aimed to establish the
procedure for an LTD4 nasal provocation test (LTD4-NAPT), and to
investigate the clinical characteristics, diagnostic value and
safety profiles of nasal response to LTD4.
Patients and methods
Subjects
Between November 2012 and May 2013, patients aged
18–30 years with recurrent symptoms of sneezing, rhinorrhea, nasal
stuffiness or itching during the preceding years were recruited
from the First Affiliated Hospital of Guangzhou Medical University
(Guangzhou, China). AR patients were positive to at least one kind
of inhaled allergens by skin prick test (ALK-Abellό, Hørsholm,
Denmark). The diagnosis of AR was based on ARIA 2008 (17). Exclusion criteria included: Smoking
status; NAR increased >30% after ethanol diluent challenge; a
past confirmed history of chronic respiratory disease other than
asthma; undergoing immunotherapy; inability to complete the test or
limited understanding of the test; acute upper and lower airway
infections two weeks prior to the enrollment; and other severe
systemic diseases, such as myocardial infarction or malignant
tumor. The patients were required to abstain from the use of oral
or nasal anti-histamines and LTRA for at least one week, and oral,
nasal and inhaled corticosteroids for at least two weeks, since
these drugs may affect the study results. The normal control
subjects were aged between 18 and 30 years, had no upper and lower
respiratory tract infections for the previous two weeks, and no
allergic or systemic diseases.
The study protocol was approved by the Ethics
Committee of the First Affiliated Hospital of Guangzhou Medical
University. The purpose, test procedures, potential benefits and
safety were explained to the participants and all subjects signed
written informed consent prior to the study.
Study design
This was an open-labeled study. Nasal airway
responsiveness was assessed by the increase of NAR and a composite
symptom score, and positive response was defined as the provocative
concentration of LTD4 causing a 60% increase in total NAR
(PC60NAR-LTD4) no more than 3.2×104 nmol/l,
or the symptom score of >3 points (pts) during the test
(18).
Rhinomanometry
Total nasal airway inspiration resistance (NAR),
left and right nasal airway inspiration resistance (RIL and RIR)
were measured by rhinomanometry (JAEGER MasterScope; CareFusion
Co., Ltd., Hoechberg, Germany). A total of at least three
repeatability (no more than five) rhinomanometry maneuvers were
performed in each nostril, with each maneuver contains at least
three respiratory cycles to calculate the mean value of NAR, under
a pressure of 150 Pa.
Preparation of diluents
The preparation of LTD4 simulative diluents was
almost the same as described elsewhere (15,16).
LTD4 (100 µg/ml in ethanol; Cayman Chemical Company, Ann Arbor, MI,
USA) was stored at −80°C. The diluent of 16 µg/ml LTD4 (solution A)
was prepared by adding 0.504 ml normal saline into 0.096 ml LTD4
aliquot. An aliquot of solution A was added to 0.3 ml normal
saline, forming 8 µg/ml LTD4 diluent (solution B). An aliquot of
solution B was added into 0.2 ml normal saline forming 4 µg/ml LTD4
diluent (solution C). Ethanol diluent control was also prepared
except that 0.096 ml ethanol absolute was adopted instead of LTD4.
To ensure the quality of the test, LTD4 diluents should be
discarded at the end of day, as stated in the manufacturers'
specifications (Cayman Chemical Company).
Nasal challenge
Diluents were delivered via nasal spray pumps
(Dezong Pharmaceutical, Co., Ltd., Foshan, China) by compressed air
(0.1 ml per spray), with provocative concentration increased in a
step-wise manner. Nasal challenge using 16% ethanol diluent, the
concentration of which corresponded to solution A, was performed in
order to exclude subjects hypersensitive to ethanol or saline. The
LTD4 challenge could be initiated provided that NAR increase was
<30%. A range between 4 and 16 µg/ml LTD4 diluents were applied
for a double-fold increment approach at intervals of 6 min. These
procedures were terminated in the case of a ≥60% NAR increase or a
composite symptom score >3, or until the use of the last
concentration of LTD4 diluents.
