Introduction
Despite advances in diagnostic imaging techniques
and the use of tumor markers, even with the development of spiral
computed tomography (CT) and positron emission tomography (PET),
the differentiation of pancreatic cancer and focal pancreatitis or
other mass lesions, such as retroperitoneal or pelvic lesions
remains problematic (1–3). Since the first report of endoscopic
ultrasound (EUS)-fine needle aspiration (FNA) of the pancreas by
Vilmann et al (4) in 1992,
EUS-FNA has been considered as a standard method for the diagnosis
of mass lesions in the pancreas because it is an effective and
accurate procedure with a low complication rate (5–8) and,
moreover, it provides cytological or pathological confirmation of
benign or malignant disease. It has also been recognized as a
minimally invasive and maximum accurate diagnostic procedure
(7,9). Furthermore, this least invasive
procedure is often suitable for use in the endoscopic procurement
of tissue from patients with unresectable tumors (10).
While EUS-FNA has been increasingly used as a
valuable diagnostic modality for mass lesions, the majority of
studies have collectively investigated primary pancreatic and
mediastinal lesions, including focal pancreatitis, pancreatic
neuroendocrine neoplasms and mediastinal lymphadenopathy (5,10–15), the
accuracy of which has previously been presented in the form of a
meta-analysis (16–18). However, very few studies have
examined other types of mass lesions, such as those affecting the
enterocoelia or retroperitoneum, due to the anatomical structural
challenges (19,20). Therefore, the aim of the present
study was to evaluate the diagnostic accuracy of EUS-FNA and to
investigate the associations of diagnostic findings with various
types of mass lesions.
Materials and methods
Patients
A total of 150 patients presenting to the Department
of Gastroenterology, Union Hospital, Tongji Medical College (Wuhan,
China) and undergoing EUS-FNA of mass lesions over a 4-year study
period, from June 2010 to March 2014, were enrolled in the study.
Suspicions of mass lesions were based on radiological findings or
abdominal imaging such as magnetic resonance imaging (MRI),
abdominal CT or transabdominal ultrasound. Targets included
gastrointestinal and extra-gastrointestinal mass lesions and
peri-gastrointestinal lymph nodes. Patients were excluded if they
had a blood coagulation disorder or had used non-steroidal
anti-inflammatory drugs or other anticoagulant drugs within 14 days
of the EUS-FNA. The study was approved by the Ethics Committee of
Tongji Medical College, Huazhong University of Science and
Technology (Wuhan, China). All patients provided signed informed
consent.
EUS technique
EUS was performed using an Olympus Ultrasound
Processor (EU-ME1) with a UCT-240 linear endoscope (Olympus
America, Inc., Center Valley, PA, USA). FNA was operated under EUS
guidance with 0, 5 or 10 ml of suction applied during aspiration
with either a 22-gauge or 19-gauge needle (22G Endocoil or 19G
Echotip; Cook Endoscopy, Winston-Salem, NC, USA). Needle passes
were processed about 1 to 6 times until the operator considered
that sample adequacy was achieved. Samples were prepared by EUS
assistants trained by cytology technicians and sent to the
pathology department for evaluation. EUS-FNA was performed by two
well-trained (>1,500 EUS procedures) endoscopists.
Data collection
Data collected included patient demographics
(gender, age and mass lesion location) and procedure details (tumor
characteristics and number of needle passes). Post-procedure
complications were defined as any symptoms requiring emergency
department evaluation, including bleeding, pneumothorax,
perforation, pancreatitis and other severe complications.
A diagnostic result was defined by cytological or
histological findings as EUS-FNA biopsy positive for tumor cells of
any type, acid-fast bacillus, mesenchymoma or leiomyoma.
Cytological or histological findings of negative for atypical cells
(inflammatory cells, phagocytes or epithelial cells), suspicious
and not obtaining adequate sample were considered non-diagnostic.
All the EUS-FNA results were compared with the gold standard of
surgical findings or follow-up examinations in non-operated cases
over a minimum period of 6 months and a final diagnosis was
determined.
Statistical analysis
Performance characteristics including sensitivity,
specificity, positive predictive value (PPV), negative predictive
value (NPV) and overall diagnostic accuracy were calculated.
Continuous variable results were reported as means with/without
standard deviation (SD). Dichotomous variables are shown as
percentages with or without 95% confidence intervals (CIs). The
χ2 test was used for comparisons of rates, and means
between two groups or three groups were assessed with
independent-samples t-tests and one-way analysis of
variance, respectively. Statistical analyses were performed with
SPSS software, version 19.0 (IBM SPSS, Armonk, NY, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
General characteristics
Data for 150 patients (57 female and 93 male)
undergoing EUS-FNA evaluation for mass lesions were entered into a
designed database. Study patients were relatively old (mean age,
54.3 years, range, 21–80 years). The mean lesion size was 3.5±1.9
cm (range, 0.4–10 cm). The numbers of pancreatic, mediastinal,
gastrointestinal, celiac and retroperitoneal lesions were 62
(41.3%), 29 (19.3%), 36 (24.0%) and 23 (15.4%) cases, respectively.
The number of passes performed per patient in this study was
between 1 and 6 with a mean of 2.4. The basic characteristics and
details of all cases (location and size of the lesions, as well as
the results of EUS-FNA) are listed in Table I.
 | Table I.Basic information of lesion
characteristics for all 150 patients, EUS-FNA results and final
diagnosis. |
Table I.
