Introduction
Electroacupuncture (EA) is a therapy for the
treatment of neurological dysfunction that functions by stimulating
specific areas in the body. This therapeutic method originated in
China >2,000 years ago, and over time EA has been accepted as
conventional medicine in clinical practice worldwide (1–4).
Statistical analyses of clinical results have indicated that the
effects of EA on strokes are significant (5). Stroke is a major cause of several
complications, including cognitive impairment, with ~25% of
patients suffering from cognitive impairment three months after a
stroke (6). In addition, up to 75%
of stroke survivors may be considered to have selective cognitive
impairment, which commonly involves memory, orientation, language
and attention (7).
Cognitive rehabilitation and medication have been
used to enhance cognition in patients who have had a stroke
(8,9); however, there is no one reliable method
or medication that has been demonstrated in clinical practice. An
increasing number of clinical trials have revealed that acupuncture
exhibits positive effects following stroke, not only as a
complementary and alternative medicine for post-stroke
rehabilitation, but also as a preventative strategy that may induce
cerebral ischemic tolerance (10–12).
Although the detailed mechanism underlying cognitive
impairment remains unclear, neuronal excitotoxicity, the
over-release of toxic neural transmitters and neuronal apoptosis
have been demonstrated to contribute to the pathological process
(8,9). Neuronal excitotoxicity is triggered by
intrinsic or extrinsic stimuli, which eventually result in the
activation of caspases and nucleases, subsequently causing cell
destruction (13).
Excitotoxicity via calcium-permeable glutamate
receptors is considered to be a critical trigger in
ischemia-induced brain damage. Experimental models have revealed
that excitotoxicity may be inhibited using glutamate receptor
antagonists, such as the non-competitive N-methyl-D-aspartate type
glutamate receptor (NMDAR) antagonist, MK-801 (14). In contrast to an NMDAR blockade, the
inhibition of specific postsynaptic NMDAR signaling by
preconditioning neuroprotection may induce neuroprotection against
cerebral ischemia-reperfusion (I/R) injury. Preconditioning has
been associated with increased phosphorylation of cyclic adenosine
monophosphate (cAMP) response element-binding (CREB) protein
(15) and CREB-dependent
transcription (16). Phosphorylation
of CREB at Ser133 is involved in the regulation of neuronal
plasticity and memory formation, and is required for glutamate- and
Ca2+-dependent neuronal survival during development
(17). In neurons of the central
nervous system, CREB phosphorylation is induced by the synaptic
activation of NMDARs, which occurs downstream of
Ca2+/CaM-dependent protein kinase (CaMK) activation
(18). CaMKII and CaMKIV are able to
regulate CREB activity; however, CaMKIV is specifically associated
with the activation and transcription of trophic CREB (19). CaMKIV is a nuclear serine/threonine
kinase that phosphorylates CREB at Ser133 and its transcription
partner, CREB binding protein (20),
thereby activating trophic gene transcription. Therefore, these
observations indicate that CaM, CaMKIV and CREB activation are
important for neuronal survival.
A previous study demonstrated that EA directly
affected the biochemical materials associated with neuronal
survival, including Ca2+, glutamate and NMDA; however,
the detailed mechanisms underlying the intracellular signaling
pathway in the hippocampus are yet to be fully elucidated (21). In the present study, EA was
hypothesized to improve cognitive impairment in cerebral
I/R-injured rats by adjusting the CaM-CaMKIV-CREB intracellular
signaling function in the hippocampus.
Materials and methods
Animals
In total, 45 female Sprague Dawley rats (weight,
270±20 g) were obtained from Shanghai SLAC Laboratory Animal Co.
Ltd. (Shanghai, China) and housed in the Animal Center of Fujian
University of Traditional Chinese Medicine (TCM; Fuzhou, China).
All the animals were housed under pathogen-free conditions with a
12-h light/dark cycle and free access to food and water. The
experimental protocol was approved by the Institutional Animal Care
and Use Committee of Fujian University of TCM.
The 45 rats were randomly divided into three groups,
which included the sham group (rats underwent sham surgery), the
middle cerebral artery occlusion (MCAO) group (rats underwent MCAO)
and the MCAO + EA group (rats underwent MCAO and received EA
intervention).
Establishment of the cerebral I/R
injured rat model
Following overnight fasting, the rats were
anesthetized with 10% chloral hydrate (3 ml/kg; Sigma-Aldrich, St.
