Introduction
Sepsis is a common complication of severe trauma,
large area burns, severe shock, serious infection and surgery.
Sepsis can progress to septic shock, acute respiratory distress
syndrome (ARDS), acute kidney injury (AKI), and multiple organ
dysfunction syndrome (MODS), which is one of the major causes of
mortality in critically ill patients (1). However, whether sepsis causes mortality
is mainly determined by the release intensity of inflammatory
factors and the response to inflammation (2). Sepsis bundle therapy is considered the
main treatment method, including removing etiological factors,
fluid resuscitation, use of vasoactive drugs, effective antibiotic
use, immune regulation and hemofiltration (3). However, the mortality rate of severe
sepsis or septic shock remains high, and ranges 30–50% (4).
Previous studies suggested that the pathogenesis of
sepsis involves inflammation, coagulation and immune reactivity
(5). Sepsis is a serious stage of
bacterial infection, which involves the release of many types of
inflammatory factors, such as tumor necrosis factor-α (TNF-α)
(6), interleukin-1β (IL-1β)
(7), IL-6 (8) and high mobility group protein box 1
(HMGB1) (9). Statins are widely used
in the regulation of lipid metabolism. Low-molecular-weight heparin
(LMWH) is widely used as an anticoagulant drug. Previous findings
showed that statins are beneficial to patients with sepsis and LMWH
improves the prognosis of patients with sepsis (9,10).
However, the mechanism and efficacy of statins combined with LMWH
in the treatment of sepsis has not been reported.
In the present study, the rats underwent cecal
ligation and puncture (CLP). We evaluated the effect of
atorvastatin combined with LMWH on early and late inflammatory
plasma cytokine levels, pulmonary pathophysiology and the
cumulative mortality rate of rats with sepsis, to clarify the
mechanism of statins combined with LMWH in the inflammatory process
of sepsis. Thus, the results provide a theoretical basis for the
clinical treatment of sepsis with combined statins and LMWH.
Materials and methods
Equipment
The DS-88 electronic scale was purchased from Wuhan
Automation Instrument Factory (Wuhan, China). The high-speed
refrigerated centrifuge was purchased from Eppendorf AG (Hamburg,
Germany) and Beckman Coulter, Inc. (Brea, CA, USA). The SHH WZI600S
digital display - three electric thermostatic water bath box was
purchased from the Shanghai Medical Equipment factory (Shanghai,
China). The Versa Max MR96A enzyme mark instrument was purchased
from Shenzhen Mindray Bio-Medical Electronics Co., Ltd. (Shenzhen,
China). NanoDrop 2000c ultraviolet spectrophotometer was purchased
from Thermo Fisher Scientific, Inc. (Wilmington, DE, USA). The
inverted microscope was purchased from Olympus (Tokyo, Japan) and
the light microscope was purchased from Carl Zeiss AG A2
(Oberkochen, Germany).
Materials and reagents
Atorvastatin (Lipitor), 20 mg/plate was purchased
from Pfizer, Inc. (Dalian, China), national drug approval no.
J20130129. LMWH (Fraxiparine), 0.4 ml/tube, was purchased from
Sanofi Winthrop Industrie SA (Paris, France), approval no.
H20140349. The anesthetic purchased was 3% pentobarbital sodium.
Surgical supplies included Ethicon braided non-absorbable sutures
(black), 15×60 cm, purchased from Johnson & Johnson
(Somerville, NJ, USA). The KGERCl02a KeyGen rat, TNF-α, and IL-1β
enzyme-linked immunosorbent assay (ELISA) kit, were purchased from
Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). The HMGB1 ELISA
kit was purchased from Beijing Wantai Biological Pharmaceutical
Co., Ltd. (Beijing, China).
Animal groupings and treatment
In total, 122 healthy male Sprague-Dawley rats
weighing 178–235 g, were procured from the Laboratory Animal Center
of Beijing Huafu Medical-Technological Co. (animal batch no.
scxk2014-0004). The rats were housed at 20–26°C, maintained in
40–70% air humidity and with a natural length of day and night
cycle in the laboratory. Five rats were placed in each cage
(500×300×200 mm) with free activity and free access to complete
formula feed (Jinan Xingkang Biotechnology Co., Ltd., Jinan, China)
and water. The rats were divided randomly into five groups,
including the sham operation group (n=10), CLP group (n=10),
atorvastatin group (n=34), LMWH group (n=34). The study was
approved by the ethics committee of Shandong University (Shandong,
China).
For the sham operation group, rats were administered
starch solution (40 mg/kg·day dissolved in 2 ml 0.9% NaCl). The
suspension was given through intragastric infusion for 5 days. The
rats were fasted 12 h prior to surgery. The rats were anesthetized
by injection of 3% pentobarbital sodium (45 mg/kg body weight,
intraperitoneally) and a sterile 2-cm ventral midline incision was
performed. The cecum was disturbed and returned to the abdomen. The
abdominal incision was closed in layers. The rats were then housed
with free activity and feed.