Basic information of lesion
characteristics for all 150 patients, EUS-FNA results and final
diagnosis.
|
| EUS-FNA results |
|---|
|
|
|
|---|
| Lesion location | Final diagnosis | Gender,
male/female | Mean age (years) | Mean size, (cm) | Mean no of.
passes | Positive | Negative |
|---|
| Pancreas (n=62) | Pancreatic
cancer | 25/7 | 58.28 | 3.19 | 2.00 | 26 | 6 |
|
| Chronic
pancreatitis | 1/2 | 51.33 | 2.34 | 2.00 | 3 | 0 |
|
| Autoimmune
pancreatitis | 2/1 | 58.00 | 3.60 | 2.00 | 1 | 2 |
|
| Solid pseudopapillary
tumor | 1/1 | 44.00 | 5.50 | 4.00 | 1 | 1 |
|
| Pancreatic
neuroendocrine neoplasm | 2/1 | 49.00 | 4.46 | 2.00 | 3 | 0 |
|
| Pancreatic
tuberculosis | 1/1 | 32.50 | 4.55 | 3.00 | 2 | 0 |
|
| Pancreatic
pseudocyst | 6/2 | 50.38 | 5.63 | 2.00 | 8 | 0 |
|
| True pancreatitis
cyst | 1/2 | 51.33 | 3.13 | 2.00 | 3 | 0 |
|
| Pancreatic
cystadenoma | 1/5 | 56.67 | 3.82 | 2.00 | 6 | 0 |
|
| Total | 40/22 | 54.68 | 3.73 | 2.63 | 53 | 9 |
| Mediastinum
(n=29) | Mediastinal lymph
node metastasis of lung cancer | 5/3 | 56.00 | 3.60 | 2.14 | 8 | 0 |
|
| Mediastinal
tumor | 5/0 | 61.80 | 4.40 | 2.60 | 5 | 0 |
|
| Lymphoma | 2/1 | 65.00 | 2.33 | 3.00 | 2 | 1 |
|
| Tuberculosis of
mediastinal lymph node | 3/1 | 35.25 | 2.86 | 2.25 | 4 | 0 |
|
| Phlogosis of
mediastinal lymph node | 3/3 | 54.83 | 2.28 | 2.60 | 4 | 2 |
|
| Sarcoidosis | 0/2 | 52.50 | 2.95 | 3.00 | 2 | 0 |
|
| Nerve sheath
tumors | 0/1 | 59.00 | 3.00 | 3.00 | 1 | 0 |
|
| Total | 18/11 | 54.69 | 2.98 | 2.46 | 26 | 3 |
| Gastrointestinal
tract (n=36) | Esophageal
cancer | 5/0 | 56.80 | 1.94 | 2.00 | 4 | 1 |
|
| Esophageal
leiomyoma | 2/1 | 40.66 | 3.25 | 3.00 | 3 | 0 |
|
| Esophageal
tuberculosis | 1/2 | 43.33 | 1.70 | 3.33 | 2 | 1 |
|
| Esophagitis | 0/1 | 59.00 | 2.20 | 2.00 | 1 | 0 |
|
| Esophageal
cyst | 0/1 | 61.00 | 1.20 | 2.00 | 0 | 1 |
|
| Physiological
thickening (esophagus, stomach and rectum) | 4/4 | 53.75 | 1.92 | 2.00 | 8 | 0 |
|
| Gastric
carcinoma | 3/1 | 55.50 | 2.78 | 3.00 | 3 | 1 |
|
| Gastrointestinal
stromal tumor | 7/0 | 56.86 | 6.48 | 2.33 | 6 | 1 |
|
| Duodenitis | 1/0 | 68.00 | 1.00 | 2.00 | 1 | 0 |
|
| Rectal
carcinoma | 3/0 | 48.33 | 1.24 | 4.20 | 2 | 1 |
|
| Total | 26/10 | 53.31 | 2.78 | 2.61 | 30 | 6 |
| Enterocoelia and
retroperitoneum (n=23) | Lymphoma | 1/5 | 58.67 | 3.50 | 1.67 | 5 | 1 |
|
| Peritoneal
tuberculosis | 0/1 | 22.00 | 3.50 | 3.00 | 1 | 0 |
|
| Celiac cyst | 2/2 | 58.00 | 4.40 | 2.33 | 0 | 4 |
|
| Liver cancer | 1/1 | 56.00 | 4.00 | 2.00 | 2 | 0 |
|
| Ewing's sarcoma of
soft tissue | 0/1 | 33.00 | 7.00 | 2.00 | 1 | 0 |
|
| Renal
carcinoma | 1/0 | 75.00 | 8.00 | 1.00 | 1 | 0 |
|
| Prostatic
cancer | 1/0 | 62.00 | 2.50 | 2.00 | 1 | 0 |
|
| Pseudomyxoma
peritonei | 1/0 | 68.00 | 3.70 | 4.00 | 1 | 0 |
|
| Nerve sheath
tumors | 0/1 | 65.00 | 4.50 | 2.00 | 1 | 0 |
|
| Celiac lymph node
metastasis of liver cancer | 2/2 | 46.00 | 2.25 | 2.00 | 4 | 0 |
|
| Omentum metastasis
of ovarian cancer | 0/1 | 49.00 | 2.30 | 2.00 | 1 | 0 |
|
| Total | 9/14 | 54.52 | 3.95 | 2.13 | 18 | 5 |
| All patients
(n=150) |
| 93/57 | 54.33 | 3.52 | 2.38 | 127 | 23 |
EUS-FNA findings
Adequate specimens for pathological assessment or
cytological examination were obtained for 136 patients (90.7%) and
23 (15.3%) cases had indeterminate results (non-diagnostic,
atypical or suspicious). The EUS-FNA diagnoses were as follows: 87
(58.0%) had malignant cytology, 3 (2%) were suspicious for
neoplasia, 6 (4.0%) had atypical cells and 40 (26.7%) were found to
be benign. There were 14 cases (9.3%) where the FNA was
non-diagnostic (an inadequate sample was obtained). Of the 23
patients with indeterminate results, 2 patients had surgical
pathology and in the remaining 21 cases, the diagnosis was based on
clinical follow-up examination. There were no false positive cases
or false negative cytological cases. The EUS-FNA findings are
detailed in Table II.