Louis, MO, USA) through intraperitoneal injection. Subsequently,
0.100 0.149 mm nylon surgical thread (Wego Holding Co., Ltd.,
Weihai, China) was inserted into the left internal carotid artery
to block the middle cerebral artery when the blunted distal end met
resistance. Following 2 h of occlusion, the thread was removed to
allow complete reperfusion of the ischemic area. A sham procedure
was carried out as aforementioned, without the occlusion of the
middle cerebral artery (21,22).
EA intervention
At 2 h after the surgery, rats in the MCAO + EA
group received EA treatment for 30 min. The complete treatment
period was 7 days. Baihui and Shenting acupuncture points in the
governing vessel were selected for this study. Needles (0.3 mm
diameter) were inserted to a depth of 2–3 mm and connected with the
EA device (Model G6805; SMIF, Shanghai, China) with a disperse wave
of 1 and 20 Hz.
Step-down avoidance test
In the step-down inhibitory avoidance task, a rat is
placed on an elevated platform in a dark compartment (20×20×60 cm),
adjacent to a wall of an arena. When the rat steps down and places
four paws onto the arena-floor grid, the rat receives a mild foot
shock (36 V) and learns to associate the exploration of the arena
with the punishment. On subsequent exposure to the same
environment, the animal may increase the latency prior to ‘stepping
down’ onto the floor grid, or may avoid stepping down. Rats were
habituated to the handling procedure on the day prior to the test
for 3 min. Following any intervention, the rats were placed onto
the platform again. The first time-period spent prior to stepping
down onto the grid (latency period) and the frequency (number of
errors) of stepping down from the platform within 3 min were
recorded. In cases where the rats did not step-down from the
platform within 3 min, the number of errors was recorded as ‘0’,
and the latency period was ‘180 sec’. Step-down latencies and
errors were recorded as a measure of memory retention (23–25).
Histopathological staining with
2,3,5-triphenyl tetrazolium chloride (TTC)
Rats from each group were decapitated following
anesthetization with 10% chloral hydrate (3 ml/kg). The brains were
immediately removed and placed in ice/water at −20°C for 15 min to
ensure rigidity. Subsequently, the brains were cut into coronal
sections of 2-mm thickness at the middle of the connecting line
between the prefrontal cortex and the optic chiasma, after which
the samples were immersed in 2% TTC (Sigma-Aldrich) (to avoid
light) for 15 min (37°C) and treated with 4% paraformaldehyde
(Sigma-Aldrich) for fixation for 24 h. Finally, images of the
brains were captured by camera (SX20; Canon, Inc., Tokyo, Japan).
Analysis of the ischemia cerebral damage was performed as described
previously (22,26).
Phosphodiesterase (PDE) activity
A modified three-step PDE-1 assay was used to
determine the CaM-dependent activation of PDE-1. In the PDE-1
assay, 3,5-cyclic-nucleotide PDE, 2-mM cAMP and 100 µM CaM
(Sigma-Aldrich) in 0.5 ml Tris buffer solution (40 mM
Tris-chloride, 0.1 mM MnCl2 and 0.01 mM CaCl2
in distilled water at pH 7.5) were incubated with increasing
concentrations of native tehranolide
(1×10−6-9×10−6 M) for 10 min at 30°C. The
reaction was stopped by placing the test tubes in a boiling water
bath for 2 min, and then cooling. The 5-AMP in the reaction product
was cleaved into adenosine and inorganic phosphate by incubation
with 5′-nucleotidase (100 µl; Sigma-Aldrich) for 10 min, and the
reaction was terminated by adding 0.05 ml trichloroacetic acid (55%
w/v) and 0.15 ml molybdic acid solution, and centrifuging (10,800 ×
g) until clear. The clear supernatants were decanted into test
tubes with Fiske-Subbarow reagent (Sigma-Aldrich). A blue color
reaction was allowed to develop in the presence of inorganic
phosphorus for 10 min, and the absorbance was measured at 660 nm
using spectrophotometry (SmartSpec Plus; Bio-Rad Laboratories,
Inc., Hercules, CA, USA) (27–29).
