Introduction
Trichloroethylene (TCE) is a ubiquitous chemical
used occupationally for various production and manufacturing
purposes; it is widely used in metal, electroplating, electronics
and other industries. Since 1990, numerous patients with
Trichloroethylene Hypersensitivity Syndrome (THS) were exposed to
TCE; this has attracted much attention worldwide (1–3). THS was
shown to occur in 1–13% of the TCE-exposed workers (3). In the Chinese prescribed occupational
disease list, THS is known as occupational medicamentosa-like
dermatitis induced by TCE. Concerns about THS have driven
epidemiological and experimental studies investigating TCE exposure
and risks associated with THS (1,4–6).
The genetic polymorphism of human leukocyte antigen
HLA-B*1301 is strongly associated with THS among exposed workers
(4). Although the mechanism
underlying TCE toxicity remains the subject of debate, THS is
suggested to be a type VI hypersensitivity (5), although types II and III
hypersensitivity may also be associated with THS (7). Current treatment strategies for THS
include hormonal therapy, administration of γ-globulin, protection
of liver and reinforcement of skin care (8,9). THS can
also be treated with glucocorticoid therapy, and the primary
therapeutic principle is to prescribe an appropriate dosage of
glucocorticoids early in the course of the disease, followed by a
tapered reduction of the dose (8,9). Despite
extensive research, studies are limited with regards to the
stability, curability and sequelae of THS. Furthermore, the nature
of the causative compound of THS was also questioned by some
researchers. Therefore, by completing a follow-up assessment of two
patients who had THS, the current study was designed to explore the
stability, curability and sequelae of THS, and to investigate the
causative compound.
Materials and methods
Subjects
The study protocol was conducted according to the
principles of the Declaration of Helsinki and approved by the
Medical Ethics Committee of the Guangdong Province Hospital for
Occupational Disease Prevention and Treatment (GDOH; Guangzhou,
China). The subjects provided their written informed consent.
In March 2011, 2 male subjects (age, 42 years) with
healing THS, who were discharged from the GDOH >10 weeks prior
to the commencement of the study, were included. The two cases were
diagnosed with THS by three occupational dermatologists of the
hospital, based on the Chinese National Diagnostic Criteria of
Occupational Disease (GBZ 185–2006; Ministry of Health, China,
2006; appendix A; http://www.moh.gov.cn/cmsresources/zwgkzt/wsbz/new/20080118111726.pdf).
Four patients with occupational noise-induced hearing loss, without
a history of dermatosis, served as controls for the patch
tests.
Questionnaire
The contents of the questionnaire questioned whether
the patients suffered from a cold, as well as skin, eye or liver
abnormalities. The subjects were questioned on disease, medication
and occupational history.
Health examination
The subjects were hospitalized for ~1 week. During
the study, the subjects underwent a series of investigations,
including the following: Physical examination; ophthalmic
examination; electrocardiogram; X-ray; abdominal color ultrasound
(liver, kidney and spleen); liver function tests (7080 Automatic
Analyzer; Hitachi, Tokyo, Japan) including the analysis of total
protein, albumin, total bilirubin, direct bilirubin, indirect
bilirubin, alkaline phosphatase, gamma glutamyl transferase, total
bile acids, aspartate aminotransferase (AST) and alanine
aminotransferase (ALT); routine blood tests (XT-1800i hematology
analyzer; Sysmex Corp., Kobe, Japan); routine urinary tests
(Mejer-600; Shenzhen Mejer Medical Science and Technology Co.,
Ltd., Shenzhen, China); autoimmune disease indicators including
anti-nuclear antibody (ANA), anti-DNA antibodies and
anti-double-stranded DNA (dsDNA); Schirmer I test (SIT) and tear
break-up time (BUT) test. These examinations were performed on
three occasions.