 | Table II.EUS-FNA cytological findings and
final diagnoses of targeted mass lesions. |
Table II.
EUS-FNA cytological findings and
final diagnoses of targeted mass lesions.
|
|
| Final diagnosis
(n) |
|
|---|
|
|
|
|
|
|---|
| EUS-FNA
diagnosis | N | Benign | Malignant | Final diagnosis of
malignancy type |
|---|
| Benign | 40 | 40 | 0 | – |
| Malignant | 87 | 0 | 87 | See Table I |
| Atypical | 6 | 2 | 4 | Pancreatic cancer
(n=3) and lymphoma in mediastinum (n=1) |
| Suspicious | 3 | 1 | 2 | Esophageal cancer
(n=1) and rectal carcinoma (n=1) |
| Non-diagnostic | 14 | 7 | 7 | Lymphoma in
retroperitoneum (n=1), pancreatic cancer (n=3), solid
pseudopapillary tumor (n=1), gastric carcinoma (n=1) and
gastrointestinal stromal tumor (n=1) |
| Total |
150 | 50 | 100 |
|
Diagnostic value of EUS-FNA
The overall diagnostic rate of EUS-FNA for all
lesions was 84.67% (127/150; 95% CI, 78.90–90.44%), and the
sensitivity, specificity, PPV, NPV, accuracy and Youden's index for
lesions at various locations are presented in Table III. The accuracy for mediastinal
lesions was the highest (89.66%); however, celiac and
retroperitoneal lesions had a diagnostic accuracy of only 78.2%,
which may be due to the presence of vital interferential
structures.
 | Table III.Diagnostic accuracy of EUS-FNA
evaluated on the basis of surgical findings or follow-up
examination. |
Table III.
Diagnostic accuracy of EUS-FNA
evaluated on the basis of surgical findings or follow-up
examination.
| Lesion
location | Lesion size
(cm) | No. of passes | Sensitivity
(%) |
Specificitya (%) | PPV (%) | NPV (%) | Accuracy (%) | Accuracy (95%
CI) | Youden's
indexa |
|---|
| Pancreas | 1.60–8.00 | 2–6 | 92.98 | – | 100.00 | 0.00 | 85.48 | 76.71–94.25 | – |
| Mediastinum | 1.10–5.00 | 2–5 | 96.30 | – | 100.00 | 0.00 | 89.66 | 78.58–100.00 | – |
|
Gastrointestinal | 0.40–7.00 | 2–6 | 91.67 | 100.00 | 100.00 | 80.00 | 83.33 | 71.15–95.51 | 0.92 |
| Celiac or
retroperitoneal | 0.60–9.60 | 1–4 | 90.00 | – | 100.00 | 0.00 | 78.23 | 61.40–95.12 | – |
| Total | 0.40–9.60 | 1–6 | 92.97 | 100.00 | 100.00 | 47.06 | 84.67 | 78.90–90.44 | 0.93 |
The masses were categorized into those associated
with parenchymal organs (n=66), luminal organs (n=36) and enlarged
lymph nodes (n=33). Parenchymal organs were more likely to have a
larger lesion diameter compared with luminal organs (P=0.03) and
enlarged lymph nodes (P=0.01). Otherwise, age, number of passes,
sensitivity and accuracy were similar among the three categories of
masses (P>0.05; Table IV). The
accuracy of EUS-FNA in the diagnosis of parenchymal organs, luminal
organs and enlarged lymph nodes was 86.36, 83.33 and 87.88%
respectively.
 | Table IV.Patient characteristics for masses of
parenchymal organs, luminal organs and enlarged lymph nodes
(n=135). |
Table IV.
