Introduction
Frequency and severity of joint loading are
important determinants for the development of joint destruction.
Osteoarthritis, which develops due to the progressive destruction
of articular cartilage, is the most common joint disease and is the
leading cause of pain and physical disability in elderly
individuals (1–3). Knee joints are particularly affected in
patients with osteoarthritis, as they are weight-bearing joints.
Previous studies investigating experimental osteoarthritis models
have demonstrated that the early changes in the metabolic and
chemical properties of cartilage matrix can be detected prior to
the appearance of radiological changes (4). Therefore, various molecular markers
have been developed as indicators of cartilage metabolism in
patients with joint disorders (5–8).
Furthermore, biomarkers are used for evaluating the effects of
disease-modifying drugs, since they specifically reflect
alterations in the metabolism of cartilage (9).
Type II collagen is one of the major constituents of
cartilage and represents 90–95% of the total collagen in cartilage
(7). Therefore, fragments of type II
collagen have been targeted as biomarkers for cartilage metabolism.
C-terminal crosslinking peptide II (CTX-II) is cleaved during the
degradation of type II collagen (10), whereas a C2C neoepitope is generated
by intrahelical cleavage at the C terminus of the 3/4 piece of
degraded type II collagen (11).
Both CTX-II and C2C are used as markers for type II collagen
degradation. In contrast, a C-terminal type II procollagen peptide
(CPII or PIICP), which is localized in newly formed type II
collagen and cleaved during processing of synthesized type II
procollagen, can be used as a marker for type II collagen synthesis
(12).
Nutritional supplements, including glucosamine,
N-acetylglucosamine, chondroitin and collagen, are used for ‘joint
health’ to treat or prevent cartilage disorders, including
osteoarthritis (13–15). Among these, glucosamine inhibits the
degradation and stimulates the synthesis of the characteristic
glycosaminoglycan polysaccharide chains of proteoglycans (16,17).
Furthermore, glucosamine suppresses the expression of
collagen-degrading enzymes, such as matrix metalloproteinases
(MMPs), whereas it increases the expression of type II collagen in
chondrocytes (18,19). Based on these observations, it has
been hypothesized that glucosamine exerts a chondroprotective
effect on cartilage disorders by retaining proteoglycans and type
II collagen in the articular cartilage, thus glucosamine may be
used to treat osteoarthritis in humans (20–22). A
previous study evaluated the effect of glucosamine on cartilage
metabolism in healthy bicycle racers (mean age, 20 years) with
normal cartilage metabolism, as assessed by the levels of CTX-II
and CPII (23). The results
indicated that oral administration of glucosamine may exert a
chondroprotective effect in healthy individuals by preventing type
II collagen degradation and simultaneously maintaining type II
collagen synthesis.
N-acetylglucosamine (GlcNAc), a derivative of
glucosamine, has been reported to stimulate hyaluronan synthesis
via the upregulation of hyaluronan synthase-2 in chondrocytes
(24), whereas it inhibits the
interleukin (IL)-1β-mediated expression of inducible nitric oxide
(NO) synthase, cyclooxygenase-2 and IL-6 in chondrocytes (25). Therefore, it is hypothesized that
GlcNAc exerts chondroprotective and antiinflammatory effects in
cartilage disorders. In previous studies investigating experimental
osteoarthritis models, intra-articular injection of GlcNAc has been
demonstrated to exhibit a chondroprotective effect (26,27).
Moreover, the administration of a GlcNAc-containing beverage has
been shown to enhance type II collagen synthesis, and resolves the
symptoms of patients with knee osteoarthritis (28). Therefore, GlcNAc is hypothesized to
exhibit a chondroprotective effect in healthy individuals by
improving cartilage metabolism.
To the best of our knowledge, no previous studies
have demonstrated the effect of GlcNAc on cartilage metabolism in
healthy individuals. Therefore, in the present study, to evaluate
the effect of GlcNAc on the joint health of healthy individuals
without symptoms of joint disorders, the effect of oral
administration of low (500 mg/day) and high (1,000 mg/day) dose
GlcNAc on cartilage metabolism in healthy middle-aged adults (mean
age,48.6±1.3 years; range, 23–64 years) by analyzing the ratio of
type II collagen degradation to synthesis using type II collagen
degradation (C2C) and synthesis (PIICP) markers.
Materials and methods
Study design
The present prospective randomized double-blind
placebo-controlled, parallel-group comparative study was designed
to assess the effects of a GlcNAc-containing test supplement and
placebo on cartilage metabolism (by proxy of type II collagen
synthesis and degradation) in healthy individuals without any joint
disorder symptoms. The safety of the test supplement was also
evaluated. This study was performed from January 2014 to August
2014, and involved three clinical service organization centers in
Japan. The study protocol was approved by the local ethics
committee of Tana Orthopedic Surgery and was conducted in
accordance with the Declaration of Helsinki and the Ethical
Guidelines for Epidemiological Research outlined by the Japanese
Government in 2008. Written informed consent was obtained from all
participants prior to their enrollment in the study. The design of
the study consisted of a 4-week run-in (screening) period, a
16-week intervention period and a subsequent 4-week follow-up
period without intervention. Subjects were screened for baseline
values at a run-in visit, which included a physical examination, a
knee radiograph according to a standardized method (29), a symptom questionnaire and routine
laboratory tests. In total, medical examinations and laboratory
tests were performed at baseline, weeks 4, 8, 12 and 16 during the
intervention, and 4 weeks after the intervention for all enrolled
subjects.