Symptom score
A composite symptom score according to Riechelmann
et al (18) was applied: 3–5
Sneezes = 1 pt; >5 sneezes = 2 pts; rhinorrhea <1 ml = 1 pt;
rhinorrhea >1 ml = 2 pts; pruritus of the palate, ears or eyes =
1 pt; and conjunctivitis, cough, urticaria or difficult breathing =
2 pts. Total scores ranged between 0 and 6 pts. The provocation
test was positive when the total score reached >3 pts, whatever
the changes of NAR.
Determination of positive
response
Positive response of LTD4 nasal provocation test was
defined as an increase in NAR of ≥60% by the concentration of LTD4
no more than 3.2×104 nmol/l and/or composite symptom
score >3 pts.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 16.0 (SPSS, Inc., Chicago, IL, USA). Dot plots
were produced using GraphPad Prism, version 5.01 (GraphPad
Software, Inc., San Diego, CA, USA). Mean ± standard deviation
(x±s) was adopted for data with normal distribution,
otherwise median (interquartile range) was used. Two-sided
t-tests were performed for comparison of baseline levels. A
dot plot was applied for distribution character of
PC60NAR-LTD4. A row-column table was analyzed via
χ2 test. The diagnostic value was assessed using
receiver operating characteristic curve (ROC), with area under
curve (AUC) and 95% confidence interval (CI) being reported.
Diagnostic value and adverse events were analyzed for efficacy and
safety outcomes. Statistical significance was defined as
P<0.05.
Results
Demographics and changes in RIR, RIL
and NAR
Among the 50 subjects that underwent screening, 8
