Introduction
Carotid atherosclerotic stenosis is one of the main
risk factor for ischemic stroke and it contributes to >20% of
incidence of ischemic stroke (1).
Ischemic stroke can be prevented by treating the carotid
atherosclerotic stenosis. The carotid artery intima stripping off
technique remains the gold standard for the treatment of carotid
atherosclerotic stenosis (1).
However, in recent years, the rapid development of neuroimaging
techniques has led to carotid angioplasty with stenting (CAS)
becoming a simple, effective and minimally invasive method used for
the treatment of extracranial carotid artery stenosis and has
considerable promise to become an alternative treatment to carotid
endarterectomy (CEA).
A previous study evaluated the risk of perioperative
stroke, myocardial infarction, and mortaltiy associated with CAS
and thus a quantitative scoring system was established (2). However, that study focused on the
assessment of high-risk patients with CEA and did not include
factors that cannot be controlled, such as age, gender, history of
cardiovascular disease, and characteristics of lesions. Another
study showed that, CAS-associated complications such as vasospasm,
low blood flow dynamics change, acute in-stent thrombosis, and
plaque prolapse, increase the episodes of major adverse cardiac and
cerebrovascular events (MACCE) (3).
In this context, the aim of the present study was to
identify the independent risk factors for MACCE, and establish a
model for risk scoring for CAS. Patients underwent CAS and were
followed up perioperatively. By considering the baseline, disease
characteristics, age, gender and postoperative complications.
Materials and methods
Patients
A total of 403 subjects were included in the study.
There were 298 males and 105 females, and the patient age range was
45–83 years, with an average of 66.73±7.03 years. The patients
underwent CAS in the Department of Neurology, Daping Hospital
affiliated to the Third Military Medical University (Chongqing,
China) between January 2010 and June 2013 were included. The
present study was approved by the Medical Ethics Committee of
Daping Hospital affiliated to the Third Military Medical
University. The purpose and methods of this study were explained to
the patients or their family members and written informed consent
was provided to undergo relevant examinations required for the
current study. The carotid atherosclerotic stenosis was measured as
described in the North American Symptomatic Carotid Endarterectomy
Trial (4). Diagnosis was made using
head and neck computed tomography (CT) angiography and
cerebrovascular digital subtraction angiography (DSA). The
diagnosed carotid atherosclerotic stenosis was stratified into
symptomatic carotid atherosclerotic stenosis (≥50% stenosis) and
asymptomatic atherosclerotic stenosis (≥70% stenosis).
Inclusion and exclusion criteria
Inclusion criteria for the study were as described
in the guidelines for carotid stenting (5): i) asymptomatic carotid atherosclerotic
stenosis patients; ii) symptomatic carotid atherosclerotic stenosis
patients; and iii) patients or their family members who expressed
willingness to participate in the study and signed the informed
consent.
Exclusion criteria for the present study were as
described by Brott et al (1):
i) patients with loss of ipsilateral brain function, and paralysis,
who did not benefit sufficiently from CAS; ii) lesion being
completely occluded and the length of the lesion being >10 mm,
and no blood flow observed at the distal DSA; iii) ipsilateral
intracranial arteriovenous malformations or aneurysms with bleeding
tendency; iv) the incidence of intracerebral hemorrhage in the
previous 3 months, or large cerebral infarction (CI) in the
previous 4 weeks or acute myocardial infarction in the previous 2
weeks; v) severe heart, liver and kidney dysfunction; vi) contrast
agent allergy and other angiographic contraindication; vii) allergy
to aspirin enteric-coated tablets, clopidogrel and other
anti-platelet agents; viii) combination of serial lesion and
anterior circulation, posterior circulation and multiple cerebral
vascular stent implantations; and ix) serious sick sinus syndrome
or atrioventricular block.
Collection of relevant patient
data
Information concerning gender, age, smoking status
and whether the patients had diseases or disorders including
hypertension, diabetes, hypercholesterolemia, coronary cardiopathy,
transient ischemic attack (TIA), symptomatic carotid
atherosclerosis stenosis and CI was collected from the medical
record of the patients.
Basis of diagnosis of disease or
disorders
One week after patients were enrolled, blood samples
were collected aseptically from the elbow vein in the morning and
subjected to analyses of total cholesterol, low-density lipoprotein
cholesterin and blood glucose.
Diagnosis of hypertension
Diagnosis of hypertension was carried out in
accordance with Lloyd-Jones et al (6). Hypertension was defined as systolic
blood pressure (SBP) greater ≥140 mm Hg or diastolic blood pressure
(DBP) greater ≥90 mm Hg, or receiving medication specifically for
the indication of hypertension.
Diabetes mellitus
The diagnostic criterion, i.e., diabetes mellitus,
established by the American Diabetes Association (7) was adhered to in diagnosing diabetes
among patients.
Diagnosis of hypercholesterolemia
The National Cholesterol Education Program Adult
Treatment Panel III (8) was adhered
to in order to identify hypercholesterolemia among patients.
Hypercholesterolemia was diagnosed as total serum cholesterolemia
≥5.20 mmol/l (or ≥200 mg/dl) or low-density lipoprotein cholesterol
≥3.40 mmol/l (or ≥130 mg/dl).
Coronary cardiopathy
Coronary cardiopathy diagnosis was established by
observing the clinical symptoms of patients and subjecting the
patients to electrocardiogram (ECG), treadmill exercise,
echocardiography, coronary CT angiography and coronary
angiography.
Acute myocardial infarction
The diagnosis of acute myocardial infarction was as
described previously (9). The
patients with an increased amount of troponin than normal value
with any of the symptoms of myocardial ischemia were considered to
have acute myocardial infarction. Myocardial ischemia symptoms are
changes in ischemic ECG, pathological Q wave of ECG, abnormalities
in ventricular wall movement or myocardial inactivation (9).
Symptomatic and asymptomatic carotid
atherosclerotic stenosis
Carotid atherosclerotic stenosis was considered
symptomatic when the patients experienced TIA or CI in the previous
6 months (4). The carotid
atherosclerotic stenosis was considered asymptomatic if it
persisted without the incidence of TIA or stroke.
Diagnosis of aortic arch
calcification
Aortic arch calcification was diagnosed through
chest radiography (Philips Digital Radiography, Utrecht, The
Netherlands). If the aortic arch was visible and >1 cm in size
with funicular and curved calcification, then it was considered as
aortic arch calcification (10), as
confirmed by two expert radiologists.
Carotid atherosclerotic plaque
typing
Carotid atherosclerotic plaque typing was performed
using head and neck CT angiography (Lightspeed 64 row spiral CT; GE
Healthcare, Piscataway, NJ, USA, and Brilliance iCT, 256 row;
Philips Healthcare, Cleveland, OH, USA). After a cervical plain
scan, the scanned images were transferred to an AW4.2 processing
workstation (64-slice CT) or EBW processing workstation (256iCT)
and analysis of multiplanar reformation, maximum intensity
projection, volume rendering, vessels was conducted and the carotid
atherosclerotic plaque CT values were measured. If the CT was ≥120
Hounsfield units (Hu), then it was considered as calcified plaque
(11), whereas ≤120 Hu was
considered as non-calcified plaque. If the contrast agent diffused
>1 mm depth along the surface of the plaque in the arterial
lumen, the plaques were considered ulcerative (12).