Subjects
Exclusion criteria were as follows:
Gout/hyperuricemia or rheumatoid arthritis; surgical treatment of
joint(s) or its necessity; clinical history of bone or cartilage
disorders (including fracture and distortion) within one year of
enrollment; routine use of health foods containing glucosamine,
chondroitin sulfate, collagen peptides or any other constituents of
the test supplement within 3 months of enrollment; hypersensitivity
or allergy to constituents of the test supplement; previous or
current medication for malignancies, hypertension, cardiac
diseases, renal diseases, thyroid diseases or cerebral infarction;
intra-articular injections of either corticosteroids or hyaluronic
acid within one year of enrollment; severe exercise with excessive
motion and exposure which places load on the joints; intake of
>60 g alcohol/day; pregnancy, nursing mothers or women of
child-bearing potential during the study period; participation in
other clinical studies within one month of enrollment; and the
presence of any clinically significant conditions judged by the
medical investigator to preclude the subject's participation in the
present study.
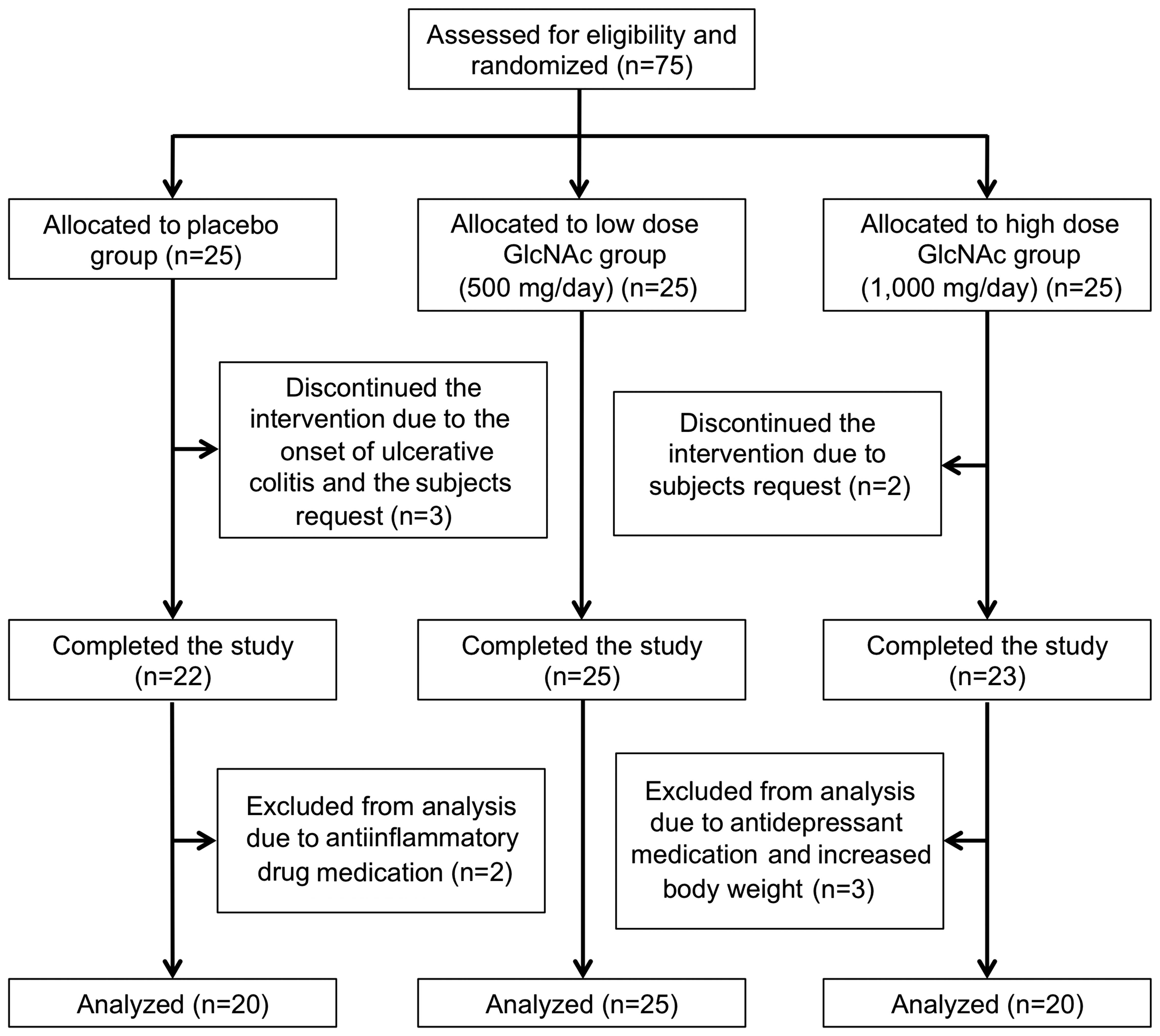
Based on the criteria outlined, 75 male and female
Japanese adults (mean age, 49.3±1.3 years; range, 23–64 years)
without clinical or radiographic evidence of knee osteoarthritis
(Kellgren and Lawrence grades 0–1, predominantly 0) (29) were enrolled as eligible subjects.
Subjects were randomly assigned to high (n=25) or low (n=25) dose
GlcNAc, or placebo (n=25) groups (Fig.
1). However, during the intervention period, five subjects
discontinued the study of their own accord or the onset of
ulcerative colitis (placebo group, n=3; high dose GlcNAc group,
n=2). Therefore, 70 subjects completed the present study. Five
subjects were subsequently excluded from the study analysis by the
medical investigator due to treatment with antidepressants for
depressive disorders and antiinflammatory agents for ankle and
Achilles-tendon pains, and increased body weight (>4 kg) during
the intervention period, as these may affect the efficacy of the
test supplement (placebo group, n=2; high dose GlcNAc group, n=3).