subjects were withdrawn for being unwilling to undergo a nasal
provocation. A total of 26 AR patients and 16 normal control
subjects were enrolled. Baseline demographics were comparable
between AR and normal controls (all P>0.05, Table I).
 | Table I.Baseline demographic characteristics
and nasal airway resistance in allergic rhinitis and normal
controls. |
Table I.
Baseline demographic characteristics
and nasal airway resistance in allergic rhinitis and normal
controls.
| Parameter | Normal control | AR | P-value |
|---|
| Patients | 16 | 26 | – |
| Age (years) |
21.1±3.2 |
22.2±6.5 | 0.440 |
| Male/female | 11/5 | 9/17 | 0.055 |
| Height (cm) | 165.8±8.5 | 163.9±8.5 | 0.493 |
| Weight (kg) |
56.5±7.4 | 53.15±7.1 | 0.151 |
| RIL |
0.41±0.17 |
0.56±0.34 | 0.123 |
| RIR |
0.47±0.40 |
0.63±0.22 | 0.097 |
| NAR |
0.22±0.08 |
0.28±0.32 | 0.061 |
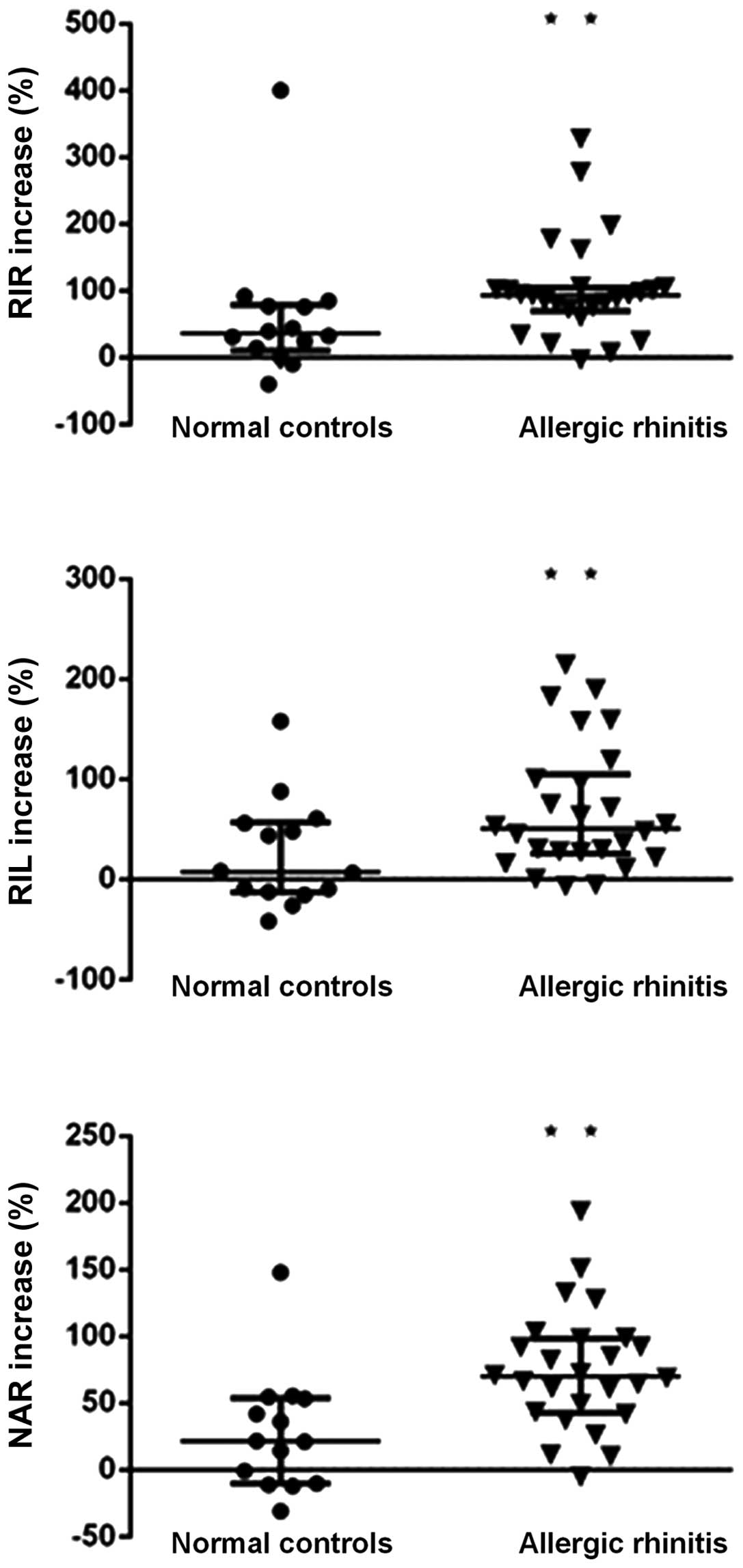
Prior to challenge, NAR was slightly higher in the
AR group compared with the controls, but without statistically
significant differences (Table I).
Following challenge, marked increases in RIL, RIR and NAR in both
groups were observed, significantly higher in AR patients compared
with the normal controls (all P<0.05, Fig. 1).
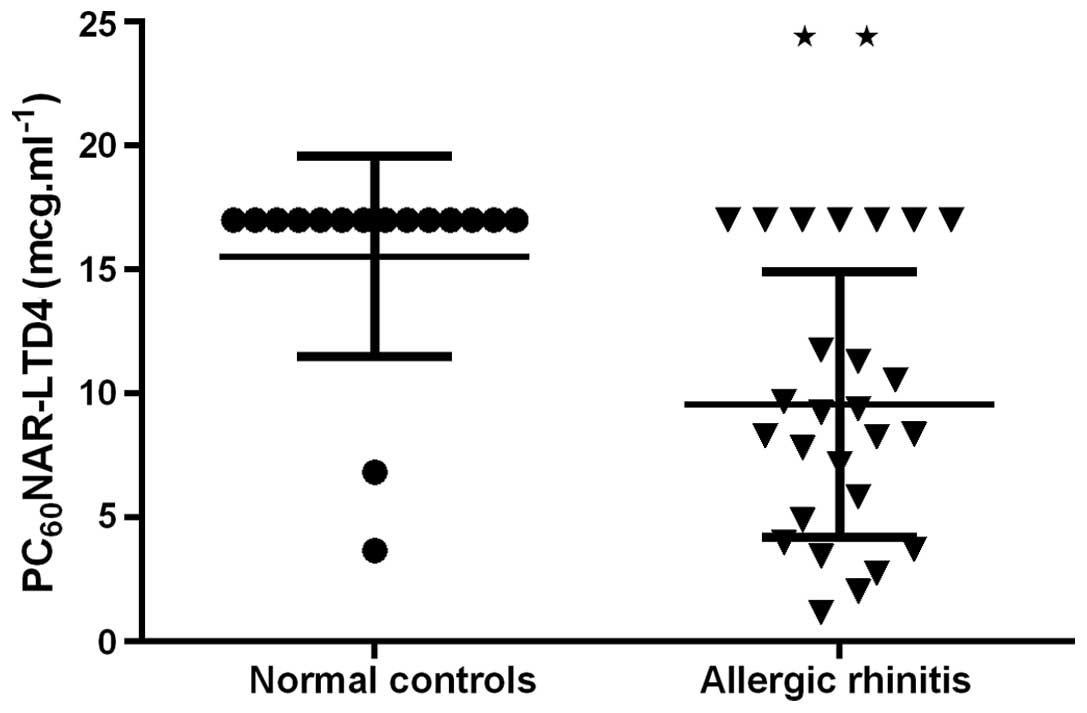
Changes in PC60NAR-LTD4 in
response to LTD4 challenge
Positive responses to LTD4 challenge were observed
in 19 AR patients and 2 normal controls (positive rate, 73.08 vs.
12.50%, P<0.001). AR had a markedly lower median of
PC60NAR-LTD4 (8.36 µg/ml), with a larger interquartile