Determination of aortic arch type
Cerebral angiography was used to identify the aortic
arch type, carotid artery stenosis rate, the extent of lesion,
carotid artery tortuosity and carotid artery occlusion. The total
arterial diameter of the left common carotid artery was taken as a
reference and the vertical distance from the top of the aortic arch
to the opening of the innominate artery was measured. If the
measured distance was similar to the common carotid artery, it was
considered a type I aortic arch; if the distance was 1–2 times more
than that of the common carotid artery, it was considered a type II
aortic arch and if the distance was >2 times that of the common
carotid artery, it was considered a type III aortic arch (13).
Determination of carotid stenosis rate
and length of lesion
The carotid artery stenosis rate was calculated as
described in NASCET (4). The carotid
artery stenosis rate (%) was calculated as: (normal carotid artery
diameter of stenosis - diameter of the most narrow section/diameter
of the normal carotid artery in the stenosis) × 100. The degree of
stenosis was divided into mild (stenosis rate 0–29%), moderate
(stenosis rate 20–69%), severe (stenosis rate 70% and above) and
occlusion (stenosis rate 100%) in which no blood flow was evident
at the distal end of the occlusion. The length of the lesion was
obtained by measuring the vertical scope of the plaque. If the
plaque was continuous, the distance from the top of the plaque to
the bottom thereof was taken to estimate the length of the lesion.
If the plaque was discontinuous, the distance from the top to the
bottom of each plaque was taken and a summation was made to
estimate the total length of the lesion.
Carotid artery tortuosity
Carotid artery tortuosity includes ‘C’-type
tortuosity, ‘S’-type kinking and ‘O’-type coiling. ‘C’-type
tortuosity is characterized by vascular wavy lines (14). ‘S’-type kinking is characterized by
vascular elongation and changed angle and ‘O’-type coiling is
characterized by excessive elongation of vascular tortuosity as ‘O’
configuration (15,16).
CAS
At least 3 days prior to surgery, clopidogrel (75
mg/l) and aspirin (100 mg-200 mg/l) were administered to the
patients daily. Phenobarbital sodium (0.1 g) was administered to
the patients intramuscularly 5 h prior to surgical resection. For 3
days after surgery, fasudil hydrochloride (30 mg) was administered
to the patients in 250 ml of 0.9% saline.
Surgery was carried out by an experienced surgeon
according to the standard procedure for carotid artery angioplasty
and stenting (17). The patient was
placed in a supine position, and after local anesthesia (1%
lidocaine hydrochloride) a Seldinger puncture of the femoral artery
was performed into the 8 F vessel sheath with intraoperative
heparinization and additional heparin (1,000 units) was also added
every 1 h. The 8 F femoral artery was replaced after cerebral
angiography and CAS. The 8F guiding catheter that was exchanged for
300 mm 0.018 guiding wire was positioned in the common carotid
artery. A distal protection device (DPD) was placed inside the
distal internal carotid artery. The DPD comprised AngioGuard
(Cordis, Miami Lakes, FL, USA) and Spider RX (ev3, Plymouth, MN,
USA) and was used according to the blood vessel type. In cases of
excessive vascular tortuosity, the DPD was not placed because it
was unable to reach the distal internal carotid artery.
The diameter of general carotid and internal carotid
arteries were measured prior to and after DPD implantation in
patients who underwent balloon predilation or stenting. Subsequent
to stenting, if the degree of improvement was not satisfactory
(>30%), balloon dilation was performed. Repeat radiography was
performed to determine whether there was any vasospasm, vascular
dissection or distal blood vessel embolization. The DPD was
recycled, the lead wire withdrawn along with the catheter and the
surgery completed.
In cases of bilateral carotid artery stenting,
surgery was performed as described above and side bracket
implantation was utilized. Intraoperative ECG, blood pressure (BP),
and pulse oxygen saturation prior to and after balloon predilation
or stent implantation was measured and BP was monitored every 2
min. The pre- and intraoperative rate and BP were recorded
according to the National Institute of Health Stroke Scale
(https://www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pdf).
Pacemakers in patients with one were monitored.
Diagnosis and removal of common
complications during surgical resection
Vasospasm
Vasospasm was diagnosed through angiography. The
appearance of rough, jagged, wavy and irregular stenosis of the
vascular wall was diagnosed as vascular spasm (18). Vasospasm was mostly localized in the
vicinity of DPD and stent. At the end of surgery, if the vasospasm
of the vascular wall was evident, papaverine hydrochloride (30
mg/20 ml of 0.9% saline) was intravenously administered. After 5
min angiography was carried out to determine whether or not the
vasospasm disappeared.
Hemodynamic depression
The diagnostic criteria for hemodynamic depression
were CAS intraoperative or postoperative symptomatic or
non-symptomatic BP decrease (systolic BP <90 mmHg) and
bradycardia (heart beat rate <60/min) (3), regardless of whether booster or heart
rate-increasing drugs or pacemaker were used. The pacing rate of
preventive use of temporary cardiac pacing in patients was set at
60/min. If intra- and postoperative ECG exhibited an explicit
pacing signal, hemodynamic depression was considered to exist. In
case of intraoperative hemodynamic depression, atropine sulfate
(0.25–0.5 mg) or dopamine hydrochloride (150 mg in 35 ml of 0.9%
saline) was administered. Atropine sulfate was administered in a
discontinuous manner when no rebound of heart rate was
observed.
Other miscellaneous complications
Acute stent thrombosis, carotid dissection, plaque
prolapse, and distal vascular embolism were also observed. However,
because of their extremely low incidence, they were considered for
analyses in the present study.
Postoperative monitoring
Parameters such as ECG, BP and pulse oxygen
saturation were monitored following surgery for ≥24 h. Of these, BP
was monitored once every 5–30 mins. The antihypertensive drugs were
discontinued for hemodialysis patients. If the heart rate was
persistent at <60/min, hydrochloric acid isopropyl epinephrine
(2 mg in 500 ml of 0.9% saline) was administered to increase the
heart rate. If systolic BP was <90 mmHg, dopamine hydrochloride
or a combination of dopamine hydrochloride and hydroxylamine was
administered. Patient consciousness and physical activity were
continuously monitored, and if abnormal, the patients' state was
evaluated through analyses of markers of myocardial injury, ECG,
magnetic resonance imaging or CT examinations. The patients were
treated with clopidogrel (75 mg/day) and aspirin (100–200 mg/day)
for ≥3 months.