Patient characteristics for masses of
parenchymal organs, luminal organs and enlarged lymph nodes
(n=135).
| Characteristic | Parenchymal organs
(n=66) | Luminal organs
(n=36) | Enlarged lymph
nodes (n=33) | P-value |
|---|
| Male | 43 | 26 | 16 | P=0.11 |
| Female | 23 | 10 | 17 |
| Mean age
(years) | 55.14 | 53.31 | 53.15 | P=0.65 |
| Mean lesion size
(cm) | 3.78 | 2.78 | 2.92 | Pa=0.03; Pb=0.01 |
| Mean no. of
passes | 2.52 | 2.61 | 2.30 | P=0.70 |
| Sensitivity
(%) | 93.44 | 91.67 | 96.67 | P=0.73 |
| Specificity
(%) | – | 100.00 | – | – |
| PPV (%) | 100.00 | 100.00 | 100.00 | – |
| NPV (%) | 0.00 | 80.00 | 0.00 | – |
| Accuracy (%) | 86.36 | 83.33 | 87.88 | P=0.85 |
Moreover, 95 (63.3%) lesions were considered as
solid masses and 22 (14.7%) as cystic masses. There was no
statistically significant difference in patient age (P=0.81) and
diagnostic sensitivity (P=1.00) between the these two types of
mass. The accuracy of diagnosis of solid masses was higher than
that of cystic masses (85.26 vs. 77.27%), although with no
statistical significance (P=0.56). Furthermore, no correlation was
observed with respect to the number of passes (P=0.38). However,
lesion size (P=0.04) and gender (P=0.03) had a statistically
significant difference between these two groups (Table V). Cystic masses were found to have a
larger diameter compared with solid masses (P<0.05). The
majority of solid masses were identified in men and cystic masses
in women.
 | Table V.Comparison of lesions and clinical
characteristics in patients with final diagnoses of solid and
cystic masses (n=150). |
Table V.
Comparison of lesions and clinical
characteristics in patients with final diagnoses of solid and
cystic masses (n=150).
| Characteristic | Solid masses
(n=95) | Cystic masses
(n=22) | P-value |
|---|
| Male | 67 | 10 | P=0.03 |
| Female | 28 | 12 |
| Age (years), mean ±
SD | 54.79±12.49 | 54.10±10.12 | P=0.81 |
| Lesion size (cm),
mean ± SD | 3.36±1.84 | 4.20±2.35 | P=0.04 |
| No. of passes, mean
± SD | 2.63±1.41 | 2.14±0.38 | P=0.38 |
| Sensitivity
(%) | 92.41 | 89.47 | P=1.00 |
| Specificity
(%) | 100.00 | – | – |
| PPV (%) | 100.00 | 100.00 | – |
| NPV (%) | 57.14 | 0.00 | – |
| Overall accuracy
(%) | 85.26 | 77.27 | P=0.56 |
Masses were also categorized into malignant and
benign masses. The final diagnosis, which was confirmed by the
pathological examination or follow-up surveillances, was malignant
in 100 cases and benign disease in 50 cases. The most frequently
observed type of malignant mass was pancreatic adenocarcinoma
(n=32; 21.3%). Other types of neoplasia included lymph node
metastasis of cancer (n=12; 8.0%), lymphoma (n=9; 6.0%) and
esophageal tumors (n=5; 3.3%). Older patients (P=0.01) and men
(P=0.03) were more likely to have malignant tumors. No association
was found between the FNA accuracy of benign (80.00%) and malignant
masses (87.00%), with a P-value of 0.26 (Table VI). No complications were identified
to be associated with the procedure in any of the 150 patients.
 | Table VI.Comparison of lesions and basic
characteristics in patients with final diagnoses of benign and
malignant masses. |
Table VI.
Comparison of lesions and basic
characteristics in patients with final diagnoses of benign and
malignant masses.
| Lesion type | Mean age
(years) | Male | Female | Lesion size
(cm) | Mean no. of
passes | Sensitivity
(%) | Specificity
(%) | Accuracy (%) |
|---|
| Benign (n=50) | 50.54 | 25 | 25 | 3.87 | 2.74 | 91.43 | 100.00 | 80.00 |
| Malignant
(n=100) | 56.22 | 68 | 32 | 3.20 | 2.35 | 93.55 | – | 87.00 |
| P-value | 0.01 | 0.03 |
| 0.08 | 0.16 | 0.98 | – | 0.26 |
Discussion
Multiple imaging modalities and techniques,
including PET, CT, MRI and ultrasound, have been used to evaluate
mass lesions; however, small or special lesions pose a diagnostic
challenge (21,22). EUS has been demonstrated to be the
most significantly advanced procedure, especially for smaller
lesions (<3 cm) (23). However,
distinguishing malignant from benign etiologies using EUS can be
difficult in certain clinical scenarios, such as chronic
pancreatitis and pancreatic neoplasm (1). EUS can only provide the tumor location,
size, shape, echo and boundary conditions, and is not able to
provide a histological diagnosis. EUS-FNA was introduced to aid in
the diagnosis and differentiation between lesion types.
Distinguishing adenocarcinoma from local pancreatitis has important
implications for prognosis and the method of treatment. A review of
the literature concerning EUS-FNA of pancreatic lesions reveals a
78–95% sensitivity, 75–100% specificity, 98–100% PPV, 46–80% NPV
and 78–95% accuracy (24–26). The present study found that the
accuracy of diagnosis of pancreatic lesions was 85.48% (53/62; 95%
CI 76.71–94.25%; Table II).
However, EUS-guided biopsy was not feasible in all cases (Table I). Nine patients were considered
non-diagnostic for pancreatic lesions because the mean number of
needle passes was insufficient (2.6 passes) and an adequate sample
was not obtained. LeBlanc et al (27) recommend that at least seven passes
with a fine needle into pancreatic lesions are required to ensure a
high degree of certainty for making a correct diagnosis.