The CLP rats underwent the same intragastric
infusion procedure as described above prior to surgery. The rat
model of sepsis was performed by CLP (12).
For the atorvastatin group, atorvastatin was ground
with a mortar. Atorvastatin suspensions were prepared (20 mg/kg·day
of atorvastatin dissolved in 2 ml 0.9% NaCl). The suspension was
given through intragastric infusion for 5 days. The rat model of
sepsis was then performed by CLP (11).
The LMWH rats were administered starch suspension
(40 mg/kg·d dissolved in 2 ml 0.9% NaCl). The suspension was given
through intragastric infusion for 5 days. LMWH heparin was injected
by hypodermic needle (100 IU/kg·day) for 5 days. The rat model of
sepsis was performed by CLP (11).
For the atorvastatin combined with LMWH group,
atorvastatin suspensions were prepared (20 mg/kg·day of
atorvastatin dissolved in 2 ml 0.9% NaCl). The suspension was given
through intragastric infusion, and LMWH was injected using a
hyperdermic needle (100 IU/kg·day) for 5 days. The rat models of
sepsis was performed by CLP.
CLP rats were fasted 12 h before surgery (12). The rats were anesthetized by
injection of 3% pentobarbital sodium (45 mg/kg body weight,
intraperitoneally) and a sterile 2-cm ventral midline incision was
performed. The cecum was exposed and ligated with 30% nitrile silk,
the intestinal mesenteric vessel was avoided while ligating, the
cecum was punctured twice (3 mm region between them) with a 9-gauge
needle. The abdominal incision was closed in layers. Normal saline
(3 ml/100 g body weight) was given subcutaneously immediately to
prevent dehydration.
Sepsis severity assessment
standards
The severity of sepsis of the animals was scored
according to modified sepsis severity assessment standards
(Table I).
 | Table I.Evaluation criterion of sepsis
severity for rats after CLP. |
Table I.
Evaluation criterion of sepsis
severity for rats after CLP.
| Observation
index | Scoring criteria | Score value |
|---|
| General
condition | Normal food intake
and activity | 1 |
|
| Eating less,
tiredness, reduced activity | 2 |
|
| Antifeedant,
somnolence, inactive | 3 |
|
| Lethargy, lower
reaction to stimuli | 4 |
| Bowel condition | Ruddy bloom,
euperistalsis | 1 |
|
| Congestion, mild
expansion, euperistalsis | 2 |
|
| Obfuscation, moderate
expansion, poor motility | 3 |
|
| Purple, high
expansion, loss of peristalsis | 4 |
| Ascites condition
(ml) | 3, clear ascites, no
smell | 1 |
|
| 3–6, opacity ascites,
no smell | 2 |
|
| >6, bloody
ascites, micro-odor, odorous | 3 |
|
| >6, purulent
ascites, severe odor | 4 |
| Cecal ligation
condition | Edema, ischemia | 1 |
|
| Black, necrosis | 2 |
|
| White, gangrene | 3 |
|
| Gangrene, surrounded
by a large amount of pus | 4 |
| Lesion encapsulation
condition | Not wrapped around,
no necrosis or exudation around | 1 |
|
| Wrapped by greater
omentum and intestine | 2 |
|
| Not wrapped, obvious
necrosis and exudation around | 3 |
|
| Pus diffusion, not
wrapped around | 4 |
| Liver volume (%,
compared to the control group) | 0–20 | 1 |
|
| 20–40 | 2 |
|
| 40–60 | 3 |
|
| >60 | 4 |
| Pulmonary weight
ratio (wet/dry) | 4.5 | 1 |
|
| 4.5–5.0 | 2 |
|
| 5.0–5.5 | 3 |
|
| >5.5 | 4 |
Specimen collection and detection
Before and after surgery and 4, 8, 12 and 24 h after
surgery, the rats were anesthetized and dissected. After finding
the jugular vein or artery, blood samples were drawn with a syringe
and left to rest for 30 min in a blood collection tube. Plasma was
separated by centrifugation (2,500 × g for 15 min) and stored at
−80°C for further analysis. Detection of TNF-α, IL-1β and HMGB1
concentration in plasma was by ELISA.
Histopathological examination
Twenty-four hours after surgery, the left lungs from
the different groups of rats were collected and immediately washed
twice with phosphate-buffered saline, then modified and fixed in
10% neutral formalin. The samples were successively dehydrated and
paraffin embedded. Tissue sections (4 µm) were fixed in ethanol at
room temperature, stained with hematoxylin and eosin, and analyzed
by light microscopy and photographed.
Calculation of cumulative
mortality
Ten animals from the different groups were kept
under observation for 7 days after surgery. The natural time of
death after surgery was recorded. The survival of rats in each
group and the cumulative mortality at 7 day was calculated.
Statistical analysis
Statistical analyses were performed using SPSS 18.0
software (IBM, Chicago, USA). Data were presented as mean ± SD.