Western blot analysis for the
determination of CaM, CaMKIV, phosphorylated (p)-CaMKIV, CREB and
p-CREB protein expression levels
Hippocampi from three groups were homogenized in
nondenaturing lysis buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 1%
Triton X-100, 1% NP-40, 2 mM sodium pyrophosphate, 25 mM
β-glycerophosphate, 1 mM EDTA, 1 mM Na3VO4,
0.5 µg/ml leupeptin) and centrifuged at 12000 × g for 15 min. The
supernatants were collected and frozen at −80°C prior to
immunoblotting. Protein concentration was determined using a
Bio-Image Analysis System (ChemiDoc™ Imaging Systems; Bio-Rad
Laboratories, Inc.). In total, a 50-µg protein sample obtained from
the CA1 region of the hippocampus was loaded onto a 12% SDS-PAGE
gel. Following electrophoresis, the proteins were
electrotransferred onto polyvinylidene difluoride membranes
(Sigma-Aldrich). The blots were blocked with 5% non-fat milk for 2
h, and subsequently incubated with primary antibodies (1:1,000
dilution) against CaM (cat. no. sc-137079; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), CaMKIV (cat. no. 4032; Cell
Signaling Technology, Inc., Danvers, MA, USA), p-CaMKIV (cat. no.
sc-28443-R; Santa Cruz Biotechnology, Inc), CREB (cat. no. 9197;
Cell Signaling Technology, Inc.), p-CREB (cat. no. 9196; Cell
Signaling Technology, Inc.) and β-actin (cat. no. 4970; Cell
Signaling Technology, Inc.) overnight at 4°C. Next, the blots were
incubated with a horseradish peroxidase-conjugated anti-rabbit or
anti-mouse secondary antibodies (1:5,000; cat. nos. 7074 and 7076;
Cell Signaling Technology, Inc.) for 50 min. β-actin was used as a
loading control. The blots were developed using a commercially
available enhanced chemiluminescence kit (Bio-Rad Laboratories,
Inc.), and examined using a Bio-Image Analysis System (ChemiDoc™
Imaging systems; Bio-Rad Laboratories, Inc.) (30).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical comparisons were conducted by one way
analysis of variance using the SPSS software package, version 18.0
(SPSS, Inc., Chicago, IL, USA), where P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of EA on the step-down
avoidance test and infarct volume
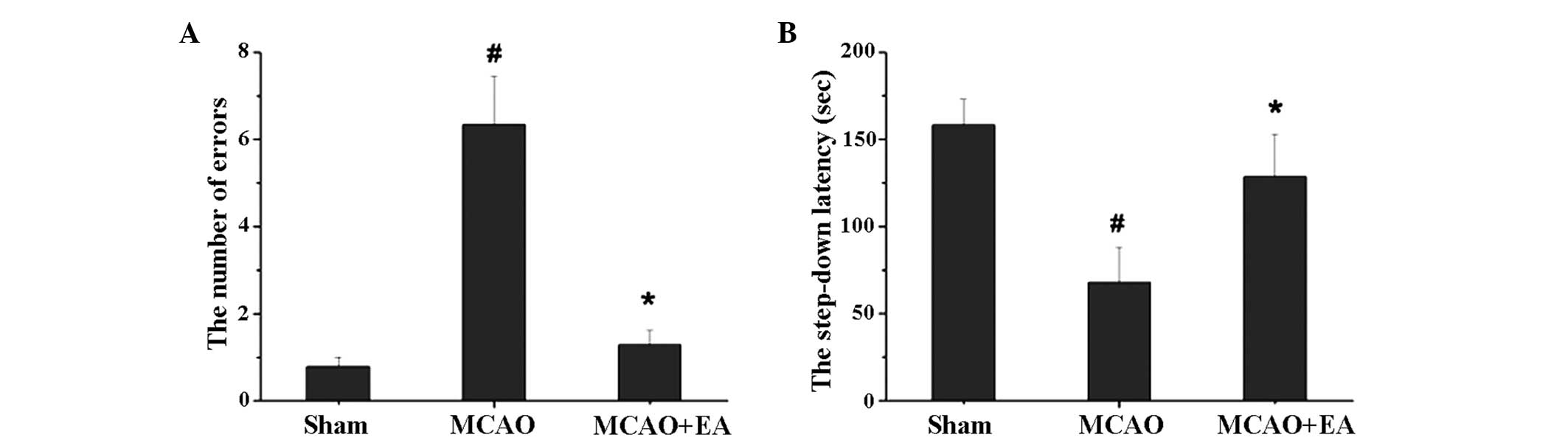
As shown in Fig. 1,
MCAO markedly affected the memory of the rats, while EA treatment
was shown to successfully repair this ability. The step-down
latency of the MCAO group rats was significantly shorter when
compared with the sham group (P<0.05), and was prolonged by EA
(P<0.05). During the 3-min test, the number of errors in the
MCAO group rats was significantly higher when compared with the
sham group and MCAO + EA group rats (Fig. 1; P<0.05).