Therefore, 65 subjects (mean age, 48.6±1.3 years) in the placebo
(n=20), low dose GlcNAc (n=25) and high dose GlcNAc (n=20) groups
were eligible for the assessment of the efficacy of the test
supplement (Table I).
 | Table I.Baseline characteristics of the
subjects in the placebo and low and high dose GlcNAc test
supplement groups. |
Table I.
Baseline characteristics of the
subjects in the placebo and low and high dose GlcNAc test
supplement groups.
|
|
| GlcNAc-containing
test supplement |
|
|---|
|
|
|
|
|
|---|
| Variable | Placebo (n=20) | Low dose (n=25) | High dose (n=20) | P-value |
|---|
| Age (years) | 48.8±2.1 | 48.0±2.2 | 49.2±2.7 | 0.927 |
| Male/female (N) | 10/10 | 10/15 | 9/11 | 0.779 |
| Height (cm) | 164.33±1.58 | 162.22±1.73 | 163.51±2.04 | 0.695 |
| Weight (kg) | 57.68±2.47 | 57.48±2.36 | 62.39±2.87 | 0.327 |
| Body mass index
(kg/m2) | 21.23±0.66 | 21.63±0.56 | 23.22±0.80 | 0.107 |
| Systolic blood
pressure (mmHg) | 120.0 ± 3.1 | 110.2±2.0 | 114.5±2.33 | 0.020 |
| Diastolic blood
pressure (mmHg) | 76.8±2.1 | 71.7±1.5 | 75.1±1.8 | 0.126 |
| Pulse rate
(beats/min) | 68.5±2.0 | 70.1±2.1 | 71.3±1.9 | 0.632 |
| Kellgren and
Lawrence grade (0:1)a |
|
|
|
|
| Right
knee | 18:2 | 22:3 | 18:2 | 1.000 |
| Left
knee | 18:2 | 21:4 | 18:2 | 0.803 |
| C2C
(ng/ml)b | 226.57±11.08 | 224.47±7.55 | 226.08±9.07 | 0.985 |
| PIICP
(ng/ml)c | 48.37±2.25 | 48.47±1.99 | 47.99±2.16 | 0.986 |
| C2C/PIICP
ratio | 4.89±0.34 | 4.87±0.29 | 4.95±0.33 | 0.983 |
Intervention and subject
assignment
The test supplement was manufactured by Q'sai Co.,
Ltd (Fukuoka, Japan) in a powdered preparation containing 1,000 mg
GlcNAc for the high dose group, and 500 mg GlcNAc and 500 mg
maltodextrin (vehicle) for the low dose group. Subjects were
randomly assigned to either the high (1,000 mg GlcNAc) or low (500
mg GlcNAc and 500 mg maltodextrin) dose GlcNAc groups, or the
placebo group, (1,000 mg maltodextrin vehicle). All subjects were
instructed to take the test supplement or placebo (dissolved in 100
ml water) once daily at any time of the day. The daily dose of the
test supplement in the present study was determined according to
the results of the previous study (16). Intervention was continued for 16
weeks, and adherence to the intervention protocol was evaluated
based on the consumption record in the study diary; <80%
adherence was considered a protocol violation.
Serum and second morning void of urine samples were
collected from subjects in a fasting state, at baseline, weeks 4,
8, 12 and 16 during intervention, and 4 weeks post-intervention.
Aliquots of serum and urine samples were stored at −80°C until use,
whereas the remaining aliquots of serum and urine samples were
immediately used for routine laboratory tests.
Evaluation of efficacy and safety
To evaluate the effect of the GlcNAc-containing test
supplement on cartilage metabolism, serum samples collected at
baseline, weeks 8, 12 and 16 during intervention, and 4 weeks after
intervention, were analyzed by assays for type II collagen
degradation and synthesis markers (C2C and PIICP, respectively).
Serum C2C and PIICP were measured using Collagen Type II Cleavege
(IBEX Pharmaceuticals Inc., Montreal, Canada) and Procollagen II
C-Terminal Propeptide (USCN Life Science Inc., Wuhan, China) ELISA
kits, respectively. In addition, the C2C/PIICP ratio was calculated
and compared among the test supplement and placebo groups.
Safety and tolerability were assessed throughout the
study on the basis of the incidence and severity of
intervention-related adverse events, as well as abnormal changes in
blood pressure, pulse rate and laboratory tests, including
hematology, biochemical profiling and urinalysis.
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Using the baseline characteristics of enrolled subjects,
the sex distribution and Kellgren and Lawrence grades were analyzed
by χ2 test, whereas other parameters were analyzed by
one-way analysis of variance among the placebo and low and high
dose GlcNAc test supplement groups. In addition, the changes in
biomarker levels from the baseline during and after intervention
were compared among the placebo and low and high dose GlcNAc test
supplement groups, and between the placebo and test supplement
groups by Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characterization of study groups
Table I presents the
baseline characteristics of the 65 subjects (placebo group, n=20;
test supplement groups: low dose GlcNAc, n=25 and high dose GlcNAc,
n=20), who fulfilled the eligibility criteria and completed the
present study. Baseline characteristics included demographic
characteristics (age and sex), physiological characteristics (body
height, body weight, body mass index, systolic and diastolic blood
pressure, and pulse rate), distribution of Kellgren and Lawrence
grades, and the levels of biomarkers for type II collagen
metabolism (C2C, PIICP and C2C/PIICP ratio). No significant
differences in baseline characteristics were detected among the
placebo and test supplement (low and high dose GlcNAc) groups, with
the exception of systolic blood pressure. Although systolic blood
pressure values were significantly different among the groups
(P<0.05), they remained within the normal range (<130 mmHg).