Normal distribution variables among different groups were compared
using analysis of variance, non-normal distribution and variables
were compared with rank-sum test. Parameters between two of the
five groups were compared using an LSD t-test. The cumulative
survival rate was calculated using the Kaplan-Meier survival
method. Enumeration data were analyzed using the Chi-square test.
P<0.05 was considered to indicate statistically significant
results.
Results
Comparison of sepsis severity of
rats
The rats in the CLP group gradually appeared less
active, were cold, had dull fur, stopped drinking water, and had
upright fur. Dyspnea occurred in some rats. In addition, some rats
appeared with double-ring eyes, had no resistance to passive supine
and became slow to respond. The mortality rate was high in the
short term.
The rats in the atorvastatin group appeared
anorectic, had vertical hair, and were less active or completely
inactive. The rats in the LMWH group behaved similarly to the
atorvastatin group.
The rats in the combined group also appeared
anorectic, with vertical hair, and were less active. The symptoms
were less severe than in the CLP group. The sepsis severity scale
in the four groups is shown in Table
II.
 | Table II.Comparison of sepsis severity scale
in the four groups (mean ± SD). |
Table II.
Comparison of sepsis severity scale
in the four groups (mean ± SD).
| Group | Case (n) | 0 h | 4 h | 8 h | 12 h | 24 h |
|---|
| Sham operation | 6 |
7.0±0.5 |
7.2±0.
5 |
7.3±0.6 |
7.1±0.5 |
7.2±0.6 |
| CLP | 6 |
7.0±0.5 |
12.2±2.0a |
16.6±2.5a |
22.8±3.0b |
27.2±4.0b |
| Atorvastatin | 6 |
7.0±0.4 |
9.0±1.5 |
12.2±2.0ac |
16.3±2.5ac |
21.2±2.5ac |
| LMWH | 6 |
7.0±0.4 |
10.0±1.8 |
11.2±2.2ac |
17.3±2.5ac |
20.2±2.0ac |
| Combined | 6 |
7.0±0.6 |
8.4±1.5c |
10.0±1.7ac |
12.20±0.8ac |
16.4±1.5bd |
Comparison of plasma TNF-α, IL-1β, and
HMGB1 concentrations in each group
At 0 h, the levels of TNF-α, IL-1β and HMGB1 in
plasma showed no significant difference in the five groups
(P>0.05). In the sham operation group, the levels of TNF-α,
IL-1β and HMGB1 in plasma had no significant changes (P>0.05).
Compared to the sham operation group, at 4, 8, 12 and 24 h, the
levels of TNF-α, IL-1β and HMGB1 in plasma of the CLP group
significantly increased (P<0.05). The concentration of TNF-α,
IL-1β and HMGB1 reached peak levels at 4, 8 and 24 h, respectively.
Compared to the CLP group, the levels of TNF-α, IL-1β and HMGB1 in
the atorvastatin, LMWH, and atorvastatin combined with LMWH group
significantly decreased (P<0.05). In addition, compared to the
atorvastatin and LMWH groups, the TNF-α concentration in plasma at
4 h, IL-1β concentration in plasma at 12 h and HMGB1 concentration
in plasma at 24 h in the atorvastatin combined with LMWH group were
significantly reduced (P<0.05) (Table III).
 | Table III.Effect on the plasma levels of TNF-α,
IL-1β and HMGB1 by atorvastatin and LMWH in sepsis rats (mean ±
SD). |
Table III.
Effect on the plasma levels of TNF-α,
IL-1β and HMGB1 by atorvastatin and LMWH in sepsis rats (mean ±
SD).