range (10.00 µg/ml) as compared with normal controls (Fig. 2). PC60NAR-LTD4 varied
between 1.17 and 11.75 µg/ml in 19 AR, by comparison,
PC60NAR-LTD4 was >16.00 µg/ml in all normal controls,
with two exceptions of 3.68 and 6.84 µg/ml. The increase of NAR was
negatively correlated with
log10(PC60NAR-LTD4) in AR (r=−0.75,
P<0.001). Mean NAR increase was 74.44±44.95% (range,
61.38–193.68%) in AR patients that were positive to LTD4
challenge.
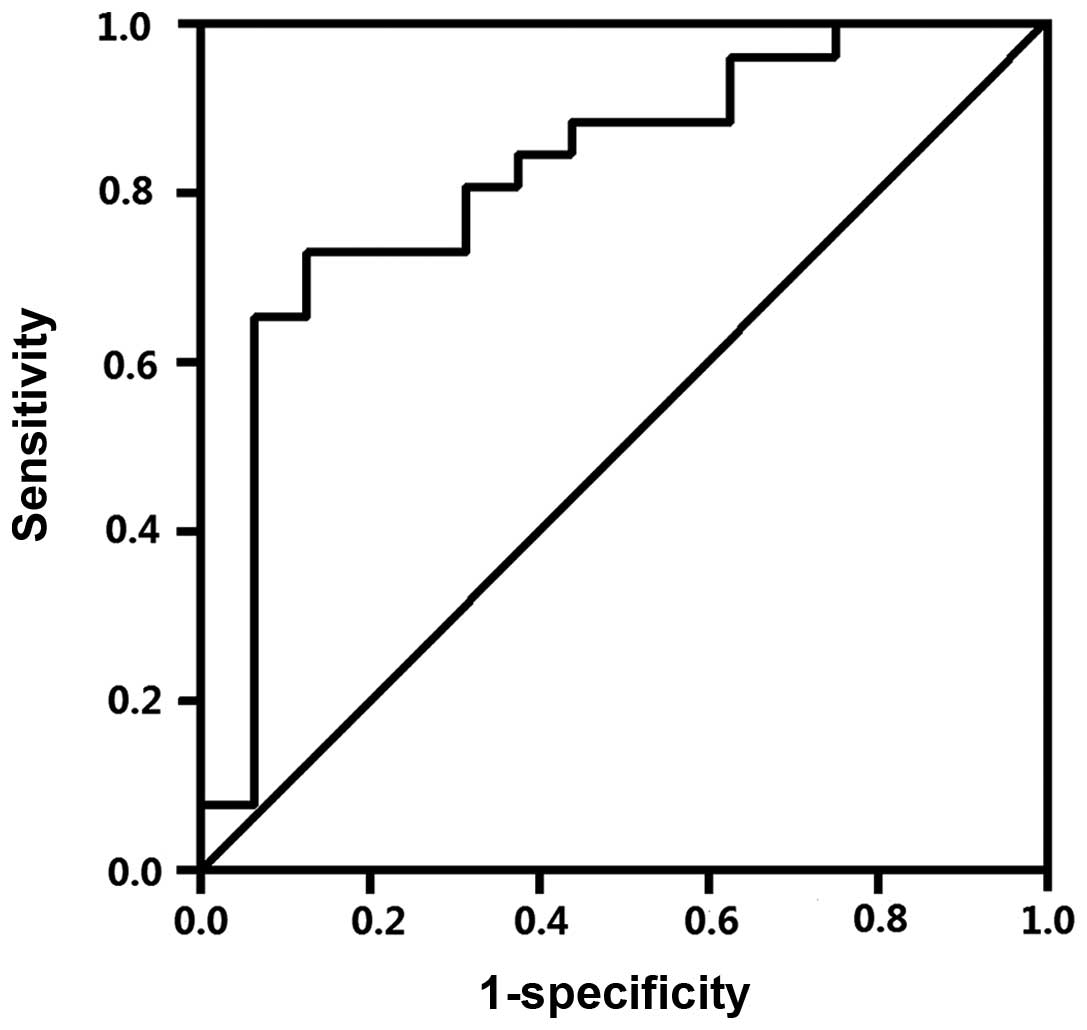
Changes in symptom scores in response
to LTD4 challenge
The symptom scores were significantly higher in AR
group compared with in the control group (1.19±0.94 vs. 0.12±0.50,
P<0.001). The symptom scores consisted of sneezing (0.12±0.34
vs. 0.00±0.00, P=0.149), rhinorrhea (0.79±0.66 vs. 0.06±0.25,
P<0.001) and chemosis or itching of the eyes (0.06±0.25 vs.
0.21±0.42, P=0.216). No symptom scores >3 pts were detected in
either group. AUC was 0.822 [95% CI, (0.665, 0.961)] (Fig. 3).
Adverse effects induced by LTD4
challenge
Adverse events were observed in 3 AR patients that
were positive to LTD4 nasal provocation. The major adverse events
by incidence were eye itching (3/3), tears (3/3) and full nasal
congestion (1/3) with breathing through mouth. All adverse events
were tolerated and recovered within an hour after challenge. No
serious adverse events (including wheezing or induced bronchia
constriction) were observed.
Discussion
The present study demonstrated that LTD4 was able to
induce an increase in NAR and nasal discharge in patients with AR
compared with normal controls. The increase of NAR was negatively