Patch test
TCE (purity, ≥99.5%; Sigma-Aldrich, St. Louis, MO,
USA), chloral hydra (CH; purity ≥99.5%; Honeywell Specialty
Chemicals Seelze GmbH, Seelze, Germany) and trichloroethanol (TCOH;
purity ≥98.0%; Sigma-Aldrich) were added to olive oil
(Sigma-Aldrich), and trichloroacetic acid (TCA; purity ≥99.5%;
Tianjin Chemical Reagent Second Factory, Tianjin, China) was added
to saline to prepare various concentrations of allergens. According
to previous trial tests (10–14), TCE
(50, 25, 10 and 5% in olive oil), chloral hydrate (15, 10 and 5% in
olive oil), TCOH (5, 0.5 and 0.05% in olive oil) and TCA (5 and
0.5% in saline) were used in the patch test. Olive oil and normal
saline served as controls. The allergen at the highest
concentration was selected as the allergen in the control group,
since it would induce the highest positive rate in the patch
test.
The patch test method and interpretation followed
the International Contact Dermatitis Research Group (ICDRG)
criteria (15). Briefly, the patch
test was performed as follows: Each well of the patch test
apparatus (Finn chamber; Beijing Baiyi Yida Science and Technology
Development Ltd., Beijing, China) was filled with 25 µl allergen,
olive oil or saline, and the apparatus was numbered and patched to
the back of the subjects. The edge of the apparatus was reinforced
by 3 M micropore permeable medical tape (Minnesota Mining and
Manufacturing Medical Equipment Co., Ltd., Shanghai, China). The
subjects did not disturb the patch test for 48 h; showering was
forbidden during the test. The patches were removed after 48 h.
Observations and images were recorded by two occupational
dermatologists of GDOH 0.5 h, 24 h and 48 h following the removal
of the patch test. The results were observed, recorded and filed
according to the ICDRG criteria by two professional
dermatologists.
The 2 cases with THS and the 4 control subjects did
not receive treatment with corticosteroids and other
immunosuppressive drugs, or anti-infection drugs for 2 weeks prior
to the patch test.
Results
Case one
The patient was exposed to TCE whilst working in a
hardware products factory between August and September 2010. His