Postoperative follow-up
The in-patients were monitored in the hospital
whereas discharged patients were monitored when they visited the
outpatient facility and via telephone enquiries for a period of 30
days post-surgery. The MACCE were considered when the patients
experienced TIA, ischemic or hemorrhagic stroke, acute myocardial
infarction or mortality during the postoperative monitoring period.
Manifestation of stroke observed was stratified into large and
minor, large stroke was 4–5 points on the modified Rankin Scale
(mRS) (19) and included patients
not capable of self-care, walking alone or were bedridden, coupled
with the presence of aphasia or hemianopsia. Minor stroke was 2–3
points on the mRS in which patients had neurological dysfunction,
self-care and absence of hemianopsia and aphasia (20).
Statistical analysis
Chi-square test and independent sample
t-test
The patients were divided into the MACCE and
non-MACCE groups. The mean values of continuous variables were
compared using the independent t-test. In cases of categorical
variables and ≥5 patients, the Pearson Chi-square test was applied.
If the number of patients were <5, the continuity corrected
Chi-square test was used, and for <1, the Fisher's exact test
was used. P<0.05 was considered to indicate a statistically
significant difference.
Unconditional logistic regression analysis
The variables which showed a significant difference
in the Chi-square test and t-test were included in the dual
unconditional logistic regression analysis. The variables were
evaluated for their effect and influence on MACCE. The odds ratio
(OR) and 95% confidence interval (CI) were calculated for
categorical variables. P<0.05 was considered statistically
significant.
Regression model construction
The regression equation was established following
examination of the regression coefficient. The likelihood ratio
test or Wald's Chi-square test was used for single regression
coefficient hypothesis testing and the contribution of the model
was determined. The test of goodness of fit of the regression model
was determined using Hosmer-Lemeshow. The regression equation was
expressed as: log (p) = β0 + β1 × 1 +… βn × n. By calculating the
normalized regression coefficients of individual variables, the
effect of the risk factors associated with the occurrence of MACCE
was analyzed.
Establishment of the risk score table
The risk score table was established from the
regression model. The score was assigned as a decimal number of the
OR value of the risk factor associated with the occurrence of MACCE
or 0.5 after the integer. This table was used to calculate the risk
score and to draw a forecast probability chart of the risk score.
The score table was also used to test the forecast effect by
drawing the receiver operating characteristic curve (ROC) and to
calculate the area under the curve (AUC). The forecast probability
chart was created through MS Excel. ROC, AUC and any other
statistical tests were carried out using SPSS 18.0 (SPSS, Inc.,
Chicago, IL, USA).
Results
Demographics of patients
Of the 433 patients that underwent CAS, 30 patients
were excluded from the present study for various reasons, including
carotid and vertebral artery stenosis in multiple sites together
with stent implantation (16 patients), surgical operation failure
(2 patients), loss of follow-up (12 patients). A total of 403
patients were followed up for 30 days subsequent to surgery.
The patient age range was 45–83 years with an
average of 66.73±7.03 years. The patients aged 60–70 years were
higher in number (40.20%, Table I).
There were 298 males and 105 females. Of the various risk factors,
hypertension was found in 66.00% of patients. Approximately 65.76%
of patients had symptomatic carotid stenosis, 31.76% had TIA and
34.00% had CI.
 | Table I.Baseline characteristics and imaging
features of CAS patients. |
Table I.
Baseline characteristics and imaging
features of CAS patients.
| Characteristics | Numerical value
(n=403) |
|---|
| Baseline
characteristics |
|
|
Demographic data |
|
|
Age (years old,
mean ± SD) | 66.73±7.03 |
|
~80 years old, no.
(%) | 5 (1.24) |
|
70–80 years old,
no. (%) | 150 (37.22) |
|
60–70 years old,
no. (%) | 162 (40.20) |
|
~60 years old, no.
(%) | 86 (21.34) |
|
Male, no. (%) | 298 (73.95) |
|
Female, (%) | 105 (26.05) |
|
Vascular risk factors |
|
|
Hypertension, no.
(%) | 266 (66.00) |
|
Diabetes mellitus,
no. (%) | 108 (26.80) |
|
Hypercholesterolemia,
no. (%) | 88 (21.84) |
|
Smoking, no.
(%) | 159 (39.45) |
|
Coronary cardiopathy, no.
(%) | 102 (25.31) |
|
Symptomatic carotid
stenosis | 265 (65.76) |
|
TIA, no. (%) | 128 (31.76) |
|
Cerebral
infarction, no. (%) | 137 (34.00) |
| Imaging
features |
|
| Aortic
arch calcification, no. (%) | 86 (21.34) |
|
Calcified plaque, no. (%) | 94 (23.33) |
| Ulcer
type plaque, no. (%) | 102 (25.31) |
| Aortic
arch typing |
|
|
Aortic arch type
I, no. (%) | 114 (28.29) |
|
Aortic arch type
II, no. (%) | 240 (59.55) |
|
Aortic arch type
III, no. (%) | 49 (12.16) |
| Carotid
artery tortuosity, no. (%) | 217 (53.85) |
| Carotid
artery stenosis |
|
|
≥70%, no. (%) | 275 (68.24) |
|
<70%, no.
(%) | 128 (31.76) |
| Lesion
length |
|
|
≥15 mm, no.
(%) |
121 (30.02) |
|
<15 mm, no.
(%) | 282 (69.98) |
|
Contralateral carotid
occlusion, no. (%) | 16 (3.97) |
| Lesions
carotid bifurcation, no. (%) | 371 (92.06) |
Clinical diagnoses
Of the 403 patients, 21.34% were found to have
aortic arch calcification, while 23.33% of patients had calcified
plaque and 25.31% had ulcerative plaque. The type II aortic arch
was identified in the majority of patients (59.55%). The
ipsilateral carotid artery tortuosity was observed in 21.34% of
patients with aortic arch calcification. The majority of patients
had severe carotid stenosis (68.24%). The carotid bifurcation
region was the most frequent site of stenosis (92.06%) and most of
the lesions were <15 mm (Table
I).
Perioperative major adverse cardio-
and cerebrovascular events
During the follow-up period, MACCE were observed in
33 (8.19%) patients. Of the 33 patients, 16 patients experienced
any of the following MACCE events: stroke, myocardial infarction or
mortality, and TIA was experienced by 17 patients. The TIA occurred
during the surgery (7 patients) and 1–3 days post-surgery in the
hospital (Table II).
 | Table II.Post-operative adverse cardiovascular
and cerebrovascular events in CAS patients. |
Table II.