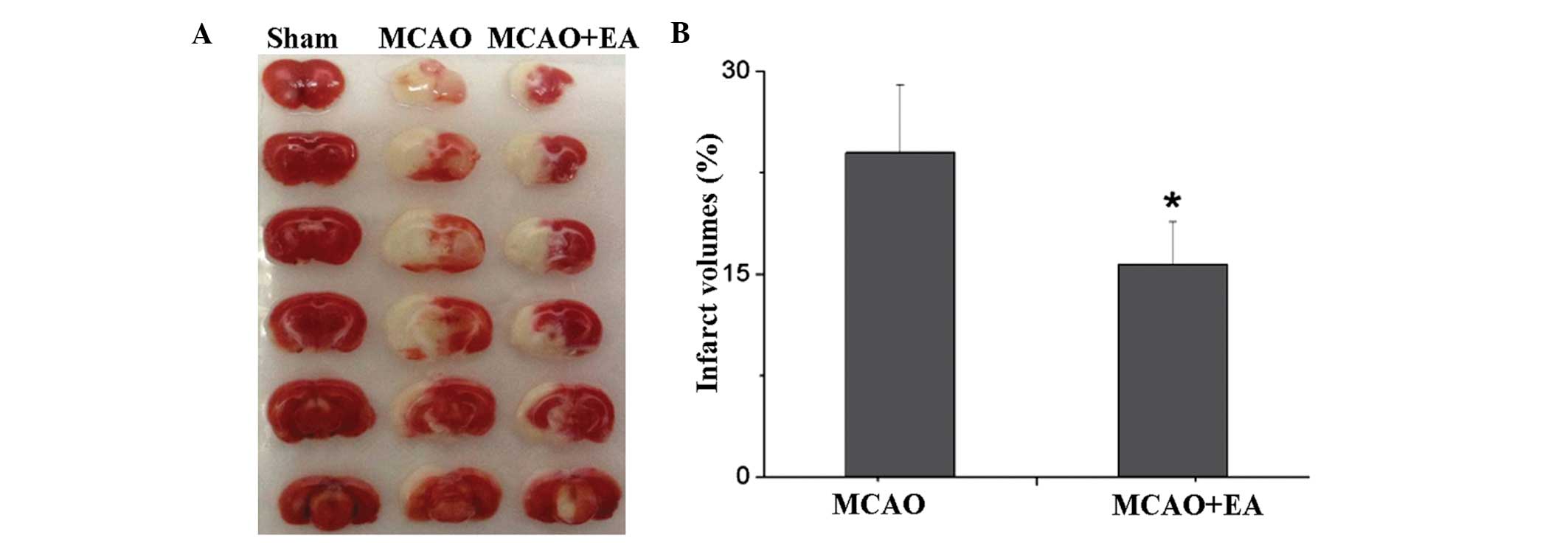
With regard to the infarct volume analyses, EA was
revealed to significantly reduce the infarct volume caused by
cerebral I/R. The sham group rats exhibited no trauma in the brain,
while the MCAO group rats exhibited a large infarct area
(23.98±5.04%; P<0.05), which was significantly decreased
following EA (15.71±3.16%; P<0.05; Fig. 2).
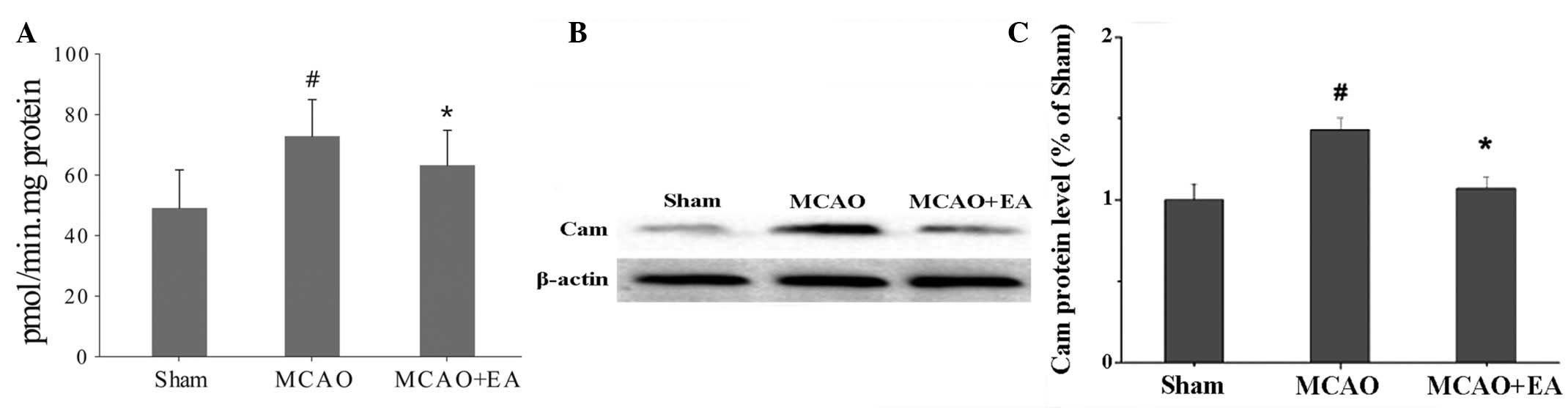
Effect of EA on the levels of CaM
activity and CaM protein expression
Notably, MCAO was found to promote CaM activity and
protein expression, whereas EA reduced these reactions. According
to the PDE analyses, CaM activity was promoted by MCAO and
inhibited by EA (P<0.05), and the same result was observed for
CaM protein expression (P<0.05; Fig.