|
| TNF-α (ng/l) | IL-1β (ng/l) | HMGB1 (µg/l) |
|---|
|
|
|
|
|
|---|
| Group | 0 h | 4 h | 8 h | 12 h | 24 h | 0 h | 4 h | 8 h | 12 h | 24 h | 0 h | 4 h | 8 h | 12 h | 24 h |
|---|
| Sham operation |
113.8±38.3 |
114.1±39.6 |
112.9±30.5 |
114.6±29.5 |
115.3±39.2 |
353.7±78.3 |
364.5±79.6 |
375.1±70.4 |
354.7±70.7 |
366.4±79.2 |
163.8±46.3 |
164.5±59.6 |
159.1±72.4 |
162.7±58.7 |
166.3±59.2 |
| CLP |
112.2±36.9 |
783.8±134.7b |
437.9±105.6b |
216.8±87.3a |
159.3±67.6 |
387.2±66.3 |
489.7±64.6 |
2873.9±295.6b |
916.7±187.4b |
459.3±167.6 |
177.5±56.3 |
209.7±74.6 |
273.0±45.2 |
316.7±67.4a |
859.3±167.5b |
| Atorvastatin |
113.8±69.5 |
668.3±124.6bc |
376.7±107.7a |
159.7±72.1a |
124.1±54.3 |
372.8±69.4 |
478.3±74.6 |
2476.7±137.8bc |
809.6±100.5b |
414.4±99.3 |
162.8±69.4 |
208.2±64.6 |
276.7±57.8 |
309.6±70.5a |
654.4±154.4bc |
| LMWH |
114.9±65.8 |
536.5±118.5bc |
360.4±70.3a |
168.6±54.6a |
129.7±54.5 |
369.0±65.4 |
506.5±108.5 |
2460.4±171.2bc |
848.6±94.6b |
439.0±84.5 |
167.9±65.4 |
206.8±78.6 |
260.4±71.2 |
348.6±54.7a |
659.0±134.6bc |
| Combined |
110.3±65.4 |
496.5±108.5bc |
324.4±72.3a |
148.6±50.6a |
122.0±34.5 |
359.0±70.4 |
478.5±90.6 |
2090.0±151.2bd |
648.5±54.7a |
429.0±39.5 |
173.8±60.4 |
236.8±58.6 |
240.0±70.3 |
308.3±58.7a |
609.4±90.5bd |
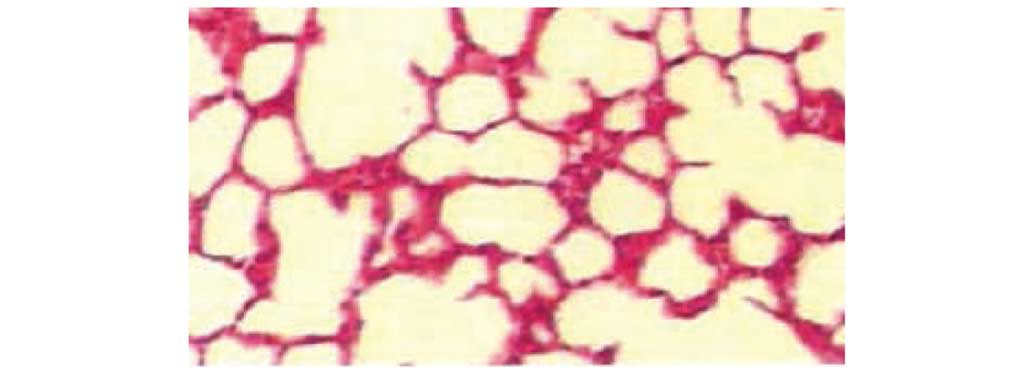
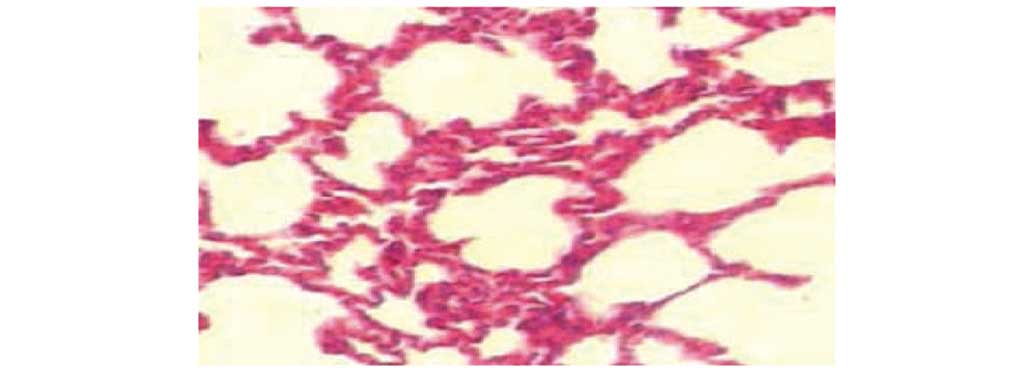
Pathological changes in the lung under
by light microscopy
Lung histology was normal in the sham operation
group by light microscopy. In the CLP group, lungs exhibited
substantial hemorrhage, inflammatory cell infiltration, obvious
consolidation, capillary congestion and thrombus formation. In the
atorvastatin and LMWH groups, lungs exhibited alveolar hemorrhage,
and more inflammatory cell infiltration and reduction in
consolidation compared to the CLP group. In the combined group,
lung tissue exhibited partial congestion and thickening, a
reduction in interstitial pulmonary edema, capillary congestion,
less inflammatory cell infiltration and mild lung consolidation
(Figs. 1–5).