correlated with log10(PC60NAR-LTD4)(r=−0.75,
P<0.001). The majority of AR patients (19/26) were nasal airway
hyperactive to LTD4, high diagnostic value of LTD4-NPT was
indicated by the ROC (AUC, 0.813). Bisgaard et al (13) demonstrated that, even in normal
control subjects, a high dose of LTD4 may induce nasal mucosal
blood flow and NAR in the absence of nasal discharge. Furthermore,
Numata et al (19) reported
that nasal mucosal swelling induced by nasal challenge was
prevented by LTRA administration.
To the best of our knowledge, the present study was
the first to directly and quantitatively evaluate the effects of
LTD4 on NAR and nasal symptoms in patients with AR. The results
supported our hypothesis that LT impacts the upper and lower
airways, and that LTD4 may be employed as a nasal provocative
mediator.
By comparison, LTD4 provocation test did not cause
sneezing and itching or other irritative symptoms in the present
study, which was similar to some previous studies (20,21).
Miadonna et al (20) and
Howarth (21) demonstrated that the
symptoms of AR such as nasal secretion, nasal obstruction in
particular, was more strongly correlated with the release of
arachidonic acid metabolites than histamine in the nasal secretion
or specimen during natural exposure. However, when stimulated with
histamine or cysLT there was a different result; sneezing, itching,
rhinorrhea and nasal obstruction could be induced by histamine
rapidly (within a few seconds), while cysLT caused a more
pronounced and longer lasting nasal obstruction (13,19).