duties included the cleaning of wax with organic solvents (90.857%
TCE). The airborne concentrations of the time-weighted average in
the working place of the subject ranged between 123.38 and 171.82
mg/m3. The onset of THS occurred in September 2010, and
the patient was transferred to the GDOH after 12 days. On
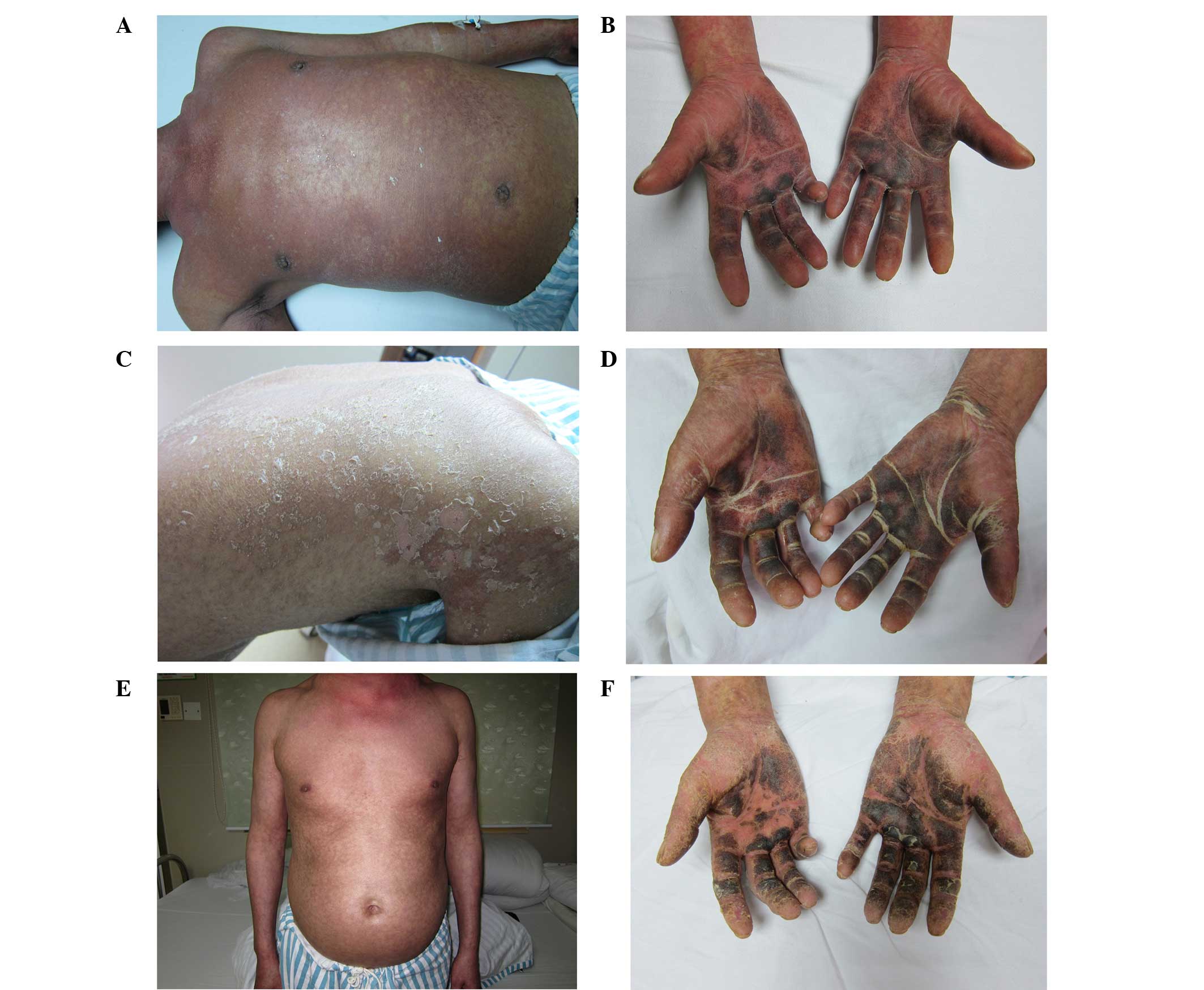
admission, the body temperature of the patient was 39.8°C. Physical
examination revealed erythematous lesions over the whole body
(Fig. 1). Dark erythematous lesions
were present over the trunk, and the majority of the rash was
confluent and resembled erythroderma. Scattered desquamated skin
was also evident, the palms and soles were pigmented and hard to
the touch, while the nature of the rash was similar to exfoliative
dermatitis. Additionally, there was lymphadenectasis and tenderness
of the submandibular, supraclavicular, inguinal and submental lymph
nodes. The patient had conjunctival congestion, scleral icterus and
itching of the eyes. The levels of ALT were 300 U/l (normal range,
<40 U/l), those of AST were 133 U/l (normal range, <40 U/l)
and the concentration of TCA in the urine was 14.10 mg/l.
Methylprednisolone (500 mg/day; Pfizer Manufacturing, Puurs,
Belgium) was administrated intravenously, and the patient also
received diammonium glycyrrhizinate (Chia Tai Tianqing
Pharmaceutical Group Co., Ltd., Lianyungang, China) for the
protection of the liver and stomach. On day 3 after admission, the
patient's body temperature returned to normal with no further
spread of the rash and liver function test results were improved.
Therefore, the methylprednisolone dose was reduced to 450 mg/day,
and after 9 days of continued improvement, the dose was tapered to
300 mg/day and then progressively reduced by 50–150 mg/day. The
total duration and dosage of methylprednisolone treatment was 81
days and 12,028 mg, respectively. When the skin recovered, the body
temperature and liver function test results returned to normal, and
the patient was discharged in January 2011. The patient returned
for a follow-up assessment in March 2011.