Post-operative adverse cardiovascular
and cerebrovascular events in CAS patients.
| Cerebral vascular
event | Values, n=403 |
|---|
| TIA, no. (%) | 17 (4.22) |
| Stroke of any
cause, no. (%) | 15 (3.72) |
| Large
stroke (operative side), no. (%) | 4
(0.99) |
| Large
stroke (non-operative side), no. (%) | 1
(0.25) |
| Minor
stroke (operation side), no. (%) | 9
(2.23) |
| Minor
stroke (non-operation side), no. (%) | 1
(0.25) |
| Myocardial
infarction, no. (%) | 1
(0.25) |
| Death of any cause,
no. (%) | 3
(0.74) |
| Stroke, myocardial
infarction and death, no. (%) | 16 (3.97) |
| TIA, stroke,
myocardial infarction and death, no. (%) | 33 (8.19) |
Comparison of baseline and clinical
parameters between the MACCE and non-MACCE groups
No significant difference in the average age of
patients between the MACCE and non-MACCE groups was identified.
However, in the subgroup of ≥70 years, MACCE was observed in 28
(84.85%) of 33 patients, which showed its significant association
with the incidence of MACCE (P<0.001). The remaining clinical
parameters significantly associated with the incidence of MACCE
included diabetes mellitus, coronary cardiopathy and symptomatic
carotid stenosis (P<0.05, Table
III).
 | Table III.Baseline characteristics of the MACCE
and non-MACCE groups. |
Table III.
Baseline characteristics of the MACCE
and non-MACCE groups.
|
Characteristics | Non-MACCE group
(n=370) | MACCE group
(n=33) | Statistic | P-value |
|---|
| Age (years old,
mean ± SD) | 69.00±6.85 | 66.53±7.02 | t=1.939 | 0.053 |
| ≥70
years old, no. (%) | 126 (34.05) | 28 (84.85) |
χ2=33.108 |
<0.001a |
| <70
years old, no. (%) | 244 (65.95) | 5 (15.15) |
|
|
| Gender |
|
|
|
|
| Male,
no. (%) | 277 (74.86) | 21 (63.64) |
χ2=1.983 | 0.159 |
| Female,
no. (%) | 93
(25.14) | 12 (36.36) |
|
|
| Hypertension, no.
(%) | 241 (65.14) | 25 (75.76) |
χ2=1.524 | 0.217 |
| Diabetes mellitus,
no. (%) | 93
(25.14) | 15 (45.45) |
χ2=6.377 | 0.012a |
| Smoking, no.
(%) | 145 (39.19) | 14 (42.42) |
χ2=0.133 | 0.716 |
|
Hypercholesterolemia, no. (%) | 78
(21.08) | 10 (30.30) |
χ2=1.510 | 0.219 |
| Coronary
cardiopathy, no. (%) | 84
(22.70) | 18 (54.55) |
χ2=16.251 |
<0.001a |
| Symptomatic carotid
stenosis (TIA, cerebral infarction), no. (%) | 237 (64.05) | 28 (84.85) |
χ2=5.818 | 0.016a |
In addition to these results, ulcerative plaque,
type III aortic arch, severe stenosis, longer lesion and carotid
artery occlusion were significantly associated with the incidence
of MACCE (P<0.05, Table IV).
 | Table IV.Comparison of imaging features of the
MACCE and non-MACCE groups. |
Table IV.
Comparison of imaging features of the
MACCE and non-MACCE groups.
|
Characteristics | Non-MACCE group
(n=370) | MACCE group
(n=33) | Statistic | P-value |
|---|
| Aortic arch
calcification, no. (%) | 78 (21.08) | 8 (24.24) |
χ2=0.180 | 0.671 |
| Patch
properties |
|
|
χ2=2.013 | 0.156 |
|
Calcified plaque, no. (%) | 83 (22.43) | 11 (33.33) |
|
|
|
Non-calcified plaque, no.
(%) | 287 (77.57) | 22 (66.67) |
|
|
| Ulcer type plaque,
no. (%) | 83 (22.43) | 19 (57.58) |
χ2=19.794 |
<0.001a |
| Aortic arch
typing |
|
|
χ2=7.700 | 0.021a |
| I, no.
(%) | 106 (28.65) | 8 (24.24) |
|
|
| II, no.
(%) | 224 (60.54) | 16 (48.49) |
|
|
| III,
no. (%) | 40 (10.81) | 9 (27.27) |
|
|
| Carotid artery
tortuosity | 194 (52.43) | 21 (63.64) |
χ2=1.528 | 0.216 |
| Carotid artery
stenosis |
|
|
χ2=4.575 | 0.032a |
| ≥70%,
no. (%) | 247 (66.76) | 28 (84.85) |
|
|
|
<70%, no. (%) | 123 (33.24) | 5 (15.15) |
|
|
| Lesion length |
|
|
χ2=5.830 | 0.016a |
| ≥15 mm,
no. (%) | 105 (28.38) | 16 (48.48) |
|
|
| <15
mm, no. (%) | 265 (71.62) | 17 (51.52) |
|
|
| Contralateral
carotid occlusion, no. (%) | 12 (3.24) | 4 (12.12) |
χ2=4.151 | 0.042a |
| Lesions cross
bifurcation, no. (%) | 343 (92.70) | 28 (84.85) |
χ2=2.557 | 0.110 |
Intraoperative situation between MACCE
and non-MACCE groups
The DPD was used in 389 (96.53%) patients but not in
14 patients because of severe tortuosity of the internal carotid
artery. A majority (18.18%) of patients in the MACCE group received
bilateral carotid stenting in comparison with patients in the
non-MACCE group (5.41%). The incidence of intraoperative
hemodynamic depression was higher in the MACCE group at 31 (93.94%)
of 33 compared to that of the non-MACCE group at 210 of 370
patients (56.76%, Table V).
 | Table V.Comparison of intraoperative
situation of the MACCE and non-MACCE groups. |
Table V.
Comparison of intraoperative
situation of the MACCE and non-MACCE groups.
|
Characteristics | Non-MACCE group
(n=370) | MACCE group
(n=33) | Statistic | P-value |
|---|
| DPD use, no.
(%) | 359 (97.03) | 30 (90.91) |
χ2=1.803 | 0.179 |
| DPD type, no.
(%) |
|
|
|
|
|
Angioguard | 164 (44.33) | 9 (27.27) |
χ2=2.757 | 0.097 |
|
Spider | 195 (52.70) | 21 (63.64) |
|
|
| Bracket type, no.
(%) |
|
|
χ2=3.350 | 0.187 |
|
Precise | 212 (57.30) | 14 (42.42) |
|
|
|
Protégé | 143 (38.65) | 16 (48.48) |
|
|
| Smart
Control | 15 (4.05) | 3 (9.09) |
|
|
| Balloon pre
expansion, no. (%) | 47 (12.70) | 8 (24.24) |
χ2=3.423 | 0.064 |
| Bilateral carotid
stenting, no. (%) | 20 (5.41) | 6 (18.18) |
χ2=8.195 | 0.004a |
| Intravascular
operation time, no. (%) |
|
|
χ2=0.988 | 0.320 |
| <30
min | 276 (74.59) | 22 (66.67) |
|
|
| ≥30
min | 94 (25.41) | 11 (33.33) |
|
|
| Vasospasm, no.