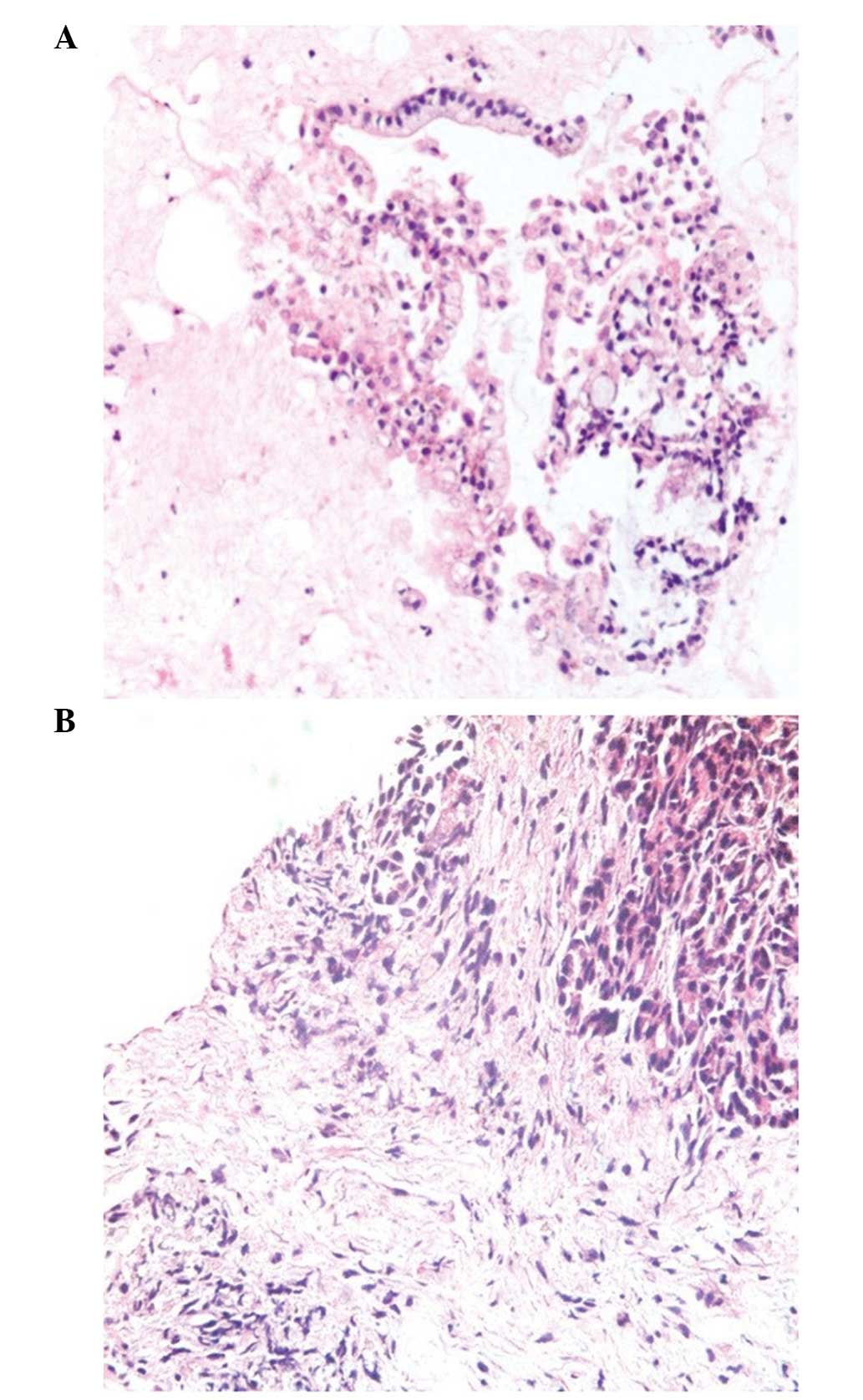
Among the patients in the present study, there was
one case that was diagnosed as a mass-forming focal chronic
pancreatitis (MFP); yet misdiagnosed as pancreatic cancer before
surgery or FNA was performed because pancreatic carcinoma could not
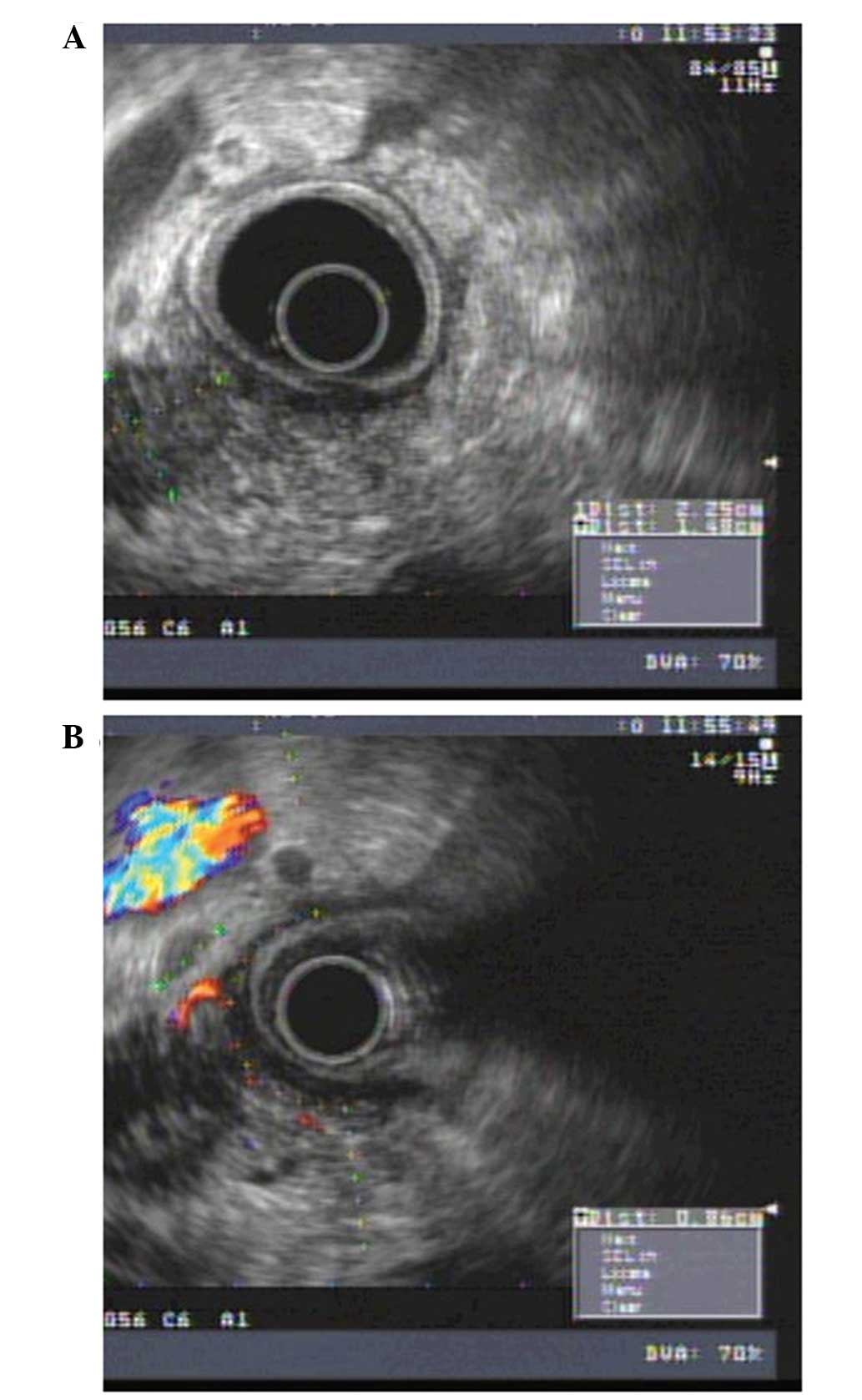
be absolutely ruled out under EUS (Figs.
1 and 2). Contrast harmonic
echo-EUS may increase the accuracy of detection of malignant
lesions in difficult cases (patients with chronic pancreatitis or
biliary stents) (28,29) and repeat EUS-FNA is able to provide a
conclusive diagnosis in the majority of cases (30).
Mediastinal lesions (lymph nodes) may be caused by
lymphoma, sarcoidosis or cancer metastasis. As there are various
potential types of pathogenesis, blind treatment, such as surgery,
may be an unnecessary burden for a patient. EUS-FNA can provide
important information for the further management of patients. In
the present study, the diameters of mediastinal lesions were
1.1–5.0 cm, and the sensitivity and accuracy of the EUS-FNA were
96.30 and 89.66%, respectively, which is concordant with previously
reported data (14). However, the
most inadequate specimens for pathological assessment or
cytological examination were obtained for celiac and
retroperitoneal lesions (5/23, 21.74%). Four cases were celiac
cysts. A study conducted by Maleki et al (31) showed that the primary tumor site for
such tumors included the colon or rectum, urinary bladder, prostate
and ovary, with EUS-FNA exhibiting 87% sensitivity and a diagnostic