Adherence to the allotted dietary supplement exceeded 96% in all of
the who completed the study (n=70).
Assessment of cartilage metabolism
using type II collagen degradation and synthesis markers
It has been suggested that the ratio of type II
collagen degradation to synthesis is suitable for the prediction of
joint damage progression in patients with knee osteoarthritis
(30,31). Therefore, to evaluate the effect of a
GlcNAc-containing test supplement on cartilage metabolism, the
C2C/PIICP ratio was assessed using serum samples collected at
baseline, weeks 8, 12 and 16 during intervention, and 4 weeks after
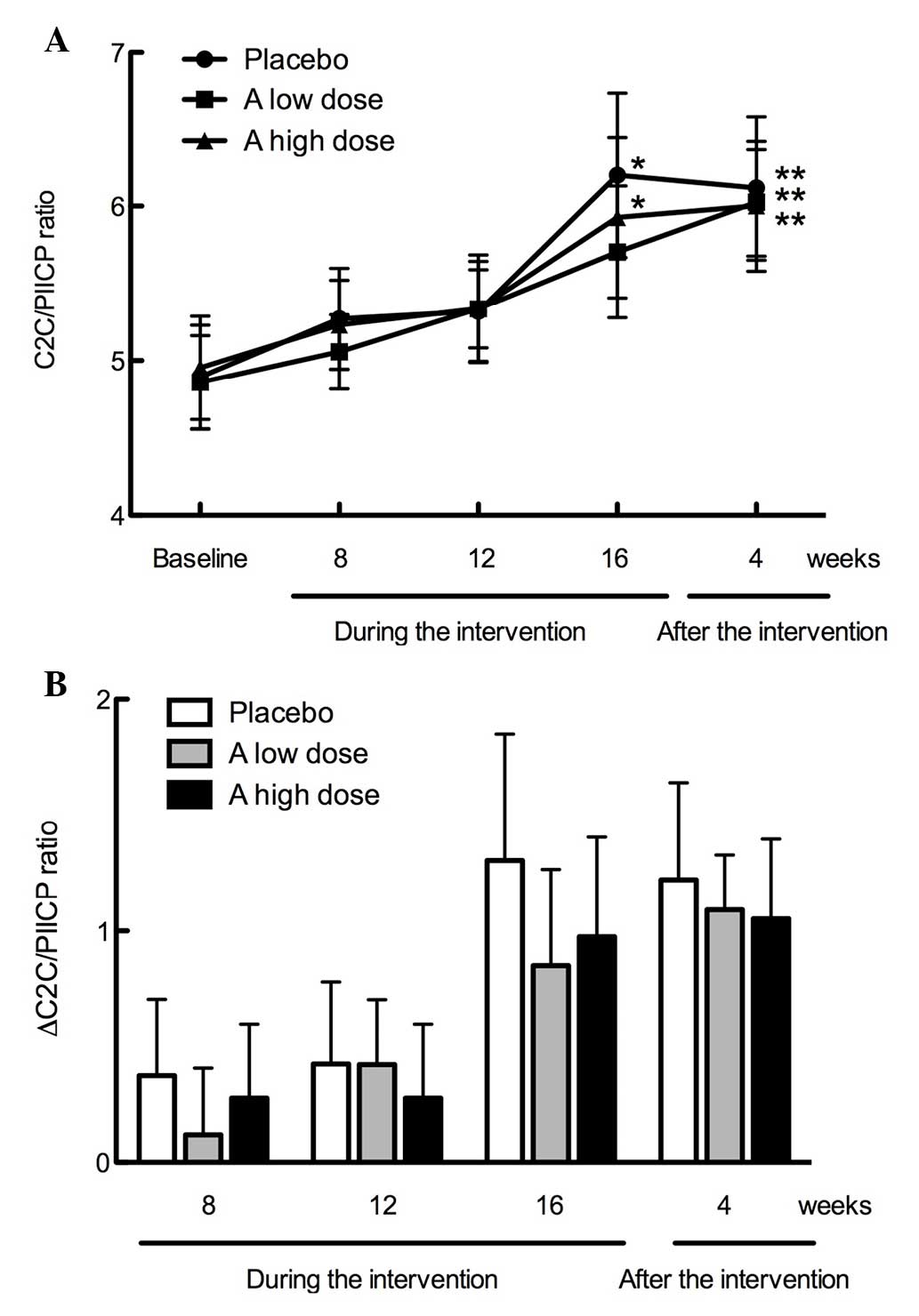
intervention. As demonstrated in Fig.
2A, C2C/PIICP ratios peaked at 16 weeks during intervention in
the placebo group and high dose of GlcNAc (P<0.05), and
maintained almost the same level 4 weeks after intervention
(P<0.01). By contrast, the C2C/PIICP ratios gradually increased
in the low dose of GlcNAc group during the 16-week intervention and
4 weeks post-intervention (P<0.01; 4 weeks after the
intervention). However, there was no significant difference among
the three groups. Following intervention, the C2C/PIICP ratios were
maintained at almost the same level (P<0.01 in the C2C/PIICP
ratios in the low and high doses of GlcNAc and placebo groups) 4
weeks after intervention, compared with the baseline. Notably, the
∆C2C/PIICP ratios from the baseline were markedly suppressed in the
low and high dose GlcNAc test supplement groups (+0.81 and +0.97,
respectively), compared with the placebo group (+1.31) at week 16
during the intervention. Furthermore, 4 weeks post-intervention,
the ∆C2C/PIICP ratio in the test supplement groups (low and high
dose GlcNAc) returned to the same level as the placebo group
(Fig. 2B).