Case two
The second subject had an employment history that
was similar to the first case. In September 2010, a widespread
pruritic rash appeared on the patient's legs and he was transferred
to GDOH for further assessment 9 days after the appearance of the
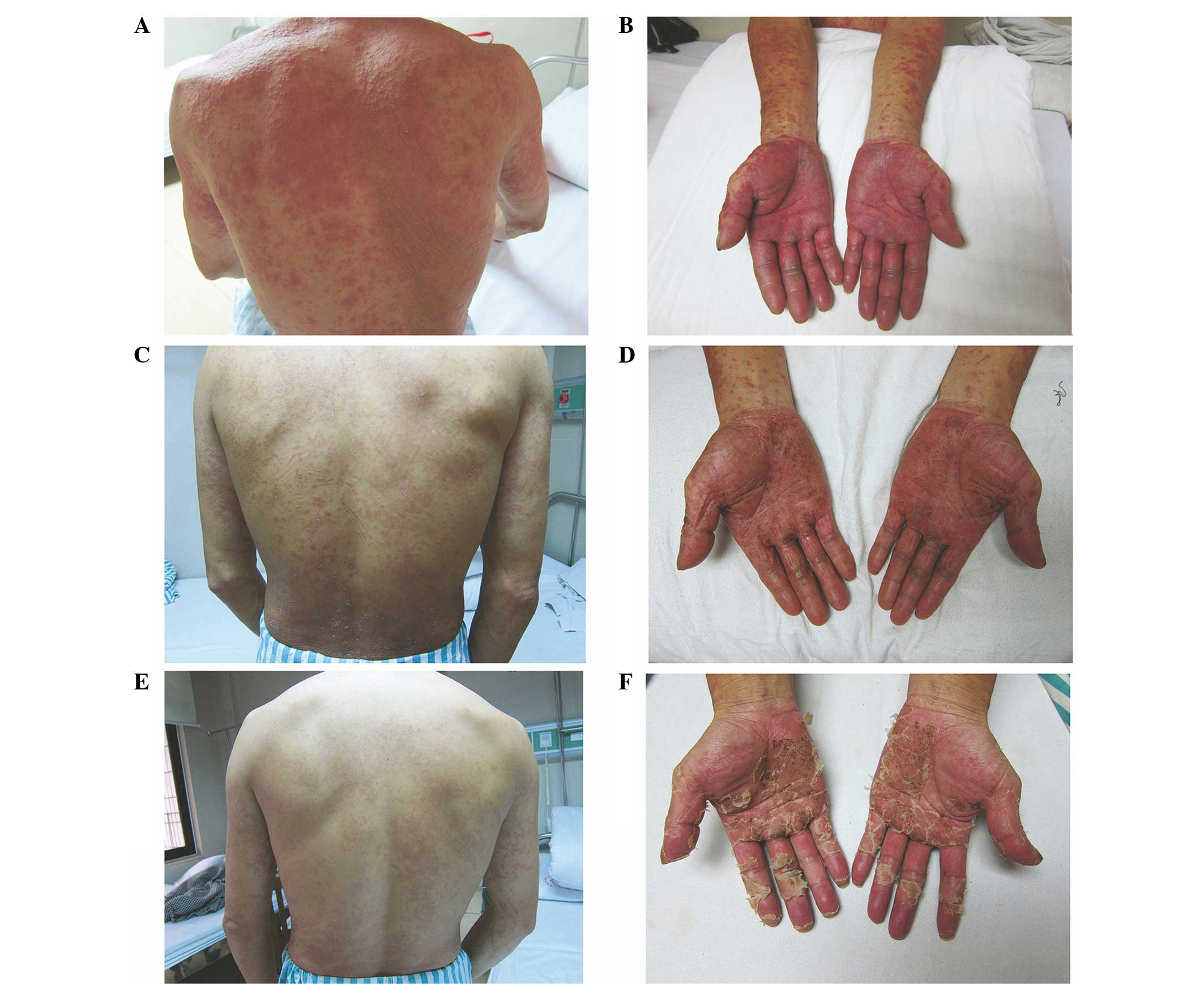
rash. On admission, the body temperature of the patient was 36.8°C.
Physical examination revealed dark erythematous skin lesions over
the majority of the body (Fig. 2).
The palms, fingers, soles and toes were swollen and tender, and the
nature of the rash was similar to exfoliative dermatitis.
Lymphadenectasis and tenderness was present in the submandibular,
throat, axillary and inguinal lymph nodes. The levels of ALT were
343 U/l and those of AST were 153 U/l, and the concentration of TCA
in the urine was 43.34 mg/l. Methylprednisolone (300 mg/day) was
administrated intravenously, and the patient also received
diammonium glycyrrhizinate for protection of the liver and stomach.
On day 3 after admission, the results of liver function tests were
improved. There was no further spread of the rash and the patient's
temperature normalized. On the day 4, the methylprednisolone dose
was reduced to 250 mg/day; the patient's condition continued to
improve and the dose was tapered progressively. The total duration
and dosage of methylprednisolone administration was 64 days and
5,237 mg, respectively. When the skin recovered, the body
temperature and liver function returned to normal, and the patient
was discharged in December 2010. The patient returned for a
follow-up assessment in March 2011.