(%) | 52 (14.05) | 6 (18.18) |
χ2=0.419 | 0.517 |
| Hemodynamic
depression, no. (%) | 210 (56.76) | 31 (93.94) |
χ2=15.912 |
<0.001a |
Additive effect of risk factors on the
incidence of MACCE
The parameters that were significantly associated
with the incidence of MACCE were included in the dual
non-conditional logistic regression analysis. After removing the
confounding factors, only age ≥70 years, ulcerative plaque, severe
stenosis, bilateral carotid stenting and hemodynamic depression
were found to be independently associated with the incidence of
MACCE (Table VI).
 | Table VI.Association of risk factors with
MACCE. |
Table VI.
Association of risk factors with
MACCE.
| Risk factor | B | SE | Wals | OR (95% CI) | P-value |
|---|
| Age (≥70)
years | 1.609 | 0.571 | 7.949 | 4.997
(1.633–15.290) | 0.005 |
| Ulcerative
plaque | 1.064 | 0.444 | 5.740 | 2.899
(1.214–6.924) | 0.017 |
| Severe
stenosis | 1.245 | 0.568 | 4.807 | 3.472
(1.141–10.566) | 0.028 |
| Bilateral carotid
stenting | 1.611 | 0.628 | 6.575 | 5.007
(1.462–17.151) | 0.010 |
| HD | 1.757 | 0.792 | 4.915 | 5.792
(1.226–27.369) | 0.027 |
The effect of the aforementioned risk factors on the
incidence of MACCE was expressed using the regression equation: Log
(MACCE incidence probability) = −8.992 + 1.609 × age ≥70 + 1.064 ×
ulcerative plaque + 1.245 × severe stenosis + 1.611 × bilateral
carotid stenting + 1.757 × hemodynamic depression
(χ2=163.478, P<0.001).
These findings showed that, age, ulcerative plaque,
severe stenosis, bilateral carotid stenting and hemodynamic
depression were significantly associated with the incidence of
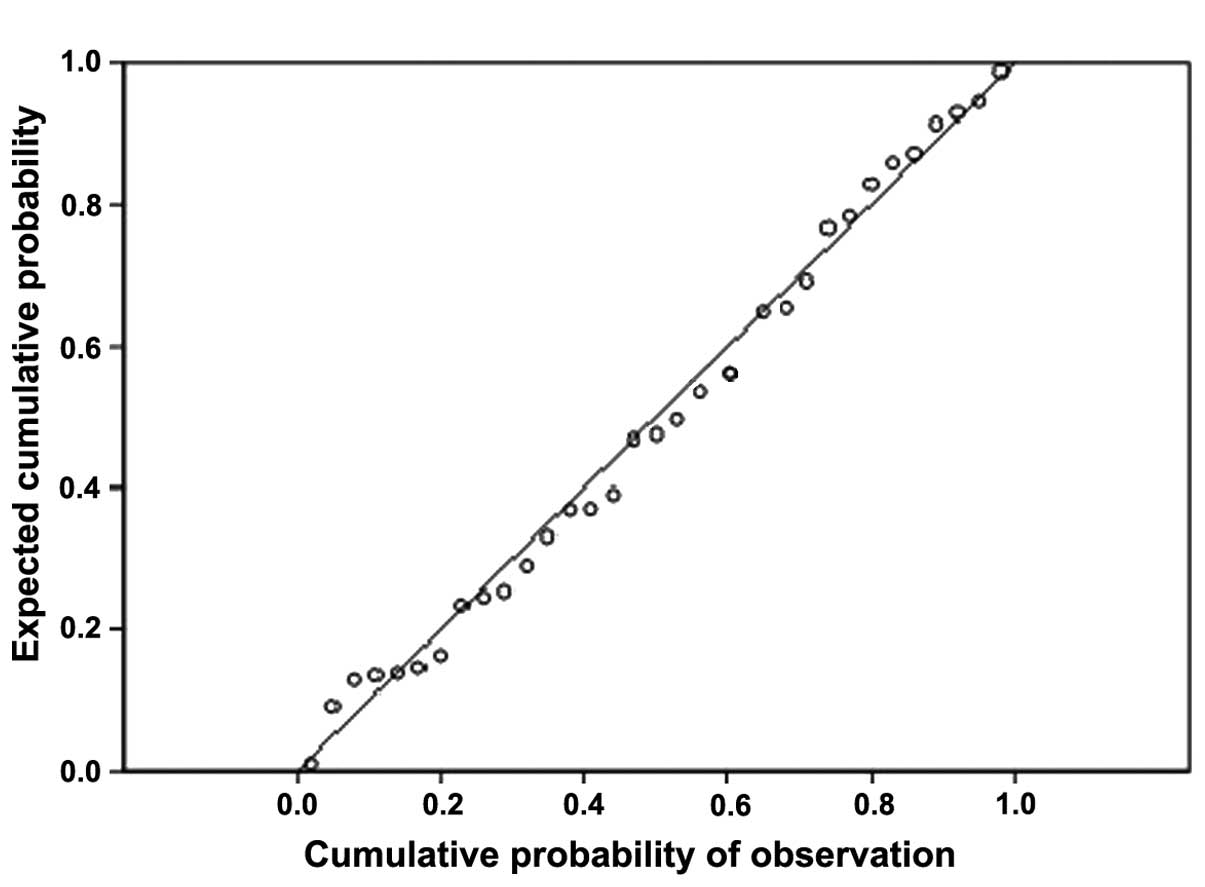
MACCE. The cumulative probability plot (Fig. 1) shows that, the scatter is basically
diagonal and the Hosmer-Lemeshow goodness-of-fit test indicated the
model has good fitting effect.
The standard regression coefficient analysis
revealed that, hemodynamic depression was the most prominent risk
factor for MACCE followed by bilateral carotid artery, age ≥70
years, severe stenosis and ulcerative plaque (Table VII).
 | Table VII.MACCE in perioperative period of
CAS. |
Table VII.
MACCE in perioperative period of
CAS.
|
Characteristics | B j | S j | Standardized
regression coefficient |
|---|
| Age (≥70)
years | 1.609 | 0.571 | 0.502 |
| Ulcerative
plaque | 1.064 | 0.444 | 0.258 |
| Severe
stenosis | 1.245 | 0.568 | 0.386 |
| Bilateral carotid
stenting | 1.611 | 0.628 | 0.553 |
| HD | 1.757 | 0.792 | 0.760 |
Risk score table for MACCE
The risk scores of the risk factors for MACCE were:
5 points for age ≥70 years, 3 points for ulcerative plaques, 3.5
points for severe stenosis, 5 points for bilateral carotid artery
stenting and 6 points for hemodynamic depression.