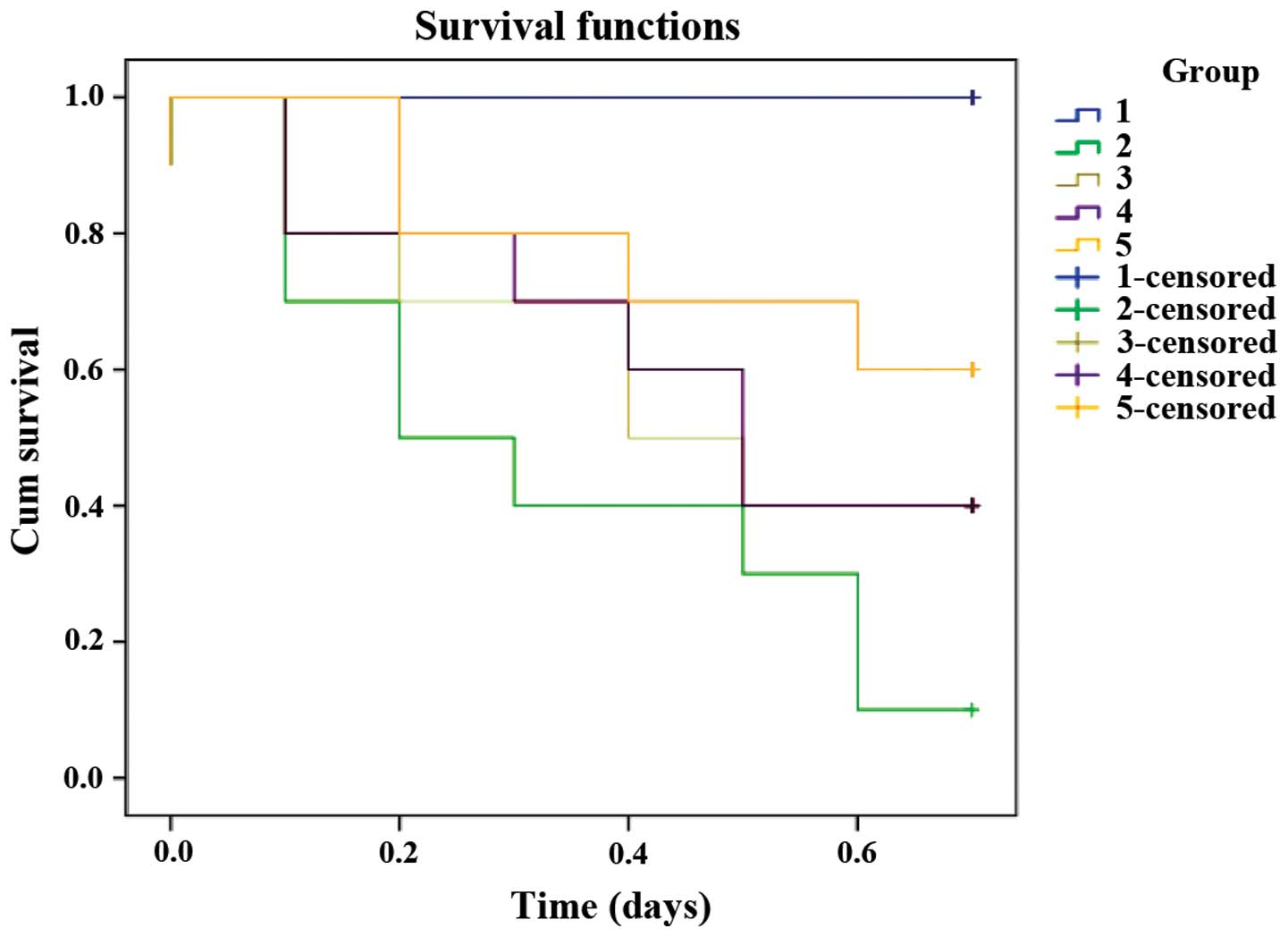
Cumulative mortality calculation
Ten animals from the different groups were kept
under observation for 7 days after surgery. No deaths occurred in
the sham operation animals. The 7-day cumulative mortality in the
CLP group was 90%. The 7-day cumulative mortality in the
atorvastatin, LMWH and combined groups were 60, 60 and 40%,
respectively, which was significantly decreased in comparison to
the CLP group (P<0.05), χ2=16.174 and P=0.003 was
determined by the log-rank test. The overall difference in the five
groups was significant by a value of α=0.05 (Fig. 6).
Discussion
Sepsis is a systemic inflammatory response syndrome
(SIRS) to infection. The process involves a series of changes in
the body, such as inflammation, cellular immunity, blood
coagulation and tissue damage. Mounting evidence indicates that the
inflammatory response induced by severe infection is closely
related to the changes in coagulation function, and inflammatory
reactions, while changes in coagulation function are closely
related to the severity and mortality of sepsis (13). At present, the imbalance of
coagulation function, inflammatory reactions and immune suppression
are considered to comprise the main pathophysiological basis of
high incidence and high mortality of sepsis. Inflammatory reactions
can activate the coagulation system. Microthrombosis in the high
coagulation state can result in vascular embolism and disseminated
intravascular coagulation (DIC), which is the basic induction of
severe sepsis and septic shock, thus coagulation disorder is an
important part of the pathogenesis of sepsis (14). Although breakthroughs have been made
in understanding the pathophysiology of the inflammatory response
in recent years and bundle therapies have been used in the
treatment of sepsis, the mortality of severe sepsis is 40%, and
>50% in patients complicated with organ failure (3). Studies suggest that sepsis is
characterized by the overexpression of pro-inflammatory cytokines
(15,6–8), such as
early inflammatory cytokine, TNF-α as well as IL-1β, IL-6, IL-8 and
HMGB1. Overexpression of inflammatory factors leads to multiple
organ dysfunction and increased mortality.