3).
Effect of EA on the protein expression
levels of CaMKIV, p-CaMKIV, CREB and p-CREB
Protein expression levels of CaMKIV, p-CaMKIV, CREB
and p-CREB were shown to decrease following MCAO and increase with
EA treatment. MCAO severely inhibited the protein expression of
CaMKIV and pCaMKIV in the hippocampus (P<0.05), while EA
repaired the expression of these proteins and promoted CaMKIV and
CREB phosphorylation (P<0.05). Furthermore, CREB and p-CREB
presented a similar variation trend where MCAO reduced CREB and
p-CREB expression (P<0.05), while EA promoted their expression
(P<0.05; Fig. 4).
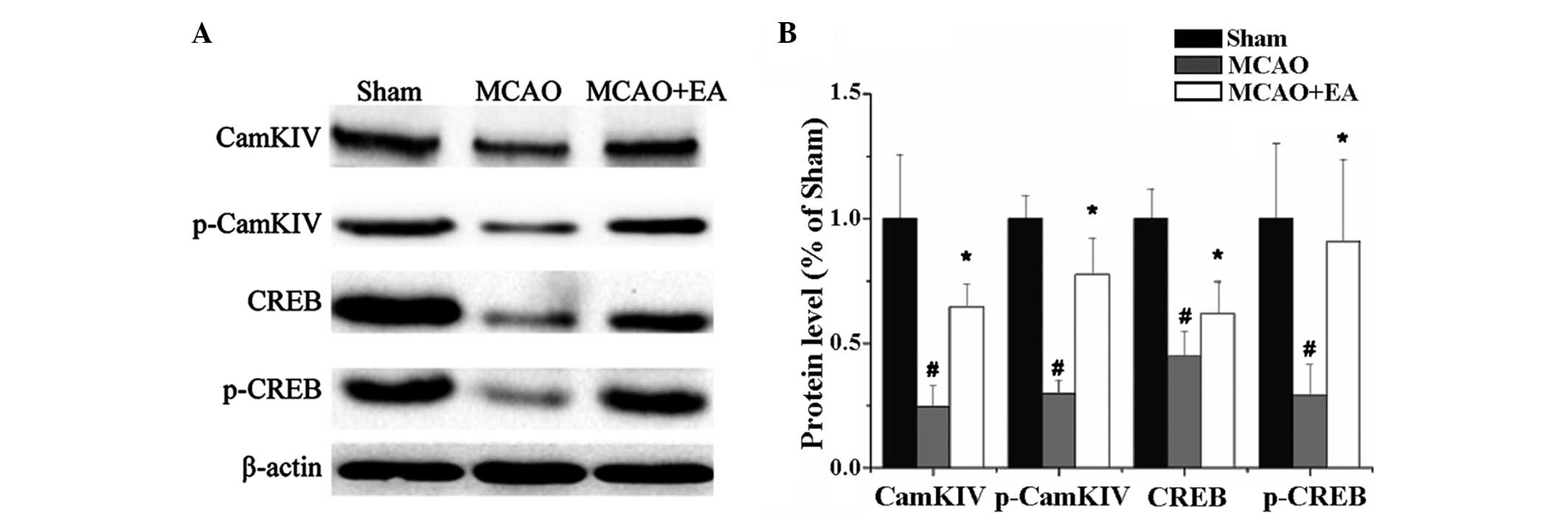 | Figure 4.(A) Protein expression levels of
CaMKIV, p-CaMKIV, CREB and p-CREB in each group. (B) Quantitative
analyses of the protein expression levels of CaMKIV, p-CaMKIV, CREB
and p-CREB, which were in accordance with the western blot analysis
results. #P<0.05, vs. sham group; *P<0.05, vs.