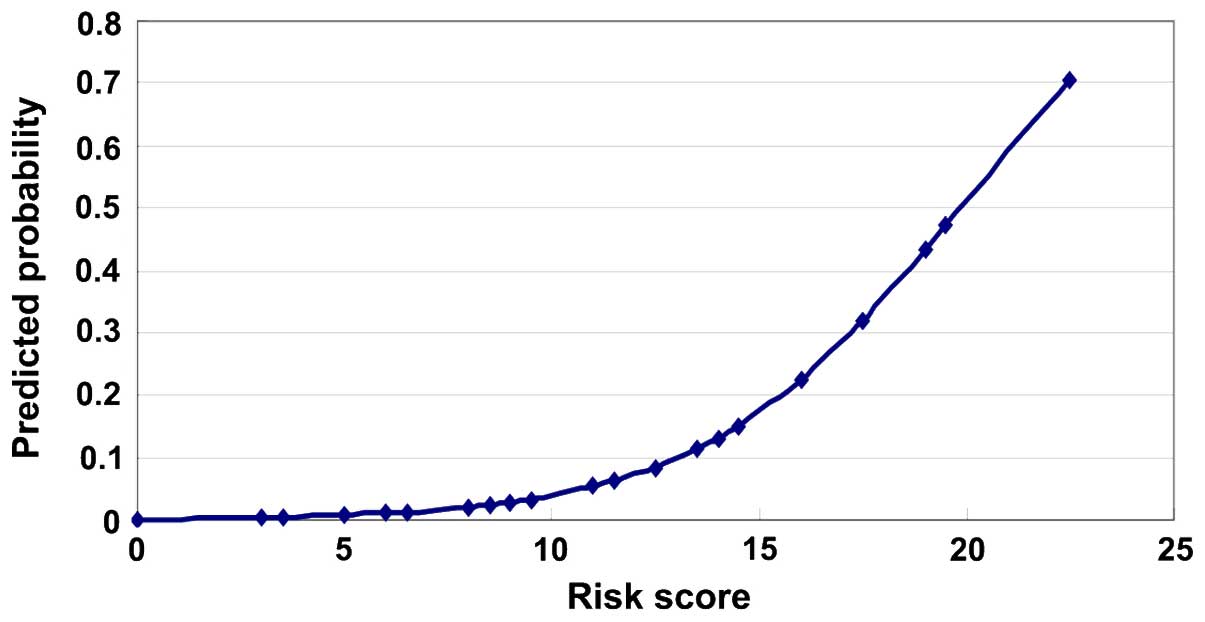
These points were used to calculate each of the 403
patient risk scores and create the forecast probability chart. The
risk of incidence of MACCE increased with an increasing risk score
(Fig. 2).
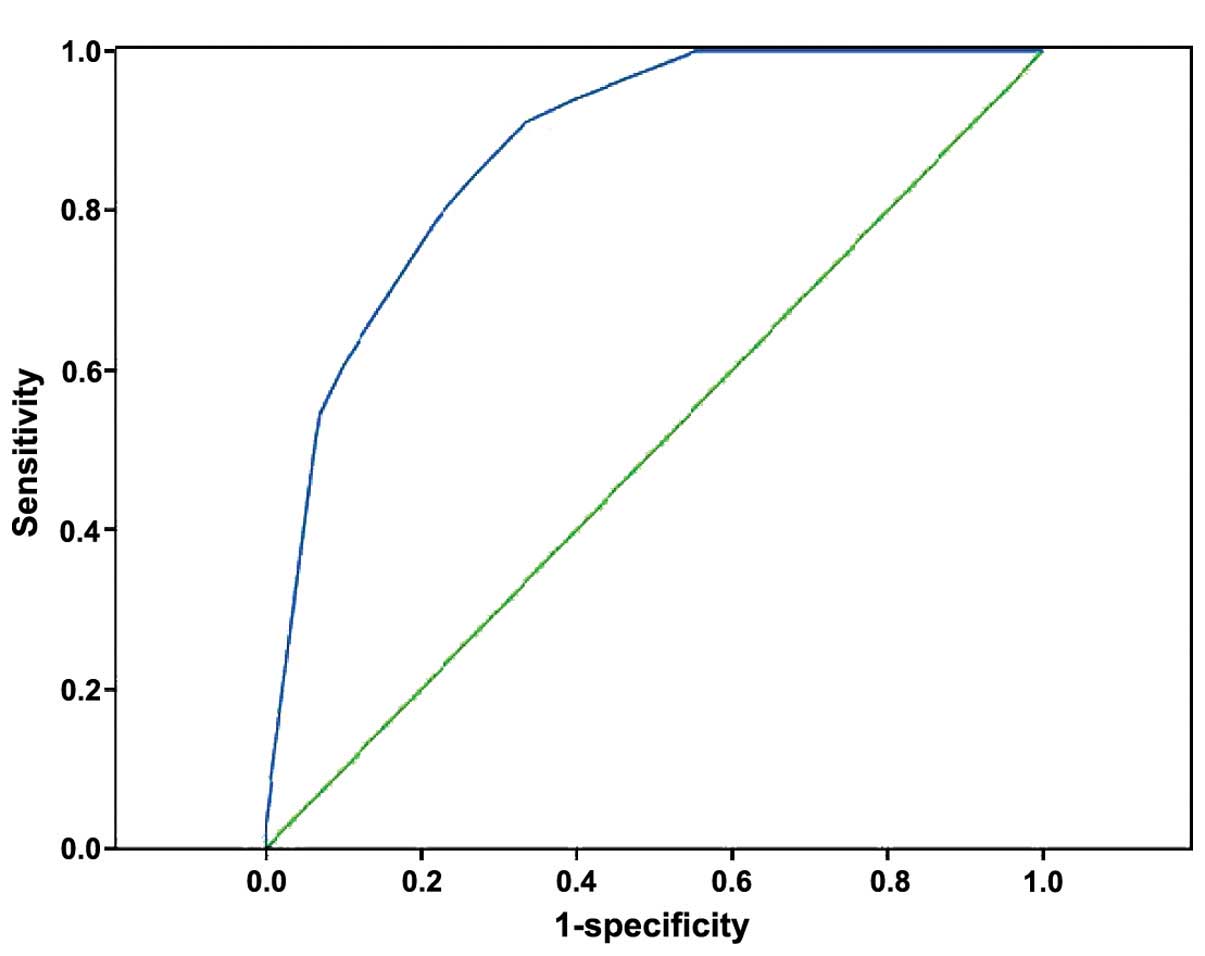
To validate the predictive effect of risk scores,
the ROC curve was drawn and AUC was found to be 0.875 (P<0.001,
95% CI: 0.825–0.925), which indicated that a good forecast effect
of this risk score model for MACCE (Fig.
3).
Discussion
Currently, stroke ranks fourth among all causes of
mortality in the United States and is recognized as a leading cause
of serious physical and cognitive long-term disability in adults.