accuracy of 90%. The accuracy determined in the present study was
lower (78.23%). This may be due to the presence of vital
interferential structures or the operating distance; power
conduction may not have been uniform, and the needle may have
failed to penetrate the mass. A further prospective study with a
large numbers of patients is necessary to confirm these results.
Real-time onsite cytopathology to increase the diagnostic yield and
reduce the number of indeterminate or unsatisfactory samples from
EUS-FNA may solve this problem (32–34).
In addition to use as a diagnostic technique,
EUS-FNA has also been developed as a therapeutic means, such as for
use in celiac plexus neurolysis, pseudocyst drainage, radiation
therapy, the delivery of antitumor agents and bile duct drainage
(35, 36). The total complication rate across the reported studies
concerning EUS-FNA ranges from 0 to 2.0% (37–39).
With the exception of some instances of mild abdominal discomfort,
no complications associated with EUS-FNA were observed in the 150
patients in the present study.
In conclusion, EUS-FNA has emerged as a powerful
modality for acquiring cytology results from types of lesion
diseases. The results of the present study demonstrated that
EUS-FNA provides an incomparable superiority for the investigation
of various masses and is able to diagnose suspected neoplastic
lesions with a high sensitivity and specificity. Furthermore, it is
a safe procedure with low complication rates, although more
high-quality, larger-scale and prospective studies are
required.