MCAO group. CaM, calmodulin; MCAO, middle cerebral artery
occlusion; EA, electroacupuncture; CaMKIV, calmodulin-dependent
protein kinase type IV; CREB, cyclic adenosine monophosphate
response elements binding protein; p-CREB, phosphorylated CREB;
p-CaMKIV, phosphorylated CaMKIV. |
Discussion
Therapeutics in clinical stroke treatment has led
researchers to question the feasibility of neuroprotection. Novel
insights into the cellular events responsible for neuronal death
and an improved understanding of the toxic and trophic roles of
excitatory neurotransmission are creating new avenues for
therapeutic research. The presence of protective signaling cascades
downstream of NMDAR activation, such as enhanced antioxidant
defenses, results in the suppression of proapoptotic signaling and
the maintenance of trophic signal events (31).
In the present study, according to the pathological
features of cerebral ischemia, where red tissues indicate normal
tissue and white sections indicate infarction, EA was demonstrated
to reduce the infarct volume. As the infarct volume decreased, the
behavior study was estimated by the step-down avoidance test. Using
this test in previous studies (23,24,32), EA
was shown to improve the memories of rats following stroke.
Previous clinical studies and meta-analyses have demonstrated that
EA exerts a positive effect on cognitive function when compared
with no acupuncture, medicine or rehabilitation (11,12,33).
Furthermore, a series of clinical trials have shown that
acupuncture regulates the release of neurochemicals, hemorheology,
cerebral microcirculation, metabolism, neuronal activity, and the
function of specific brain regions (10,34,35).
Animal studies have revealed that the effects of acupuncture
therapy on stroke may possibly be mediated through the inhibition
of post-ischemic inflammatory reactions, the stimulation of
neurogenesis and angiogenesis, and the influence on neural
plasticity (36,37).
Recently, improvement in cognitive dysfunction by EA
following stroke has attracted increasing interest. Physical
rehabilitation is not the only treatment aim, but also cognitive
improvement is closely associated with the quality of daily life
(38).
Transport of Ca2+/CaM from the surface
membrane to the nucleus activates CaMK kinase (CaMKK) and the
substrate, CaMKIV, the CREB kinase. This classical cellular
signaling pathway is considered to be closely associated with
cognitive function in the hippocampus. Ca2+ binding to
CaM, and the consequent activation of Ca2+/CaM-dependent
protein kinases combined with the CaM kinase family, contribute
strongly to synaptic potentiation, learning and memory. Additional
CaM kinases and CaMKIV form a CaMK cascade within the nucleus.
Neuronal activity and Ca2+/CaM drive CaMKK to
phosphorylate and activate nuclear CaMKIV, which phosphorylates
CREB and CREB-binding protein (39).
In the present study, a notable finding was that the expression and
activity of CaM in the MCAO group was significantly increased, in
contrast to the sham and EA groups. Thus, the pathological process
is yet to be fully elucidated. However, the expression levels of
the additional proteins varied as predicted. EA promoted CaMKIV,
p-CaMKIV, CREB and p-CREB protein expression. According to a
previous study, the inhibitory effect of EA on NF-κB activation led
to the inhibition of cerebral cell apoptosis and an improved
cognitive ability (21). Therefore,
the CaM-CaMKIV-CREB pathway may be an additional important cellular
signaling pathway involved in cognitive improvement.
The present study is preliminary investigation that
used rat models; thus, the effect of EA on post-stroke cognitive
impairment requires detailed evaluation in clinical practice. The
aim of the present study was to explain the detailed mechanism
underlying the effects of EA on cognitive impairment from a novel
perspective; however, the mechanisms of EA on stroke are complex,
comprehensive and wide. Therefore, this cellular signaling pathway
may not be the only neuronal signaling pathway involved in
cognitive impairment.
In conclusion, to the best of our knowledge, the
present study is the first to demonstrate that EA exhibits
excellent cognitive repair properties, with the underlying
mechanism closely associated with the CaM-CaMKIV-CREB signaling
pathway.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81273835).
References
|
1
|
Sator-Katzenschlager SM and
Michalek-Sauberer A: P-Stim auricular electroacupuncture
stimulation device for pain relief. Expert Rev Med Devices.