To further elucidate the effects of the
GlcNAc-containing test supplement, the subjects with impaired
cartilage metabolism were assessed. For this purpose, subjects with
reduced type II collagen degradation (<220 ng/ml C2C) and
enhanced type II collagen synthesis (≥60 ng/ml PIICP) were
excluded, and subjects with ≥220 ng/ml C2C and <60 ng/ml PIICP
were evaluated. Table II presents
the baseline characteristics of these subjects, including
demographic and physiological characteristics, Kellgren and
Lawrence grade distribution, and the levels of biomarkers for type
II collagen metabolism. Among the placebo (n=7) and low (n=12) and
high (n=10) dose GlcNAc test supplement groups, these parameters
were not significantly different, with the exception of systolic
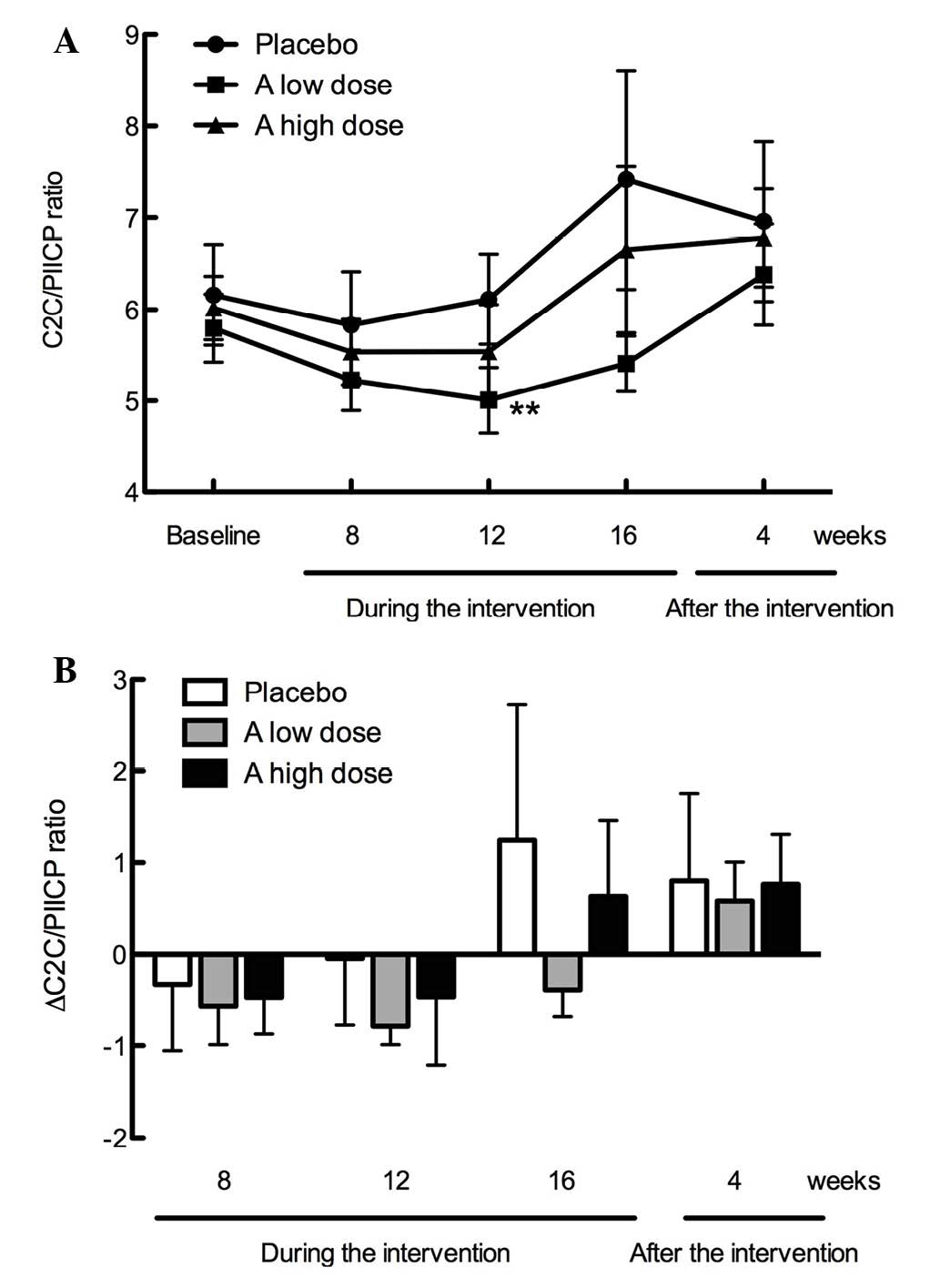
blood pressure. As shown in Fig. 3A,
the C2C/PIICP ratios were not significantly different among the
three groups during intervention (16 weeks) and after intervention
(4 weeks); however, the C2C/PIICP ratio was significantly decreased
at week 12 of intervention in the low dose GlcNAc group
(P<0.01). As shown in Fig. 3B,
the ∆C2C/PIICP ratios from the baseline were markedly suppressed in
the low and high dose GlcNAc groups (week 12, −0.79 and −0.47; and
week 16, −0.39 and +0.63), as compared with the placebo group (week
12, −0.05 and week 16, +1.25). The ∆C2C/PIICP ratios in the test
supplement groups returned to the same levels as the placebo group
4 weeks after intervention (Fig.
3B).
 | Table II.Baseline characteristics of subjects
with ≥220 ng/ml C2C and <60 ng/ml PIICP in the placebo and low
and high dose GlcNAc test supplement groups. |
Table II.