Acknowledgements
This study was supported in part by the National
Natural Science Foundation of China (grant nos. 81000160, 81272656,
30900664, 81470039 and 81170373).
References
|
1
|
Harewood GC and Wiersema MJ:
Endosonography-guided fine needle aspiration biopsy in the
evaluation of pancreatic masses. Am J Gastroenterol. 97:1386–1391.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sengupta S, Pal S, Biswas BK, Chakrabarti
S, Bose K and Jana S: Fine-needle aspiration cytology of
retroperitoneal lesions: A 5-year experience with an emphasis on
cytohistological discrepancy. Acta Cytol. 58:138–144. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han C, Lin R, Liu J, Hou X, Qian W and
Ding Z: Endoscopic Ultrasonography-Guided Biopsy for
Differentiation of Benign and Malignant Pelvic Lesions: A
Systematic Review and Meta-Analysis. Dig Dis Sci. 60:3771–3781.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vilmann P, Jacobsen GK, Henriksen FW and
Hancke S: Endoscopic ultrasonography with guided fine needle
aspiration biopsy in pancreatic disease. Gastrointest Endosc.
38:172–173. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen J, Yang R, Lu Y, Xia Y and Zhou H:
Diagnostic accuracy of endoscopic ultrasound-guided fine-needle
aspiration for solid pancreatic lesion: A systematic review. J
Cancer Res Clin Oncol. 138:1433–1441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho C, Dewitt J and Al-Haddad M:
Echo-endoscopy: New therapeutic frontiers. Minerva Gastroenterol
Dietol. 57:139–158. 2011.PubMed/NCBI
|
|
7
|
Krishna NB, LaBundy JL, Saripalli S,
Safdar R and Agarwal B: Diagnostic value of EUS-FNA in patients
suspected of having pancreatic cancer with a focal lesion on CT
scan/MRI but without obstructive jaundice. Pancreas. 38:625–630.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eloubeidi MA, Tamhane A, Varadarajulu S
and Wilcox CM: Frequency of major complications after EUS-guided
FNA of solid pancreatic masses: A prospective evaluation.
Gastrointest Endosc. 63:622–629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beane JD, House MG, Coté GA, DeWitt JM,
Al-Haddad M, LeBlanc JK, McHenry L, Sherman S, Schmidt CM, Zyromski
NJ, et al: Outcomes after preoperative endoscopic ultrasonography
and biopsy in patients undergoing distal pancreatectomy. Surgery.
150:844–853. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zeppa P, Barra E, Napolitano V, Cozzolino
I, Troncone G, Picardi M, De Renzo A, Mainenti PP, Vetrani A and
Palombini L: Impact of endoscopic ultrasound-guided fine needle
aspiration (EUS-FNA) in lymph nodal and mediastinal lesions: A
multicenter experience. Diagn Cytopathol. 39:723–729. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Turner BG, Cizginer S, Agarwal D, Yang J,
Pitman MB and Brugge WR: Diagnosis of pancreatic neoplasia with EUS
and FNA: A report of accuracy. Gastrointest Endosc. 71:91–98. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maker AV, Katabi N, Gonen M, DeMatteo RP,
D'Angelica MI, Fong Y, Jarnagin WR, Brennan MF and Allen PJ:
Pancreatic cyst fluid and serum mucin levels predict dysplasia in
intraductal papillary mucinous neoplasms of the pancreas. Ann Surg
Oncol. 18:199–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nguyen TQ, Kalade A, Prasad S, Desmond P,
Wright G, Hart D, Conron M and Chen RY: Endoscopic ultrasound
guided fine needle aspiration (EUS-FNA) of mediastinal lesions. ANZ
J Surg. 81:75–78. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dubravcsik Z, Serényi P, Madacsy L and
Szepes A: Endoscopic ultrasound-guided fine needle aspiration
cytology in the mediastinum. Orvosi Hetill. 154:338–344. 2013.(In
Hungarian). View Article : Google Scholar
|
|
15
|
Han C, Lin R, Yu J, Zhang Q, Zhang Y, Liu
J, Ding Z and Hou X: A case report of esophageal bronchogenic cyst
and review of the literature with an emphasis on endoscopic
ultrasonography appearance. Medicine (Baltimore). 95:e31112016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen G, Liu S, Zhao Y, Dai M and Zhang T:
Diagnostic accuracy of endoscopic ultrasound-guided fine-needle
aspiration for pancreatic cancer: A meta-analysis. Pancreatology.
13:298–304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Puli SR, Bechtold ML, Buxbaum JL and
Eloubeidi MA: How good is endoscopic ultrasound-guided fine-needle
aspiration in diagnosing the correct etiology for a solid
pancreatic mass? A meta-analysis and systematic review. Pancreas.