4:23–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toosizadeh N, Lei H, Schwenk M, Sherman
SJ, Sternberg E, Mohler J and Najafi B: Does integrative medicine
enhance balance in aging adults? Proof of concept for the benefit
of electroacupuncture therapy in Parkinson's disease. Gerontology.
61:3–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu HJ, Zhu JG, Shen P, Shi LH, Shi YC and
Chen F: Electroacupuncture decreases the urinary bladder pressure
in patients with acute gastrointestinal injury. Genet Mol Res.
14:34–39. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zyloney CE, Jensen K, Polich G, Loiotile
RE, Cheetham A, LaViolette PS, Tu P, Kaptchuk TJ, Gollub RL and
Kong J: Imaging the functional connectivity of the Periaqueductal
Gray during genuine and sham electroacupuncture treatment. Mol
Pain. 6:802010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inoue I, Fukunaga M, Koga K, Wang HD and
Ishikawa M: Scalp acupuncture effects of stroke studied with
magnetic resonance imaging: Different actions in the two stroke
model rats. Acupunct Med. 27:155–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haring HP: Cognitive impairment after
stroke. Curr Opin Neurol. 15:79–84. 2002.PubMed/NCBI
|
|
7
|
Cumming TB, Marshall RS and Lazar RM:
Stroke, cognitive deficits, and rehabilitation: Still an incomplete
picture. Int J Stroke. 8:38–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hachinski V and Munoz D: Vascular factors
in cognitive impairment - where are we now? Ann N Y Acad Sci.
903:1–5. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tatemichi TK, Desmond DW, Stern Y, Paik M,
Sano M and Bagiella E: Cognitive impairment after stroke:
Frequency, patterns and relationship to functional abilities. J
Neurol Neurosurg Psychiatry. 57:202–207. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li X and Wang Q: Acupuncture therapy for
stroke patients. Int Rev Neurobiol. 111:159–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li L, Zhang H, Meng SQ and Qian HZ: An
updated meta-analysis of the efficacy and safety of acupuncture
treatment of cerebral infarction. PLoS One. 9:e1140572014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shih CC, Hsu YT, Wang HH, Chen TL, Tsai
CC, Lane HL, Yeh CC, Sung FC, Chiu WT, Cherng YG and Liao CC:
Decreased risk of stroke in patients with traumatic brain injury
receiving acupuncture treatment: A population-based retrospective
cohort study. PLoS One. 9:e892082014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davis SM, Lees KR, Albers GW, Diener HC,
Markabi S, Karlsson G and Norris J: Selfotel in acute ischemic
stroke: Possible neurotoxic effects of an NMDA antagonist. Stroke.
31:347–354. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meller R, Minami M, Cameron JA, Impey S,
Chen D, Lan JQ, Henshall DC and Simon RP: CREB-mediated Bcl-2
protein expression after ischemic preconditioning. J Cereb Blood
Flow Metab. 25:234–246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kitagawa K: CREB and cAMP response
element-mediated gene expression in the ischemic brain. FEBS J.
274:3210–3217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ao H, Ko SW and Zhuo M: CREB activity
maintains the survival of cingulate cortical pyramidal neurons in
the adult mouse brain. Mol Pain. 2:152006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deisseroth K, Bito H and Tsien RW:
Signaling from synapse to nucleus: Postsynaptic CREB
phosphorylation during multiple forms of hippocampal synaptic
plasticity. Neuron. 16:89–101. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bok J, Wang Q, Huang J and Green SH:
CaMKII and CaMKIV mediate distinct prosurvival signaling pathways
in response to depolarization in neurons. Mol Cell Neurosci.