Almost 800,000 US residents experience an incident or recurrent
stroke each year (21). In China,
the annual incidence of stroke is approximately 2 million, of which
1.5 million patients succumb, while the majority of survivors (75%)
become disabled. Additionally, the 5-year survival rate is 41%
(22). Ischemic stroke was involved
in 75–85% of all stroke incidences for which carotid
atherosclerotic stenosis is one of the major contributing factors.
After decades of clinical practices, CEA was considered the gold
standard for the treatment of carotid atherosclerotic stenosis.
Continuous advancements in the field of neuroimaging and vascular
operation technology in the last decade have led to CAS potentially
becoming an alternative treatment to CEA for carotid
atherosclerotic stenosis (23).
However, there is a lack of sufficient evidence to verify this
hypothesis as the MACCE were not less in CAS than CEA during the
perioperative period (24).
Consequently, CAS is often recommended as an alternative treatment
method to CEA only in high-risk patients (25).
Previous findings have suggested that, the incidence
of ipsilateral stroke was extremely low in patients who underwent
CAS in comparison with CEA (26).
Therefore, we carried out risk assessment for MACCE during the
perioperative period of CAS that would reduce MACCE during the
perioperative period of CAS by providing intervention to
complications that were significantly associated with MACCE.
However, investigations pertaining to the risk of stroke,
myocardial infarction and death during the CAS perioperative period
are scarce (2).
In the present study, MACCE were observed in 8.19%
of patients who underwent CAS, of which 3.97% experienced stroke,
myocardial infarction or mortality. This incidence rate was similar
to that reported worldwide (27).
Although the univariate analysis revealed 11 risk factors (age,
diabetes, coronary heart disease, symptomatic carotid artery
stenosis, ulcerative plaque, type III aortic arch, severe carotid
artery stenosis, longer lesion, carotid artery occlusion, bilateral
carotid artery stenting and hypertension) associated with the
incidence of MACCE, the multivariate analysis revealed the
association of only five risk factors (age ≥70, ulcerative plaque,
bilateral carotid artery stenting, severe stenosis and
hypertension) for the incidence of MACCE and a potent risk
model.
In a previous study, age was found to be an
independent risk factor associated with postoperative complications
(28), because elderly patients are
more prone to artery tortuosity and vascular calcification
(29). The instability of ulcerative
plaque increases the risk of TIA and ischemic stroke caused by
thromboembolism during or after surgical resection (30). There is a high risk of
thromboembolism during endovascular surgery where the catheter,
guidewire and DPD are used to reach the lesion.
The present study also showed that bilateral carotid
artery stenting is a risk factor of MACCE in CAS patients. The
operation time in the blood vessels was prolonged subsequent to
bilateral stent release, thus, cerebral perfusion increased, which
ultimately leads to increased risk of thrombosis or cerebral
hemorrhage. Severe carotid stenosis was also found to be a risk
factor for MACCE. Due to preoperative cerebral hypoperfusion and
ascertaining adverse collateral circulation following the discharge
of bracket, severe carotid stenosis is more prone to cerebral
hyperperfusion leading to hemorrhagic stroke.
Previous findings have shown that, hypertension is
linked with other conditions such as coronary disease (31), type of aortic arch (32), carotid artery tortuosity,
calcification (33), balloon
predilation, bilateral stent implantation and other factors
(34). In the present study, the
interaction between these factors constitutes hypertension as a
potent risk factor. A possible reason for observing CI and
myocardial infarction in the current study was the decreased BP and
bradycardia caused by the intra- and postoperative slowdown of
clearance of emboli and plaque debris that weaken the stenosis
vascular tortuosity of collateral circulation, thereby increasing
the risk of cerebral ischemia and myocardial infarction.
The results of the present study established a risk
score model for MACCE that may be used to quantify the risk for
MACCE in CAS patients through which effective measures can be taken
to reduce the incidence of MACCE in CAS patients during their
perioperative period.
References
|
1
|
Brott TG, Halperin JL, Abbara S, Bacharach
JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G, et
al: American College of Cardiology Foundation/American Heart
Association Task Force on Practice Guidelines: American Stroke
Association; American Association of Neuroscience Nurses; American
Association of Neurological Surgeons; American College of
Radiology; American Society of Neuroradiology; Congress of
Neurological Surgeons; Society of Atherosclerosis Imaging and
Prevention; Society for Cardiovascular Angiography and
Interventions; Society of Interventional Radiology; Society of
NeuroInterventional Surgery; Society for Vascular Medicine; Society
for Vascular Surgery; American Academy of Neurology and Society of
Cardiovascular Computed Tomography: 2011
ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/CAI/SIR/SNIS/SVM/SVS
guideline on the management of patients with extracranial carotid
and vertebral artery disease: executive summary. Stroke.
42:e420–e463. 2011.PubMed/NCBI
|
|
2
|
Setacci C, Chisci E, Setacci F, Iacoponi
F, de Donato G and Rossi A: Siena carotid artery stenting score: a
risk modelling study for individual patients. Stroke. 41:1259–1265.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta R, Abou-Chebl A, Bajzer CT,
Schumacher HC and Yadav JS: Rate, predictors, and consequences of
hemodynamic depression after carotid artery stenting. J Am Coll
Cardiol. 47:1538–1543. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
North American Symptomatic Carotid
Endarterectomy Trial Collaborators: Beneficial effect of carotid
endarterectomy in symptomatic patients with high-grade carotid
stenosis. N Engl J Med. 325:445–453. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Interventional radiology group of
Radiology Branch of Chinese Medical Association: Interventional
treatment for carotid stenosis (expert consensus). Zhonghua Fang
She Xue Za Zhi. 44:995–998. 2010.
|
|
6
|
Lloyd-Jones DM, Evans JC and Levy D:
Hypertension in adults across the age spectrum: Current outcomes
and control in the community. JAMA. 294:466–472. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Expert Committee on the Diagnosis and
Clasification of Diabetes Mellitus: American Diabetes Association:
clinical practice recommendations 2002. Diabetes Care. 25(Suppl 1):
S1–S147. 2002.PubMed/NCBI
|
|
8
|
National Cholesterol Education Program
(NCEP) Expert Panel on Detection, Evaluation, and Treatment of High
Blood Cholesterol in Adults (Adult Treatment Panel III): hird
Report of the National Cholesterol Education Program (NCEP) Expert
Panel on Detection, Evaluation, and Treatment of High Blood
Cholesterol in Adults (Adult Treatment Panel III) final report.
Circulation. 106:3143–3421. 2002.PubMed/NCBI
|
|
9
|
No authors listed: Myocardial infarction
redefined – a consensus document of The Joint European Society of
Cardiology/American College of Cardiology Committee for the
redefinition of myocardial infarction. Eur Heart J. 21:1502–1513.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li J, Galvin HK, Johnson SC, Langston CS,
Sclamberg J and Preston CA: Aortic calcification on plain chest
radiography increases risk for coronary artery disease. Chest.