In this study, sepsis was induced by CLP. Bacteria
that released into the peritoneal cavity and stimulated monocytes,
macrophages and endothelial cells to produce a variety of
inflammatory cytokines, leading to metabolic, hormonal and
neuroendocrine changes in the body, which resulted in abnormal cell
function and organ failure. TNF-α is an important initiator in
sepsis. It induces the release of inflammatory mediators, which
results in a ‘waterfall pattern’ cascade reaction. When bacteria
enter the body, TNF-α appears early in the circulation and quickly
reaches peak levels, inducing a series of inflammatory changes in
vascular endothelial cells and the microcirculation (16). The levels of plasma TNF-α in infected
animals significantly increased at 1 h after infection, reached
peak levels at 2 h and returned to control levels at 4 h. The
concentration of TNF-α positively correlated with the severity of
pathological changes and tissue damage. TNF-α is involved in
metabolic processes and immune responses, activates the coagulation
process and stimulates macrophages to produce IL-lβ, IL-6 as well
as other inflammatory factors (17).
IL-1β and IL-6 are considered to be closely related to SIRS
severity and mortality (18,19). HMGB1 is a late inflammatory mediator,
mainly released by monocytes and macrophages under stimulation by
infection and trauma, and the release intensity is dose- and
time-dependent with TNF-α and IL-lβ (20). HMGB1 as a late inflammatory mediator,
and has been recognized as a key factor in the lethal effect of
sepsis, and its level in vivo will directly affect the
severity of the body's response and prognosis of sepsis (21).
Previous findings have indicated that in the process
of sepsis, HMGB1 has a role in coagulation and thrombosis, which is
consistent with the cross activation of inflammation and
coagulation in the process of sepsis (22). It has been reported that HMGBl
reaches peak levels at 12–24 h. Compared with early inflammatory
mediators including IL-lβ and TNF-α which return to normal levels
6–12 h later, the therapeutic time window for HMGBl is relatively
larger, thus the targeting therapeutic effect is important
(23).
The current results have shown that compared to the
sham operation group, at 4, 8, 12 and 24 h, the concentration of
inflammatory cytokines in the CLP group had increased
significantly, where the TNF-α concentration in plasma peaked at 4
h, the IL-1β concentration in plasma peaked at 8 h, and the HMGB1
concentration in plasma peaked at 24 h. Compared to the CLP group,
the concentration of inflammatory cytokines in the atorvastatin,
LMWH, and atorvastatin combined with LMWH groups decreased
significantly, and there were significant differences in the four
groups. Compared to the atorvastatin and LMWH groups, the TNF-α
concentration in plasma at 4 h, IL-1β concentration in plasma at 12
h and HMGB1 concentration in plasma at 24 h in the atorvastatin
combined with LMWH group decreased significantly (P<0.05). The
results showed that atorvastatin and LMWH had a significant
inhibitory effect on the release of inflammatory factors, and the
two had a synergistic effect.
Statins, 3-hydroxy-3-methyl-glutaryl-CoA reductase
inhibitors, possess effects including anti-inflammatory properties,
immune regulatory properties, antioxidant and anticoagulant
properties as well as can stabilize the endothelial cells of blood
vessels. These effects are referred to as the pleiotropic effects
of statins (24), which are
independent of their lipid lowering effect and, can be used for
treatment of sepsis. Clinical studies demonstrated that statins are
beneficial for sepsis (25). In
basic research, sepsis was induced by CLP in mice, and the average
survival rate of mice treated with statins was 4-fold higher than
that in the control group (26).
Crosstalk exists between inflammatory reactions and coagulation
disorders, both of which play important roles in the pathogenesis
of sepsis as initiating factors. Thus, the intervention of
coagulation disorders may be a new therapeutic area for sepsis
treatment (27). Heparin can inhibit
HMGBl pro-inflammatory activity by changing the conformation of
HMGB1 by combining with 6–12 amino acid residues in its N-terminal
region (28–30). LMWH plays a role in alleviating the
inflammatory response likely by blocking NF-κB-mediated
inflammatory effects, which may be one of the mechanisms for
improving the prognosis in critically ill patients. LMWH can reduce
the APACHE II score in elderly patients with severe pneumonia,
shorten the mechanical ventilation time and ICU stay time, thereby
improving the prognosis of patients (31). The efficacy and mechanism of combined
treatment with the two drugs in sepsis have not been reported. In
this study, we found that the sepsis severity scores of rats
decreased after atorvastatin or LMWH treatment, and their
combination afforded better effects. Our findings also showed that
in the sham operation group, the lung tissue was normal by light
microscopy. In the CLP group, lungs exhibited substantial alveolar
hemorrhage, a large amount of inflammatory cell infiltration,
obvious consolidation, capillary congestion and thrombus formation.