42:20–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Puli SR, Batapati Krishna Reddy J,
Bechtold ML, Ibdah JA, Antillon D, Singh S, Olyaee M and Antillon
MR: Endoscopic ultrasound: It's accuracy in evaluating mediastinal
lymphadenopathy? A meta-analysis and systematic review. World J
Gastroenterol. 14:3028–3037. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Korenblit J, Anantharama A, Loren DE,
Kowalski TE and Siddiqui AA: The role of endoscopic
ultrasound-guided fine needle aspiration (EUS-FNA) for the
diagnosis of intra-abdominal lymphadenopathy of unknown origin. J
Interv Gastroenterol. 2:172–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anderson B, Singh J and Jafri SF: Tumor
seeding following endoscopic ultrasonography-guided fine-needle
aspiration of a celiac lymph node. Dig Endosc. 25:344–345. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huh JW, Kwon SY, Lee JH and Kim HR:
Comparison of restaging accuracy of repeat FDG-PET/CT with pelvic
MRI after preoperative chemoradiation in patients with rectal
cancer. J Cancer Res Clin Oncol. 141:353–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moon JH, Choi HJ and Lee YN: Endoscopic
retrograde cholangiopancreatography. Endoscopy. 46:775–778. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kedia P, Gaidhane M and Kahaleh M:
Technical advances in endoscopic ultrasound (EUS)-guided tissue
acquisition for pancreatic cancers: How can we get the best results
with EUS-guided fine needle aspiration? Clin Endosc. 46:552–562.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Volmar KE, Vollmer RT, Jowell PS, Nelson
RC and Xie HB: Pancreatic FNA in 1000 cases: A comparison of
imaging modalities. Gastrointest Endosc. 61:854–861. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilson JL, Kalade A, Prasad S, Cade R,
Thomson B, Banting S, Mackay S, Desmond PV and Chen RY: Diagnosis
of solid pancreatic masses by endoscopic ultrasound-guided
fine-needle aspiration. Intern Med J. 39:32–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshinaga S, Suzuki H, Oda I and Saito Y:
Role of endoscopic ultrasound-guided fine needle aspiration
(EUS-FNA) for diagnosis of solid pancreatic masses. Dig Endosc.
23(Suppl 1): 29–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
LeBlanc JK, Ciaccia D, Al-Assi MT, McGrath
K, Imperiale T, Tao LC, Vallery S, DeWitt J, Sherman S and Collins
E: Optimal number of EUS-guided fine needle passes needed to obtain
a correct diagnosis. Gastrointest Endosc. 59:475–481. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fusaroli P, Spada A, Mancino MG and
Caletti G: Contrast harmonic echo-endoscopic ultrasound improves
accuracy in diagnosis of solid pancreatic masses. Clin
Gastroenterol Hepatol. 8:629–634, e622. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Napoleon B, Alvarez-Sanchez MV, Gincoul R,
Pujol B, Lefort C, Lepilliez V, Labadie M, Souquet J, Queneau PE,
Scoazec JY, et al: Contrast-enhanced harmonic endoscopic ultrasound
in solid lesions of the pancreas: Results of a pilot study.
Endoscopy. 42:564–570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun B, Yang X, Ping B, He Y and Zhang Z:
Impact of inconclusive endoscopic ultrasound-guided fine-needle
aspiration results in the management and outcome of patients with
solid pancreatic masses. Dig Endosc. 27:130–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maleki Z, Erozan Y, Geddes S and Li QK:
Endorectal ultrasound-guided fine-needle aspiration: A useful
diagnostic tool for perirectal and intraluminal lesions. Acta
Cytol. 57:9–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Iglesias-Garcia J, Dominguez-Munoz JE,
Abdulkader I, Larino-Noia J, Eugenyeva E, Lozano-Leon A and
Forteza-Vila J: Influence of on-site cytopathology evaluation on
the diagnostic accuracy of endoscopic ultrasound-guided fine needle
aspiration (EUS-FNA) of solid pancreatic masses. Am J
Gastroenterol. 106:1705–1710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alsohaibani F, Girgis S and Sandha GS:
Does onsite cytotechnology evaluation improve the accuracy of
endoscopic ultrasound-guided fine-needle aspiration biopsy? Can J
Gastroenterol. 23:26–30. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Campisi P, Accinelli G, De Angelis C,
Pacchioni D and Bussolati G: On-site evaluation and triage for
endoscopic ultrasound-guided fine needle aspiration cytology. The
Turin experience. Minerva Med. 98:395–400. 2007.(In Italian).
PubMed/NCBI
|
|
35
|
Al-Haddad M and Eloubeidi MA:
Interventional EUS for the diagnosis and treatment of locally
advanced pancreatic cancer. JOP. 11:1–7. 2010.PubMed/NCBI
|
|
36
|
Widmer JL and Michel K: Endoscopic
ultrasound-guided treatment beyond drainage: Hemostasis,
anastomosis and others. Clin Endosc. 47:432–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fisher L, Segarajasingam DS, Stewart C,
Deboer WB and Yusoff IF: Endoscopic ultrasound guided fine needle
aspiration of solid pancreatic lesions: Performance and outcomes. J
Gastroenterol Hepatol. 24:90–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Möller K, Papanikolaou IS, Toermer T,
Delicha EM, Sarbia M, Schenck U, Koch M, Al-Abadi H, Meining A,
Schmidt H, et al: EUS-guided FNA of solid pancreatic masses: High
yield of 2 passes with combined histologic-cytologic analysis.
Gastrointest Endosc. 70:60–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hwang CY, Lee SS, Song TJ, Moon SH, Lee D,
Park DH, Seo DW, Lee SK and Kim MH: Endoscopic ultrasound guided
fine needle aspiration biopsy in diagnosis of pancreatic and
peripancreatic lesions: A single center experience in Korea. Gut
Liver. 3:116–121. 2009. View Article : Google Scholar : PubMed/NCBI
|