Follow-up assessment
Survey findings
Skin itching and xerosis were the primary complaints
of both patients; no other symptoms were reported.
Health examination findings
The body temperature of both patients was normal.
Skin examination revealed that spread pigmentation, but no new
rashes or lymphadenectasis were apparent. The abdominal color
ultrasound (liver, kidney and spleen), electrocardiogram and X-ray
did not detect any abnormal changes. The blood tests, urinary test
and liver function test were normal. Autoimmune disease indicators
including ANA, anti-DNA antibodies and dsDNA were negative. The SIT
results for patient one were as follows: Right eye 8.0 mm/5 min and
left eye 18.0 mm/5 min; the SIT results for patient two were as
follows: Right eye 2.0 mm/5 min and left eye 5.0 mm/5 min. A normal
result is <10 mm/5 min. The BUT results for patient one were as
follows: Right eye 4.0 sec and left eye 5.0 sec; the BUT results
for patient two were as follows: Right eye 3.0 sec and left eye 4.0
sec. A normal result is >10 sec.
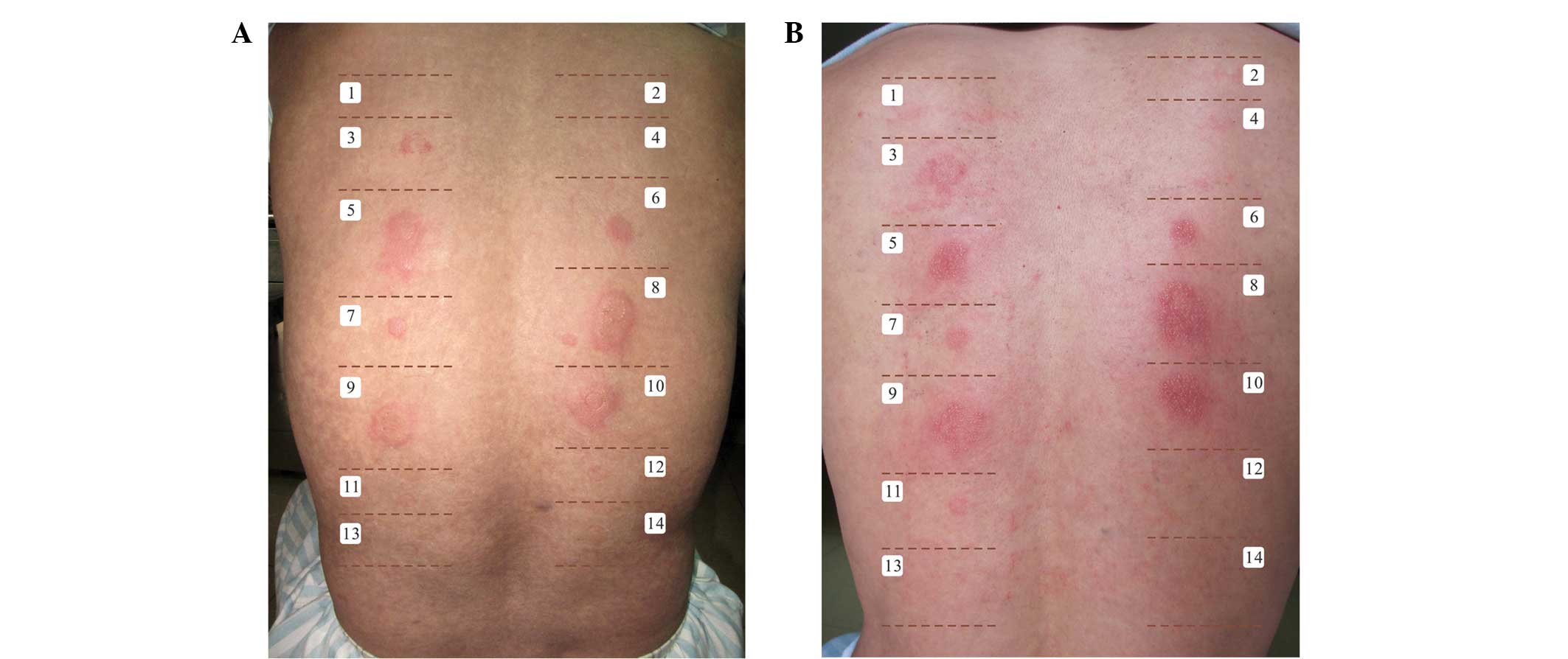
Patch test results
No adverse reactions were observed during the patch
test. Patches of skin with olive oil, saline and adhesive paste did
not show abnormal changes such as reddening of the skin and
swelling. The patch tests were positive for all mass concentrations
of CH and TCOH, were weakly positive for 5.0% TCA, and were
negative for 0.5% TCA and all mass concentrations of TCE. The four
control patches also returned negative results. The results are
presented in Table I and Fig. 3.