In the atorvastatin and LMWH heparin groups, lungs exhibited
alveolar hemorrhage, inflammatory cell infiltration, and a
reduction in consolidation compared to the CLP group. In the
combined therapy group, lungs exhibited partial congestion and
thickening, a reduction in interstitial pulmonary edema, less
capillary congestion, less inflammatory cell infiltration and mild
lung consolidation. No deaths occurred in the sham operation
animals. The 7-day cumulative mortality in the CLP group was 90%.
The 7-day cumulative mortality in the atorvastatin group, LMWH
group and combined group were 60, 60 and 40%, respectively, which
significantly decreased in comparison to the CLP group.
The results of the present study showed that
combined treatment with statins and LMWH reduced sepsis-associated
mortality significantly by protecting and improving the functions
of tissues and organs at multiple levels by different mechanisms,
and exerting anti-inflammatory effects. Lung, as the most involved
and important organ in the pathophysiology of sepsis, mainly
exhibits alveolar capillary permeability changes and hypoxia
(32). The present findings show
that the combined treatment of statins and LMWH in lung injury
attenuated pathologic destruction, greatly decreased inflammatory
cell accumulation and maintained the alveolar structure. These
results show that the combined treatment of statins and LMWH can
reduce the lung injury in sepsis and that the two have a
synergistic effect. A possible mechanism is that atorvastatin and
LMWH have inhibitory effects on the expression of early and late
inflammatory factors, thereby reducing the inflammatory reaction of
rats with sepsis.
In conclusion, combined treatment with atorvastatin
and LMWH inhibited the release of inflammatory cytokines, decreased
the sepsis severity score, and lowered the mortality rate and the
two had a synergistic effect. However, whether the treatment is
valid in clinic requires further prospective randomized controlled
studies of large sample sizes.
Acknowledgements
This study was supported by grants from Shandong
Provincial Natural Science Foundation, China (no. ZR2013HM062) and
the Shandong Provincial Science and Technology Development Plan of
Medical and Health (no. 2013WS0110).
References
|
1
|
Sakr Y, Elia C, Mascia L, Barberis B,
Cardellino S, Livigni S, Fiore G, Filippini C and Ranieri VM:
Epidemiology and outcome of sepsis syndromes in Italian ICUs: a
muticentre, observational cohort study in the region of Piedmont.
Minerva Anestesiol. 79:993–1002. 2013.PubMed/NCBI
|
|
2
|
Kobashi H, Toshimori J and Yamamoto K:
Sepsis-associated liver injury: incidence, classification and the
clinical significance. Hepatol Res. 43:255–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Diament D, Salomão R, Rigatto O, Gomes B,
Silva E, Carvalho NB and Machado FR: Guidelines for the treatment
of severe sepsis and septic shock - management of the infectious
agent - diagnosis. Rev Bras Ter Intensiva. 23:134–144. 2011.(In
Portuguese). PubMed/NCBI
|
|
4
|
Dellinger RP, Levy MM, Rhodes A, Annane D,
Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke
R, et al: Surviving Sepsis Campaign Guidelines Committee including
The Pediatric Subgroup: Sepsis Campaign: international guidelines
for management of severe sepsis and septic shock, 2012. Intensive
Care Med. 39:165–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fukushima H, Nishio K, Asai H, Watanabe T,
Seki T, Matsui H, Sugimoto M, Matsumoto M, Fujimura Y and Okuchi K:
Ratio of von Willebrand factor propeptide to ADAMTS13 is associated
with severity of sepsis. Shock. 39:409–414. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hatherill M, Tibby SM, Turner C, Ratnavel
N and Murdoch IA: Procalcitonin and cytokine levels: relationship
to organ failure and mortality in pediatric septic shock. Crit Care
Med. 28:2591–2594. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
King EG, Bauzá GJ, Mella JR and Remick DG:
Pathophysiologic mechanisms in septic shock. Lab Invest. 94:4–12.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aslani F, Schuppe HC, Guazzone VA, Bhushan
S, Wahle E, Lochnit G, Lustig L, Meinhardt A and Fijak M: Targeting
high mobility group box protein 1 ameliorates testicular
inflammation in experimental autoimmune orchitis. Hum Reprod.
30:417–431. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Subramani J, Kathirvel K, Leo MD,
Kuntamallappanavar G, Uttam Singh T and Mishra SK: Atorvastatin
restores the impaired vascular endothelium-dependent relaxations
mediated by nitric oxide and endothelium-derived hyperpolarizing
factors but not hypotension in sepsis. J Cardiovasc Pharmacol.
54:526–534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu X, Zhao L and Xu YH: Low molecular
weight heparin prevents CLP-induced acute lung injury in rats by
anti-inflammatory coagulation. Bosn J Basic Med Sci. 13:50–56.
2013.PubMed/NCBI
|
|
11
|
Liu FY and Liu TF: Experimental zoology.
Beijing China Science and Technology Press. Beijing: 209–210.