121:1468–1471. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Das M, Braunschweig T, Mühlenbruch G,
Mahnken AH, Krings T, Langer S, Koeppel T, Jacobs M, Günther RW and
Mommertz G: Carotid plaque analysis: Comparison of dual-source
computed tomography (CT) findings and histopathological
correlation. Eur J Vasc Endovasc Surg. 38:14–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi W, Guo W, Yue M and Guo Q: Use 256
slice spiral CT angiography of carotid artery to evaluate ulcer
plaque. Zhongguo Jieru Yingxiang Yu Zhiliaoxue. 9:354–357.
2012.
|
|
13
|
Madhwal S, Rajagopal V, Bhatt DL, Bajzer
CT, Whitlow P and Kapadia SR: Predictors of difficult carotid
stenting as determined by aortic arch angiography. J Invasive
Cardiol. 20:200–204. 2008.PubMed/NCBI
|
|
14
|
Metz H, Murray-Leslie RM, Bannister RG,
Bull JW and Marshall J: Kinking of the internal carotid artery.
Lancet. 1:424–426. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weibel J and Fields WS: Tortuosity,
coiling, and kinking of the internal carotid artery. II.
Relationship of morphological variation to cerebrovascular
insufficiency. Neurology. 15:462–468. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jia X: DSA imaging findings, clinical
features and interventional therapy of intracranial and
intracranial arteriosclerosis. Third Military Medical University.
Chongqing: 2010.
|
|
17
|
Powers CJ, Hirsch JA, Hussain MS,
Patsalides AT, Blackham KA, Narayanan S, Lee SK, Fraser JF, Bulsara
KR, Prestigiacomo CJ, Gandhi CD, Abruzzo T, Do HM, Meyers PM,
Albuquerque FC, Frei D, Kelly ME, Pride GL and Jayaraman MV:
Standards and Guidelines committee of the Society of
NeuroInterventional Surgery: Standards of practice and reporting
standards for carotid artery angioplasty and stenting. J
Neurointerv Surg. 6:87–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vijayvergiya R, Otaal PS, Bagga S and Modi
M: Symptomatic carotid vasospasm caused by a distal-protection
device during stent angioplasty of the right internal carotid
artery. Tex Heart Inst J. 37:226–229. 2010.PubMed/NCBI
|
|
19
|
van Swieten JC, Koudstaal PJ, Visser MC,
Schouten HJ and van Gijn J: Interobserver agreement for the
assessment of handicap in stroke patients. Stroke. 19:604–607.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Topakian R, Strasak AM, Sonnberger M,
Haring HP, Nussbaumer K, Trenkler J and Aichner FT: Timing of
stenting of symptomatic carotid stenosis is predictive of 30-day
outcome. Eur J Neurol. 14:672–678. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koton S, Schneider AL, Rosamond WD, Shahar
E, Sang Y, Gottesman RF and Coresh J: Stroke incidence and
mortality trends in US communities, 1987 to 2011. JAMA.
312:259–268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Neurology branch of Chinese Medical
Association: China cerebrovascular disease prevention and control
guide. People's Health Press. Beijing: 52007.
|
|
23
|
van der Vaart MG, Meerwaldt R, Reijnen MM,
Tio RA and Zeebregts CJ: Endarterectomy or carotid artery stenting:
the quest continues. Am J Surg. 195:259–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ederle J, Featherstone RL and Brown MM:
Randomized controlled trials comparing endarterectomy and
endovascular treatment for carotid artery stenosis: A Cochrane
systematic review. Stroke. 40:1373–1380. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
European Stroke Organisation (ESO)
Executive Committee and ESO Writing Committee: Guidelines for
management of ischaemic stroke and transient ischaemic attack 2008.
Cerebrovasc Dis. 25:457–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goldstein LB, Adams R, Alberts MJ, Appel
LJ, Brass LM, Bushnell CD, Culebras A, Degraba TJ, Gorelick PB,
Guyton JR, et al: American Heart Association/American Stroke
Association Stroke Council; Atherosclerotic Peripheral Vascular
Disease Interdisciplinary Working Group; Cardiovascular Nursing
Council; Clinical Cardiology Council; Nutrition, Physical Activity,
and Metabolism Council; Quality of Care and Outcomes Research
Interdisciplinary Working Group; American Academy of Neurology:
Primary prevention of ischemic stroke: a guideline from the
American Heart Association/American Stroke Association Stroke
Council: cosponsored by the Atherosclerotic Peripheral Vascular
Disease Interdisciplinary Working Group; Cardiovascular Nursing
Council; Clinical Cardiology Council; Nutrition, Physical Activity,
and Metabolism Council; and the Quality of Care and Outcomes
Research Interdisciplinary Working Group: the American Academy of
Neurology affirms the value of this guideline. Stroke.
37:1583–1633. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Touzé E, Trinquart L, Chatellier G and Mas
JL: Systematic review of the perioperative risks of stroke or death
after carotid angioplasty and stenting. Stroke. 40:e683–e693. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kastrup A, Gröschel K, Schnaudigel S,
Nägele T, Schmidt F and Ernemann U: Target lesion ulceration and
arch calcification are associated with increased incidence of
carotid stenting-associated ischemic lesions in octogenarians. J
Vasc Surg. 47:88–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bazan HA, Pradhan S, Mojibian H,
Kyriakides T and Dardik A: Increased aortic arch calcification in
patients older than 75 years: Implications for carotid artery
stenting in elderly patients. J Vasc Surg. 46:841–845. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Montorsi P, Caputi L, Galli S, Ciceri E,
Ballerini G, Agrifoglio M, Ravagnani P, Trabattoni D, Pontone G,
Fabbiocchi F, et al: Microembolization during carotid artery
stenting in patients with high-risk, lipid-rich plaque. A
randomized trial of proximal versus distal cerebral protection. J
Am Coll Cardiol. 58:1656–1663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mlekusch W, Schillinger M, Sabeti S,
Nachtmann T, Lang W, Ahmadi R and Minar E: Hypotension and
bradycardia after elective carotid stenting: Frequency and risk
factors. J Endovasc Ther. 10:851–859; discussion 860–861. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Taha MM, Toma N, Sakaida H, Hori K, Maeda
M, Asakura F, Fujimoto M, Matsushima S and Taki W: Periprocedural
hemodynamic instability with carotid angioplasty and stenting. Surg
Neurol. 70:279–285; discussion 285–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jeon JS, Sheen SH and Hwang G: Hemodynamic
instability during carotid angioplasty and stenting-relationship of
calcified plaque and its characteristics. Yonsei Med J. 54:295–300.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lian X, Lin M, Zhu S, Liu W, Li M, Sun W,
Yin Q, Xu G, Zhang R and Liu X: Risk factors associated with
haemodynamic depression during and after carotid artery stenting. J
Clin Neurosci. 18:1325–1328. 2011. View Article : Google Scholar : PubMed/NCBI
|