 | Table I.Patch test results for TCE and its
metabolites. |
Table I.
Patch test results for TCE and its
metabolites.
|
|
| Reactions at 48
h | Reactions at 72
h |
|---|
|
|
|
|
|
|---|
| Patch test no. | Chemical and
concentration | Case one | Case two | Case one | Case two |
|---|
| 1 | Control (NS) | − | − | − | − |
| 2 | Control (OO) | − | − | − | − |
| 3 | TCA 5% in NS | + | + | + | + |
| 4 | TCA 0.5% in NS | − | − | − | − |
| 5 | TCOH 5% in NS | ++ | ++ | ++ | ++ |
| 6 | TCOH 0.5% in NS | ++ | ++ | ++ | ++ |
| 7 | TCOH 0.05% in NS | ++ | ++ | ++ | ++ |
| 8 | CH 15% in NS | ++ | ++ | ++ | ++ |
| 9 | CH 10% in NS | ++ | ++ | ++ | ++ |
| 10 | CH 5% in NS | ++ | ++ | ++ | ++ |
| 11 | TCE 50% in OO | − | − | − | − |
| 12 | TCE 25% in OO | − | − | − | − |
| 13 | TCE 10% in OO | − | − | − | − |
| 14 | TCE 5% in OO | − | − | − | − |
Discussion
In the present study, THS was caused in both
patients by exposure to TCE without any previous history. To the
best of our knowledge, this is the first study to describe the
simultaneous THS onset in two patients that were exposed to the
same work environment (same factory), and to perform follow-up
assessment in THS cases. The patients presented predominantly with
symptoms of skin involvement, as well as fever, lymphadenectasis
and liver dysfunction (2,3,16,17). The
primary therapeutic principle was to prescribe an appropriate
dosage of glucocorticoid early in the course of the disease,
followed by a tapered dose reduction. However, it is important to
protect the liver and stomach from adverse effects during the
treatment of THS (9). In the present
study, the symptoms of the patients markedly improved when
glucocorticoid therapy was administered. Following the discharge of
patients one and two for 11 and 15 weeks, respectively, health
examinations demonstrated that both patients were healthy. However,
skin examination revealed pigmentation, itching and xerosis, but no
new rash. These results suggested that the curative effect of
glucocorticoid therapy is stable and that patients do not relapse
following healing.
SIT and BUT are critical dry eye tests (18). The SIT and BUT results were abnormal
in both patients, suggesting that dry eye syndrome may be a
sequelae for THS. It has previously been reported that
Stevens-Johnson syndrome results in a lack of lacrimal gland
secretion, resulting in various severe ocular surface disorders
manifesting dry eye (19,20). Considering that the clinical
manifestations and mechanism underlying THS resemble those of
Stevens-Johnson syndrome, dry eye syndrome may be one of the
primary sequelae of THS; this requires further study.
TCE is predominantly metabolized by cytochrome P450
(10). Two active metabolic
compounds of TCE are chloral and CH; these metabolites have similar
biological properties as the former metabolite hydrates rapidly to
CH. CH is easily reduced to TCOH (a reversible reaction) and then
to TCA (21). A patch test is now
considered to be a recognized method for confirming an allergic
contact dermatitis diagnosis and distinguishing the causal
allergen(s), as well as identifying a type IV (cell delayed)
hypersensitivity reaction (22–24). In
the present study, the patch test was positive for CH, TCOH and
TCA, but negative for all mass concentrations of TCE, in both
patients, results which were consistent with those of previous
investigations (11–14,16,25).
This suggested that the causal allergens for THS were metabolites
of TCE, not TCE itself; it can be hypothesized that the mechanism
underlying THS is cell delayed-type hypersensitivity induced by TCE
exposure.