2005.(In Chinese).
|
|
12
|
Wichterman KA, Baue AE and Chaudry IH:
Sepsis and septic shock - a review of laboratory models and a
proposal. J Surg Res. 29:189–201. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Levi M, van der Poll T and Schultz M: New
insights into pathways that determine the link between infection
and thrombosis. Neth J Med. 70:114–120. 2012.PubMed/NCBI
|
|
14
|
Schouten M, Wiersinga WJ, Levi M and van
der Poll T: Inflammation, endothelium, and coagulation in sepsis. J
Leukoc Biol. 83:536–545. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang L and An YZ: The stage-biomarkers of
the sepsis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 23:509–512.
2011.(In Chinese). PubMed/NCBI
|
|
16
|
Khalid U, Jenkins RH, Pino-Chavez G, Bowen
T, Fraser DJ and Chavez R: A localized ischemic preconditioning
regimen increases tumor necrosis factor α expression in a rat model
of kidney ischemia-reperfusion injury. Exp Clin Transplant.
13:535–542. 2015.PubMed/NCBI
|
|
17
|
Zou JL, Yin ZP, Zhang LQ, Wang YQ and Qi
GX: Expression of TNG-α and IL-6 in tissues and serums in the early
stage of myocardial ischemia-reperfusion in rat. J China Med Univ.
25:541–543. 2013.(In Chinese).
|
|
18
|
Zhao L, An R, Yang Y, Yang X, Liu H, Yue
L, Li X, Lin Y, Reiter RJ and Qu Y: Melatonin alleviates brain
injury in mice subjected to cecal ligation and puncture via
attenuating inflammation, apoptosis, and oxidative stress: the role
of SIRT1 signaling. J Pineal Res. 59:230–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bosmann M, Russkamp NF and Ward PA:
Fingerprinting of the TLR4-induced acute inflammatory response. Exp
Mol Pathol. 93:319–323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu H, Cai PP, Yin X and Zhu J: Role of
high mobility group protein B1 in acute lung injury in rats with
sepsis. Chin J Crit Care Med. 23:253–254. 2011.(In Chinese).
|
|
21
|
Ito T, Kawahara K, Nakamura T, Yamada S,
Nakamura T, Abeyama K, Hashiguchi T and Maruyama I: High-mobility
group box 1 protein promotes development of microvascular
thrombosis in rats. J Thromb Haemost. 5:109–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H and Liu D: Baicalin inhibits
high-mobility group box 1 release and improves survival in
experimental sepsis. Shock. 41:324–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SA, Kwak MS, Kim S and Shin JS: The
role of high mobility group box 1 in innate immunity. Yonsei Med J.
55:1165–1176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kouroumichakis I, Papanas N, Proikaki S,
Zarogoulidis P and Maltezos E: Statins in prevention and treatment
of severe sepsis and septic shock. Eur J Intern Med. 22:125–133.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Janda S, Young A, Fitzgerald JM, Etminan M
and Swiston J: The effect of statins on mortality from severe
infections and sepsis: a systematic review and meta-analysis. J
Crit Care. 25:656.e7–22. 2010. View Article : Google Scholar
|
|
26
|
Beffa DC, Fischman AJ, Fagan SP, Hamrahi
VF, Paul KW, Kaneki M, Yu YM, Tompkins RG and Carter EA:
Simvastatin treatment improves survival in a murine model of burn
sepsis: role of interleukin 6. Burns. 37:222–226. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jaimes F, De La Rosa G, Morales C, Fortich
F, Arango C, Aguirre D and Muñoz A: Unfractioned heparin for
treatment of sepsis: a randomized clinical trial (The HETRASE
Study). Crit Care Med. 37:1185–1196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao D, Ding R, Mao Y, Wang L, Zhang Z and
Ma X: Heparin rescues sepsis-associated acute lung injury and
lethality through the suppression of inflammatory responses.
Inflammation. 35:1825–1832. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Spratte J, Meyer zu Schwabedissen H,
Endlich N, Zygmunt M and Fluhr H: Heparin inhibits TNF-α signaling
in human endometrial stromal cells by interaction with NF-κB. Mol
Hum Reprod. 19:227–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu ZY, Zhu H and Ma XC: Systematic
evaluation of heparin treatment in sepsis. Chin J Crit Care Med.
26:135–141. 2014.(In Chinese).
|
|
31
|
Wang P, Wang X, Zhang LJ, Yang F, Wang GX,
Li XL and Huang X: Effect of low molecular weight heparin on the
prognosis of elderly patients with severe pneumonia. Chin J Crit
Care Med. 25:734–737. 2013.(In Chinese).
|
|
32
|
Gill SE, Taneja R, Rohan M, Wang L and
Mehta S: Pulmonary microvascular albumin leak is associated with
endothelial cell death in murine sepsis-induced lung injury in
vivo. PLoS One. 9:e885012014. View Article : Google Scholar : PubMed/NCBI
|