Baseline characteristics of subjects
with ≥220 ng/ml C2C and <60 ng/ml PIICP in the placebo and low
and high dose GlcNAc test supplement groups.
|
|
| GlcNAc-containing
test supplement |
|
|---|
|
|
|
|
|
|---|
| Variable | Placebo (n=7) | Low dose
(n=12) | High dose
(n=10) | P-value |
|---|
| Ages (years) | 51.7±3.8 | 46.5 ± 4.0 | 50.0 ± 3.8 | 0.647 |
| Male/female
(N) | 4/3 | 3/9 | 5/5 | 0.393 |
| Height (cm) | 165.39±3.32 | 161.40±2.52 | 166.26±2.86 | 0.408 |
| Weight (kg) | 55.01±3.72 | 58.68±4.04 | 60.79±4.74 | 0.689 |
| Body mass index
(kg/m2) | 19.98±0.77 | 22.25±1.01 | 21.71±1.02 | 0.318 |
| Systolic blood
pressure (mmHg) | 122.9±5.2 | 108.5±2.8 | 117.4±3.5 | 0.032 |
| Diastolic blood
pressure (mmHg) | 77.9±4.3 | 71.8±2.0 | 77.6±2.5 | 0.196 |
| Pulse rate
(beats/min) | 68.9±2.4 | 68.1±2.6 | 73.4±3.2 | 0.356 |
| Kellgren and
Lawrence grade (0:1)a |
|
|
|
|
| Right
knee | 7:0 | 11:1 | 9:1 | 1.000 |
| Left
knee | 7:0 | 10:2 | 9:1 | 0.770 |
| C2C
(ng/ml)b | 277.63±16.09 | 249.77±8.58 | 255.79±10.93 | 0.248 |
| PIICP
(ng/ml)c | 46.43±3.25 | 44.58±2.45 | 43.12±1.66 | 0.678 |
| C2C/PIICP
ratio | 6.16±0.55 | 5.80±0.37 | 6.02±0.35 | 0.825 |
Notably, the change in the ∆C2C/PIICP ratios from
the baseline was markedly suppressed in the low dose GlcNAc group
(−0.79 and −0.39, respectively), as compared with the high dose
GlcNAc group (−0.47 and +0.63, respectively) at weeks 12 and 16
during intervention. This may be due to the difference in the mean
body weight between the two groups, since heavy body weight may
place an increased load on the joints, thereby affecting the
response of cartilage metabolism to the test supplement. Based on
this hypothesis, subjects with a body weight of ≥70 kg were
excluded, and subjects weighing <70 kg were subsequently
evaluated. Table III presents the
baseline characteristics of the subjects who weighed <70 kg and
exhibited ≥220 ng/ml C2C and <60 ng/ml PIICP. No significant
differences in demographic and physiological characteristics,
Kellgren and Lawrence grade distribution, and the levels of
biomarkers for type II collagen metabolism were detected among the
placebo (n=7) and test supplement (low dose GlcNAc, n=10 and high
dose GlcNAc, n=7) groups, with the exception of systolic blood
pressure and C2C.
 | Table III.Baseline characteristics of subjects
weighing <70 kg with ≥220 ng/ml C2C and <60 ng/ml PIICP in
the placebo and low and high dose GlcNAc test supplement
groups. |
Table III.
Baseline characteristics of subjects
weighing <70 kg with ≥220 ng/ml C2C and <60 ng/ml PIICP in
the placebo and low and high dose GlcNAc test supplement
groups.
|
|
| GlcNAc-containing
test supplement |
|
|---|
|
|
|
|
|
|---|
| Variable | Placebo (n=7) | Low dose
(n=10) | High dose
(n=7) | P-value |
|---|
| Ages (years) | 51.7±3.8 | 44.1±4.3 | 48.9±5.1 | 0.472 |
| Male/female
(N) | 4/3 | 2/8 | 2/5 | 0.319 |
| Height (cm) | 165.39±3.32 | 159.53±2.64 | 161.51±2.24 | 0.337 |
| Weight (kg) | 55.01±3.72 | 54.29±3.28 | 51.91±1.65 | 0.791 |