36:13–26. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Impey S, Fong AL, Wang Y, Cardinaux JR,
Fass DM, Obrietan K, Wayman GA, Storm DR, Soderling TR and Goodman
RH: Phosphorylation of CBP mediates transcriptional activation by
neural activity and CaM kinase IV. Neuron. 34:235–244. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng X, Yang S, Liu J, Huang J, Peng J,
Lin J, Tao J and Chen L: Electroacupuncture ameliorates cognitive
impairment through inhibition of NF-κB-mediated neuronal cell
apoptosis in cerebral ischemia-reperfusion injured rats. Mol Med
Rep. 7:1516–1522. 2013.PubMed/NCBI
|
|
22
|
Xue X, You Y, Tao J, Ye X, Huang J, Yang
S, Lin Z, Hong Z, Peng J and Chen L: Electro-acupuncture at points
of Zusanli and Quchi exerts anti-apoptotic effect through the
modulation of PI3K/Akt signaling pathway. Neurosci Lett. 558:14–19.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu L, Zhang L, Zhan LB, Lu X, Peng J,
Liang L, Liu Y, Zheng L, Zhang F and Liu Q: The effects of Zibu
Piyin Recipe components on scopolamine-induced learning and memory
impairment in the mouse. J Ethnopharmacol. 151:576–582. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsapakis EM, Fernandes C, Moran-Gates T,
Basu A, Sugden K, Aitchison KJ and Tarazi FI: Effects of
antidepressant drug exposure on gene expression in the developing
cerebral cortex. Synapse. 68:209–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Heo YM, Shin MS, Lee JM, Kim CJ, Baek SB,
Kim KH and Baek SS: Treadmill exercise ameliorates short-term
memory disturbance in scopolamine-induced amnesia rats. Int
Neurourol J. 18:16–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou H, Zhang Z, Wei H, Wang F, Guo F, Gao
Z, Marsicano G, Wang Q and Xiong L: Activation of STAT3 is involved
in neuroprotection by electroacupuncture pretreatment via
cannabinoid CB1 receptors in rats. Brain Res. 1529:154–164. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan H, West JA, Ramsay JP, Monson RE,
Griffin JL, Toth IK and Salmond GP: Comprehensive overexpression
analysis of cyclic-di-GMP signalling proteins in the phytopathogen
Pectobacterium atrosepticum reveals diverse effects on
motility and virulence phenotypes. Microbiology. 160:1427–1439.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu CP, Kuo MS, Wu BN, Chai CY, Huang HT,
Chung PW and Chen IJ: NO-releasing xanthine KMUP-1 bonded by
simvastatin attenuates bleomycin-induced lung inflammation and
delayed fibrosis. Pulm Pharacol Ther. 27:17–28. 2014. View Article : Google Scholar
|
|
29
|
Bowman PB and Puett D: Electron
paramagnetic resonance spectroscopy of nitroxide-labeled
calmodulin. Protein J. 33:267–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Willard SS and Koochekpour S: Glutamate,
glutamate receptors and downstream signaling pathways. Int J Biol
Sci. 9:948–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Balazs R: Trophic effect of glutamate.
Curr Top Med Chem. 6:961–968. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jing XH, Chen SL, Shi H, Cai H and Jin ZG:
Electroacupuncture restores learning and memory impairment induced
by both diabetes mellitus and cerebral ischemia in rats. Neurosci
Lett. 443:193–198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu F, Li ZM, Jiang YJ and Chen LD: A
meta-analysis of acupuncture use in the treatment of cognitive
impairment after stroke. J Altern Complem Med. 20:535–544. 2014.
View Article : Google Scholar
|
|
34
|
Chu Q, Wang L and Liu GZ: Effect of
acupuncture on hemorheology in patients with diabetic nephropathy.
Zhen Ci Yan Jiu. 32:335–337. 2007.(In Chinese). PubMed/NCBI
|
|
35
|
Zhang SQ, Wang YJ, Zhang JP, Chen JQ, Wu
CX, Li ZP, Chen JR, Ouyang HL, Huang Y and Tang CZ: Brain
activation and inhibition after acupuncture at Taichong and Taixi:
Resting-state functional magnetic resonance imaging. Neural Regen
Res. 10:292–297. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang C, Wen Y, Fan X, Yang S, Tian G,
Zhou X, Chen Y and Meng Z: A microarray study of middle cerebral
occlusion rat brain with acupuncture intervention. Evid Based
Complement Alternat Med. 2015:4969322015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Leung MC, Yip KK, Lam CT, Lam KS, Lau W,
Yu WL, Leung AK and So KF: Acupuncture improves cognitive function:
A systematic review. Neural Regen Res. 8:1673–1684. 2013.PubMed/NCBI
|
|
38
|
Chou PC, Chu HY and Lin JG: Effects of
electroacupuncture treatment on impaired cognition and quality of
life in Taiwanese stroke patients. J Altern Complem Med.
15:1067–1073. 2009.
|
|
39
|
Ma H, Groth RD, Cohen SM, Emery JF, Li B,
Hoedt E, Zhang G, Neubert TA and Tsien RW: γCaMKII shuttles
Ca(2+)/CaM to the nucleus to trigger CREB phosphorylation and gene
expression. Cell. 159:281–294. 2014. View Article : Google Scholar : PubMed/NCBI
|