These phenomena implied that not only one, probably many mediators
were involved in the development of symptoms, even involved with
nervous reflex.
To date, a number of methods, such as the
measurement of LTs in urinal, nasal lavage or blood, have been used
to identify the patients with AR or asthma that are sensitive to
LTRA (22,23); however, none of them has been
successfully used in clinical practice.
Although the cost of LTD4-NPT is higher than that of
histamine/methacholine-NPT, LTD4-NPT may provide improved
predictive efficacy of LTRA among AR patients with distinct
inflammatory phenotypes compared with conventional provocation
agents (such as histamine) or for identifying patients who are
highly sensitivity to LTs. Furthermore, by measuring the
alterations in upper and lower airway responsiveness and
inflammatory mediators to LTD4-NPT, more may be elucidated
regarding the association between AR and asthma. These critiques
will be investigated in our future studies.
There are a number of limitations in the present
study, owing to high thermosensitivity and reducibility in aqueous
environment; the LTD4 diluents were valid for only one day, which
may limit the widely use of it. Furthermore, it is necessary to
increase the population size in future studies.
In conclusion, the established procedure of LTD4
nasal provocation test is effective in the evaluation of nasal
airway hyperresponsiveness, and is well tolerated. LTD4-NAPT may be
applied in the diagnosis of AR and provide a useful tool for
testing the effectiveness of LTRA in future.
Acknowledgements
The authors would like to thank Dr Xu Shi and Mr.
Wenhua Jian from the First Affiliated Hospital of Guangzhou Medical
University; Mrs. Qingxia Liu from Qingyuan People's Hospital
(Qingyuan, China); Miss E Guo from Xiangyang Central Hospital
(Xiangyang, China); Miss Zhiyu Liang from Yuexiu People's Hospital
(Guangzhou, China); and Miss Linting Luo, Mr. Diteng Luo, Mr.
Xiangong Xu, Mr. Huayi Huang, Miss Yongqing Ye and Miss Xianmiao Ye
from Guangzhou Medical University for their assistance with
recruiting participants. This study was supported by Changjiang
Scholars and Innovative Research Team in University (grant no.
ITR0961), The National Key Technology R&D Program of the 12th
National Five-year Development Plan (grant no. 2012BAI05B00) and
Guangzhou Medical University Doctor Startup Items (grant no.
2011C39).
References
|
1
|
Bisgaard H, Robinson C, Rømeling F, Mygind
N, Church M and Holgate ST: Leukotriene C4 and histamine in early
allergic reaction in the nose. Allergy. 43:219–227. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kunitomo M and Otsuka H: Comparison of
antigen-induced leukotriene and histamine release from nasal
scrapings in allergic rhinitis. Rhinology. 43:199–204.
2005.PubMed/NCBI
|
|
3
|
Busse WW: The role of leukotrienes in
asthma and allergic rhinitis. Clin Exp Allergy. 26:868–879. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Howarth PH, Salagean M and Dokic D:
Allergic rhinitis: Not purely a histamine-related disease. Allergy.
55(Suppl 64): S7–S16. 2000. View Article : Google Scholar
|
|
5
|
Price DB, Swern A, Tozzi CA, Philip G and
Polos P: Effect of montelukast on lung function in asthma patients
with allergic rhinitis: Analysis from the COMPACT trial. Allergy.
61:737–742. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patel P, Philip G, Yang W, Call R, Horak
F, LaForce C, Gilles L, Garrett GC, Dass SB, Knorr BA and Reiss TF:
Randomized, double-blind, placebo-controlled study of montelukast
for treating perennial allergic rhinitis. Ann Allergy Asthma
Immunol. 95:551–557. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Philip G, Williams-Herman D, Patel P,
Weinstein SF, Alon A, Gilles L, Tozzi CA, Dass SB and Reiss TF:
Efficacy of montelukast for treating perennial allergic rhinitis.