| Body mass index
(kg/m2) | 19.98±0.77 | 21.16±0.80 | 19.91±0.54 | 0.400 |
| Systolic blood
pressure (mmHg) | 122.9±5.2 | 106.3±2.3 | 114.7±3.9 | 0.013 |
| Diastolic blood
pressure (mmHg) | 77.9±4.3 | 69.7±1.7 | 75.4±3.2 | 0.139 |
| Pulse rate
(beats/min) | 68.9±2.4 | 69.6±2.8 | 77.3±2.8 | 0.100 |
| Kellgren and
Lawrence grade (0:1)a |
|
|
|
|
| Right
knee | 7:0 | 9:1 | 6:1 | 1.000 |
| Left
knee | 7:0 | 8:2 | 7:1 | 0.315 |
| C2C
(ng/ml)b | 277.63±16.09 | 238.62±5.00 | 258.00±11.63 | 0.048 |
| PIICP
(ng/ml)c | 46.43±3.25 | 44.23±2.95 | 42.73±2.14 | 0.702 |
| C2C/PIICP
ratio | 6.16±0.55 | 5.64±0.43 | 6.13±0.41 | 0.654 |
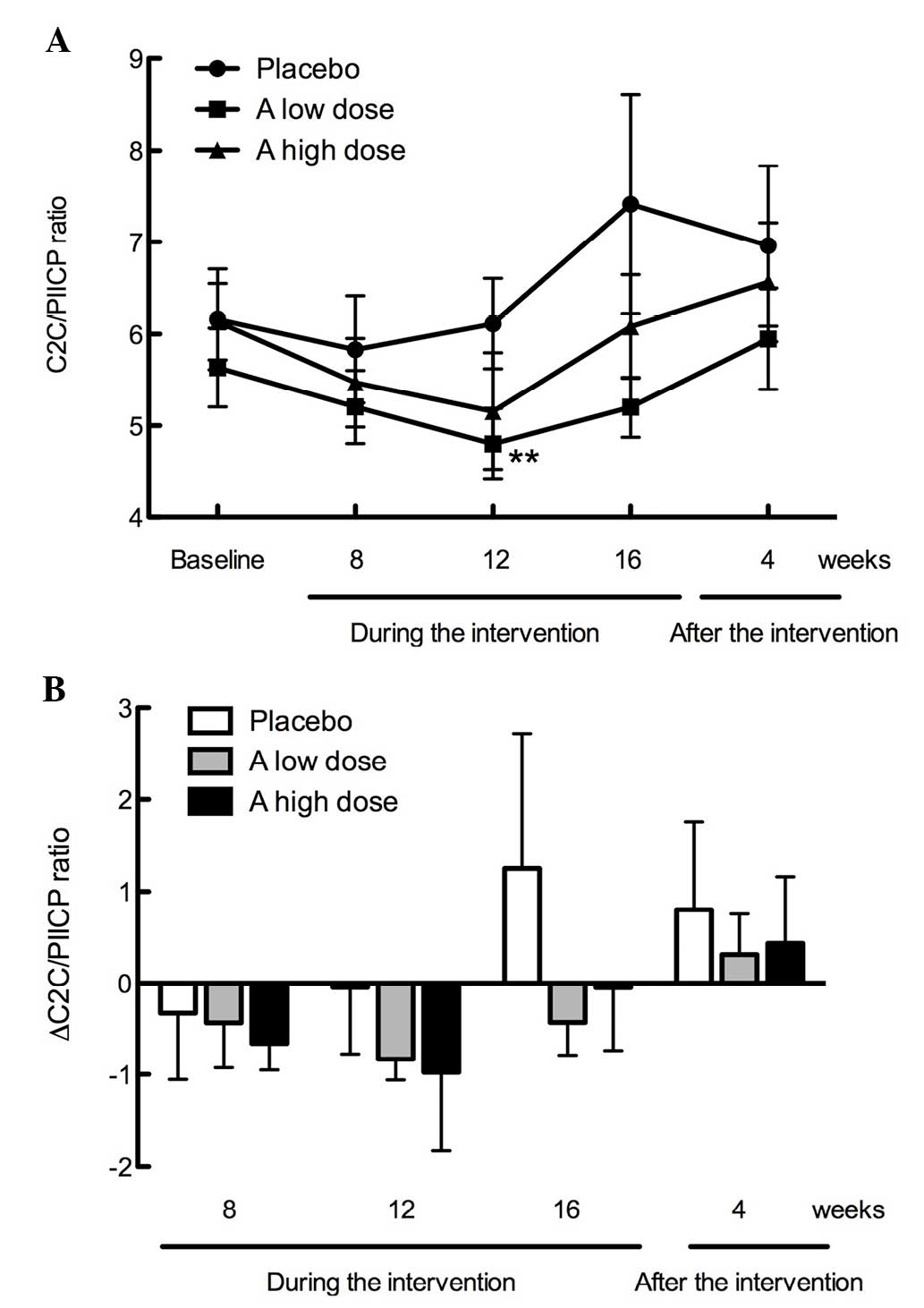
As demonstrated in Fig.
4A, the C2C/PIICP ratios were not significantly different among
the three groups during intervention (16 weeks) and after
intervention (4 weeks); however, the C2C/PIICP ratio was
significantly decreased at week 12 during the intervention in the
low dose GlcNAc group (P<0.01). As shown in Fig. 4B, the ∆C2C/PIICP ratios from the
baseline were similarly suppressed in the low and high dose GlcNAc
groups (week 12, −0.83 and −0.98; and week 16, −0.43 and −0.05,
respectively), as compared with the placebo group (week 12, −0.05
and week 16, +1.25). The ∆C2C/PIICP ratios in the test supplement
groups returned to the same levels as the placebo group 4 weeks
after intervention (Fig. 4B).
These findings suggest that the oral administration
of the test supplement with low and high doses of GlcNAc exhibited
a protective effect on cartilage metabolism in healthy individuals
without any symptoms of joint disorders, by improving the C2C/PIICP
ratio (relatively reduced type II degradation and increased type II
collagen synthesis).
Assessment of safety and
tolerability
Among the 75 subjects enrolled, seven subjects (28%)
in the low dose GlcNAc group (n=25), eight subjects (32%) in the
high dose GlcNAc group (n=25) and eight subjects in the placebo
group (n=25) experienced one or more adverse events during the
intervention period. The total number of adverse events reported
was eight in the low dose GlcNAc group, 31 in the high dose GlcNAc
group, and 18 in the placebo group, No significant differences in
the frequency of adverse events were detected among the three
groups. Adverse events reported from the study subjects
predominantly included respiratory symptoms (sore throat, cough,
rhinorrhea and/or fever), joint pain (shoulder or elbow) and neck
stiffness. All adverse events were of mild intensity and were
judged by an independent medical investigator who was blinded to
the intervention.
Furthermore, no significant differences in physical
measurement parameters (body weight and body mass index),
physiological examinations (systolic and diastolic blood pressures,
and pulse rate) and laboratory tests (urinalysis, hematology and
blood chemistry) were detected between the baseline and during and
after the intervention in the three groups.