It has been reported that THS patients may relapse
following re-exposure to TCE (8).
The patch test was positive for CH, TCOH and TCA following healing
for >10 weeks, illustrating that the hypersensitivity state in
patients who had THS may be sustainable over a long period of time.
Therefore, in order to avoid a relapse, patients who previously had
THS should be advised to avoid re-exposing themselves to TCE and
its metabolites. However, as the study period was short in the
follow-up assessment in the present study, the sustainable period
of hypersensitivity state remains unclear.
In conclusion, the follow-up assessment in the
current study suggested that THS does not recur following healing,
and that the curative effect of glucocorticoid therapy is stable;
however, the results suggested that dry eye syndrome may continue
as sequelae for THS. The mechanism underlying THS may be cell
delayed-type hypersensitivity induced by TCE exposure; the fact
that the hypersensitivity state in patients with THS remained over
a long period of time indicates that the causal allergens for THS
were the metabolites of TCE. Therefore, it can be suggested that
patients who previously had THS do not re-expose themselves to TCE
and its metabolites, and avoid receiving antipyretic, hypnotic or
anticonvulsive medicines in which CH is a primary ingredient. Due
to the sample and follow-up period limitations in the present
study, further studies are required to verify these
conclusions.
Acknowledgements
The authors are grateful to Dr. Jianxun Huang and
Dr. Zhenlie Huang for their help with the present study. The study
was supported by the Guangdong Provincial Key Laboratory of
Occupational Disease Prevention and Treatment (grant no.
2012A061400007), the National Key Technologies R&D Program of
China during the 12th Five-Year Plan Period (grant no.
2014BAI12B01) and National Natural Science Foundation of China
(grant no. 81502769).
References
|
1
|
Huang Z, Yue F, Yang X, Xia L, Chen C, Qiu
X, Huang J, Li L, Kamijima M, Nakajima T and Huang H: Upregulation
of calprotectin and downregulation of retinol binding protein in
the serum of workers with trichloroethylene-induced
hypersensitivity dermatitis. J Occup Health. 54:299–309.
2012.PubMed/NCBI
|
|
2
|
Xu X, Yang R, Wu N, Zhong P, Ke Y, Zhou L,
Yuan J, Li G, Huang H and Wu B: Severe hypersensitivity dermatitis
and liver dysfunction induced by occupational exposure to
trichloroethylene. Ind Health. 47:107–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamijima M, Hisanaga N, Wang H and
Nakajima T: Occupational trichloroethylene exposure as a cause of
idiosyncratic generalized skin disorders and accompanying hepatitis
similar to drug hypersensitivities. Int Arch Occup Environ Health.
80:357–370. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li H, Dai Y, Huang H, Li L, Leng S, Cheng
J, Niu Y, Duan H, Liu Q, Zhang X, et al: HLA-B* 1301 as a biomarker
for genetic susceptibility to hypersensitivity dermatitis induced
by trichloroethylene among workers in China. Environ Health
Perspect. 115:1553–1556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang X, Que B, Song X, Li S, Yang X, Wang
H, Huang H, Kamijima M, Nakajima T, Lin Y and Li L:
Characterization of liver injury associated with hypersensitive
skin reactions induced by trichloroethylene in the guinea pig
maximization test. J Occup Health. 50:114–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamijima M, Wang H, Yamanoshita O, Ito Y,
Xia L, Yanagiba Y, Chen C, Okamura A, Huang Z, Qiu X, et al:
Occupational trichloroethylene hypersensitivity syndrome: Human
herpesvirus 6 reactivation and rash phenotypes. J Dermatol Sci.
72:218–224. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao N, Wang HL, Yue F, Zeng ZM, Li HL,
Huang YS and Chen RT: Studying the changes of the related serum
complement immune indexes in patients with occupational
medicamentosa-like dermatitis induced by trichloroethylene and
workers occupationally exposed to trichloroethylene. Zhonghua Lao
Dong Wei Sheng Zhi Ye Bing Za Zhi. 30:284–288. 2012.(In Chinese).
PubMed/NCBI
|
|
8
|
Liu J: Clinical analysis of seven cases of
trichloroethylene medicamentose-like dermatitis. Ind Health.