Allergy Asthma Proc. 28:296–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pinar E, Eryigit O, Oncel S, Calli C,
Yilmaz O and Yuksel H: Efficacy of nasal corticosteroids alone or
combined with antihistamines or montelukast in treatment of
allergic rhinitis. Auris Nasus Larynx. 35:61–66. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nayak A and Langdon RB: Montelukast in the
treatment of allergic rhinitis: An evidence-based review. Drugs.
67:887–901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Virchow JC and Bachert C: Efficacy and
safety of montelukast in adults with asthma and allergic rhinitis.
Respir Med. 100:1952–1959. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Drazen JM: Leukotrienes as mediators of
airway obstruction. Am J Respir Crit Care Med. 158:S193–S200. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee TH, Woszczek G and Farooque SP:
Leukotriene E4: Perspective on the forgotten mediator. J Allergy
Clin Immunol. 124:417–421. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bisgaard H, Olsson P and Bende M: Effect
of leukotriene D4 on nasal mucosal blood flow, nasal airway
resistance and nasal secretion in humans. Clin Allergy. 16:289–297.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miadonna A, Tedeschi A, Leggieri E, Lorini
M, Folco G, Sala A, Qualizza R, Froldi M and Zanussi C: Behavior
and clinical relevance of histamine and leukotrienes C4 and B4 in
grass pollen-induced rhinitis. Am Rev Respir Dis. 136:357–362.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guan WJ, Zheng JP, Gao Y, Jiang CY, An JY,
Yu XX and Liu WT: Leukotriene D4 bronchial provocation test:
Methodology and diagnostic value. Curr Med Res Opin. 28:797–803.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guan WJ, Zheng JP, Gao Y, Jiang C, Xie Y,
An J, Yu X, Liu W and Zhong N: Leukotriene D4 and methacholine
bronchial provocation test for identifying
leukotriene-responsiveness subtypes. J Allergy Clin Immunol.
131:332–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bousquet J, Khaltaev N, Cruz AA, Denburg
J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica
GW, van Weel C, et al: Allergic rhinitis and its impact on asthma
(ARIA) 2008 Update (in collaboration with the World Health
Organization, GA(2)LEN and AllerGen). Allergy. 63(Suppl 86): 8–160.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Riechelmann H, Bachert C, Goldschmidt O,
Hauswald B, Klimek L, Schlenter WW, Tasman AJ and Wagenmann M:
German Society for Allergology and Clinical Immunology (ENT
Section); Working Team for Clinical Immunology: Application of the
nasal provocation test on diseases of the upper airways. Position
paper of the German society for allergology and clinical immunology
(ENT Section) in cooperation with the working Team for Clinical
Immunology. Laryngorhinootologie. 82:183–188. 2003.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Numata T, Hanazawa T, Konno A, Terada N,
Yamakoshi T and Nagata H: Comparative role of peptide leukotrienes
and histamine in the development of nasal mucosal swelling in nasal
allergy. Ann Otol Rhinol Laryngol. 108:467–473. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Miadonna A, Tedeschi A, Leggieri E, Lorini
M, Folco G, Sala A, Qualizza R, Froldi M and Zanussi C: Behavior
and clinical relevance of histamine and leukotrienes C4 and B4 in
grass pollen-induced rhinitis. Am Rev Respir Dis. 136:357–362.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Howarth PH: Mediators of nasal blockage in
allergic rhinitis. Allergy. 52(Suppl 40): S12–S18. 1997. View Article : Google Scholar
|
|
22
|
Serrano CD, Valero A, Bartra J,
Roca-Ferrer J, Muñoz-Cano R, Sánchez-López J, Mullol J and Picado
C: Nasal and bronchial inflammation after nasal allergen challenge:
Assessment using noninvasive methods. J Investig Allergol Clin
Immunol. 22:351–356. 2012.PubMed/NCBI
|
|
23
|
Baek HS, Cho J, Kim JH, Oh JW and Lee HB:
Ratio of leukotriene e(4) to exhaled nitric oxide and the
therapeutic response in children with exercise-induced
bronchoconstriction. Allergy Asthma Immunol Res. 5:26–33. 2013.
View Article : Google Scholar : PubMed/NCBI
|