47:685–688. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia LH, Huang HL, Kuang SR, Liu HF and
Kong LZ: A clinical analysis of 50 cases of medicament-like
dermatitis due to trichloroethylene. Zhonghua Lao Dong Wei Sheng
Zhi Ye Bing Za Zhi. 22:207–210. 2004.(In Chinese). PubMed/NCBI
|
|
10
|
Chiu WA, Jinot J, Scott CS, Makris SL,
Cooper GS, Dzubow RC, Bale AS, Evans MV, Guyton KZ, Keshava N, et
al: Human health effects of trichloroethylene: Key findings and
scientific issues. Environ Health Perspect. 121:303–311. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chae H, Lee S, Lee K, Kim J, Lee S, Shin
D, et al: Exfoliative dermatitis and toxic hepatitis associated
with occupational exposure to trichloroethylene. Korean J Occup
Environ Med. 15:111–117. 2003.(In Korean).
|
|
12
|
Chittasobhaktra T, Wannanukul W,
Wattanakrai P, Pramoolsinsap C, Sohonslitdsuk A and Nitiyanant P:
Fever, skin rash, jaundice and lymphadenopathy after
trichloroethylene exposure: A case report. J Med Assoc Thai.
80(Suppl 1): S144–S148. 1997.PubMed/NCBI
|
|
13
|
Phoon WH, Chan MO, Rajan VS, Tan KJ,
Thirumoorthy T and Goh CL: Stevens-Johnson syndrome associated with
occupational exposure to trichloroethylene. Contact Dermatitis.
10:270–276. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Conde-Salazar L, Guimaraens D, Romero LV
and Sanchez Yus E: Subcorneal pustular eruption and erythema from
occupational exposure to trichloroethylene. Contact Dermatitis.
9:235–237. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohtoshi S, Kitami Y, Sueki H and Nakada T:
Utility of patch testing for patients with drug eruption. Clin Exp
Dermatol. 39:279–283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Y and Huang H: Research progress oil
immune injury resulted from occupational medicamentose-like
dermatitis induced by trichloroethylene. Zhong Guo Zhi Ye Yi Xue.
37:157–162. 2010.(In Chinese).
|
|
17
|
Huang H, Kamijima M, Wang H, Li S,
Yoshikawa T, Lai G, Huang Z, Liu H, Chen J, Takeuchi Y, et al:
Human herpesvirus 6 reactivation in trichloroethylene-exposed
workers suffering from generalized skin disorders accompanied by
hepatic dysfunction. J Occup Health. 48:417–423. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu H, Wang Y, Dong N, Yang F, Lin Z, Shang
X and Li C: Meibomian gland dysfunction determines the severity of
the dry eye conditions in visual display terminal workers. PLoS
One. 9:e1055752014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schrader S, Liu L, Kasper K and Geerling
G: Generation of two- and three-dimensional lacrimal gland
constructs. Dev Ophthalmol. 45:49–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong N, Li W, Lin H, Wu H, Li C, Chen W,
Qin W, Quyang L, Wang H and Liu Z: Abnormal epithelial
differentiation and tear film alteration in pinguecula. Invest
Ophthalmol Vis Sci. 50:2710–2715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lash LH, Fisher JW, Lipscomb JC and Parker
JC: Metabolism of trichloroethylene. Environ Health Perspect 108
Suppl. 2:177–200. 2000. View Article : Google Scholar
|
|
22
|
Gawkrodger DJ: Patch testing in
occupational dermatology. Occup Environ Med. 58:823–828. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shear NH, Milpied B, Bruynzeel DP and
Phillips EJ: A review of drug patch testing and implications for
HIV clinicians. AIDS. 22:999–1007. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Slodownik D, Williams J, Frowen K, Palmer
A, Matheson M and Nixon R: The additive value of patch testing with
patients' own products at an occupational dermatology clinic.
Contact Dermatitis. 61:231–235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Watanabe H, Tohyama M, Kamijima M,
Nakajima T, Yoshida T, Hashimoto K and Iijima M: Occupational
trichloroethylene hypersensitivity syndrome with human
herpesvirus-6 and cytomegalovirus reactivation. Dermatology.
221:17–22. 2010. View Article : Google Scholar : PubMed/NCBI
|