Discussion
Accumulating evidence indicates that biomarkers for
cartilage metabolism, particularly type II collagen metabolism, can
be used to screen for individuals at risk of progressive joint
destruction, and for monitoring the effects of structure-modifying
agents or therapies on osteoarthritis (8). For example, previous studies have
demonstrated the use of type II collagen degradation biomarkers,
including CTX-II and C2C, to evaluate the effects of
chondroprotective agents such as glucosamine (32,33) and
chondroitin sulfate (34).
Subsequently, type II collagen synthesis biomarkers, such as CPII
(PIICP), have been used alone or in combination with type II
collagen degradation biomarkers (CTX-II and C2C) to monitor the
disease state and progression of osteoarthritis, since the ratio of
type II collagen degradation to synthesis has been demonstrated to
be more effective than measuring a single biomarker for monitoring
the effect of chondroprotective agents (30,31).
Based on these findings, in the present study, in order to evaluate
the effect of GlcNAc on joint health of healthy individuals without
symptoms of arthritis, a randomized double-blind placebo-controlled
clinical trial was performed to investigate the effect of oral
GlcNAc administration (low dose, 500 mg/day and high dose, 1,000
mg/day) on cartilage metabolism in healthy middle-aged adults (mean
age, 48.6±1.3 years) by analyzing the ratio of type II collagen
degradation to synthesis using type II collagen degradation (C2C)
and synthesis (PIICP) markers.
The results indicated that the changes in the
C2C/PIICP ratios from the baseline were slightly suppressed in the
low and high dose GlcNAc groups (+0.81 and +0.97, respectively), as
compared with the placebo group (+1.31) at week 16 during
intervention. The ∆C2C/PIICP ratios in the test supplement groups
returned to the same level as the placebo group 4 weeks after
intervention. To further elucidate the effect of the
GlcNAc-containing test supplement, subjects with impaired cartilage
metabolism (≥220 ng/ml C2C and <60 ng/ml PIICP) were evaluated.
Notably, the changes in the C2C/PIICP ratios from the baseline were
markedly suppressed in the low and high dose GlcNAc groups (week
12, −0.79 and −0.47; week 16, −0.39 and +0.63, respectively), as
compared with the placebo group (week 12, −0.05 and week 16,
+1.25). The ∆C2C/PIICP ratios in the test supplement groups
returned to the same levels as the placebo group 4 weeks after
intervention. Finally, to exclude the effect of heavy body weight
on joint loading, subjects weighing <70 kg with ≥220 ng/ml C2C
and <60 ng/ml PIICP were analyzed. Notably, the changes in the
C2C/PIICP ratios from the baseline were markedly suppressed in the
low and high dose GlcNAc groups (week 12, −0.83 and −0.98; week 16,
−0.43 and −0.05, respectively), as compared with the placebo group
(week 12, −0.05 and week 16, +1.25), and the ∆C2C/PIICP ratios in
the test supplement groups returned to the same level as the
placebo group 4 weeks after intervention. Moreover, no test
supplement-related adverse events were observed during or after the
intervention. Together, these observations suggest that oral
administration of GlcNAc at doses of 500 mg and 1,000 mg/day
induces a chondroprotective effect on the healthy individuals
without any apparent adverse effect, by lowering the C2C/PIICP
ratio (relatively reducing type II degradation and increasing type
II collagen synthesis) and improving cartilage metabolism. However,
this effect is reversible and disappears after withdrawal of the
administration.
The mechanism by which the GlcNAc-containing test
supplement exerts a protective effect on the cartilage metabolism
remains to be clarified. In this context, it is interesting to note
that GlcNAc stimulates hyaluronan synthesis via the upregulation of
hyaluronan synthase-2 in chondrocytes (24). Hyaluronan is reported to inhibit
IL-1β-induced MMP-13 expression via its principal receptor, CD44,
and subsequent signaling of p38 mitogen-activated protein kinase
(MAPK) in arthritic chondrocytes (35). In addition, hyaluronan suppresses
aggrecan degradation by downregulating IL-1α-induced expression a
disintegrin-and metalloproteinase with thrombospondin motifs
(ADAMTS)-4, which is an aggrecanase, through the CD44 signaling in
osteoarthritic chondrocytes (36).
Hyaluronan also suppresses the IL-1β-induced expression of MMP-3,
MMP-13, ADAMTS-4 and ADAMTS-5 in osteoblasts (37). Notably, GlcNAc inhibits the
IL-1β-mediated expression of inducible NO synthase,
cyclooxygenase-2 and IL-6 via the inhibition of MAPKs including
c-jun N-terminal kinase, extracellular signal-related kinase and
p38MAPK activation in chondrocytes (25). Therefore, GlcNAc may improve
cartilage metabolism by reducing the C2C/PIICP ratio (relatively
reducing type II collagen degradation and increasing type II
collagen synthesis) due to its chondroprotective and
antiinflammatory effects based on the suppression of cartilage
degrading enzymes, such as MMPs and ADAMTSs, potentially via the
production of hyaluronan. However, a detailed mechanism outlining
how GlcNAc effects cartilage metabolism via type II collagen
degradation and synthesis remains to be elucidated.
The present study had a limitation. The number of
subjects enrolled in study was small; thus, it was difficult to
detect significant differences among the three groups, particularly
when subjects with impaired cartilage metabolism were selected and
analyzed. In future studies, the number of subjects enrolled should
be increased when demonstrating the potential of a test supplement
to improve cartilage metabolism in healthy individuals.
To the best of our knowledge, this study was first
to evaluate the effect of oral GlcNAc administration on cartilage
metabolism in healthy individuals. However, it has previously been
demonstrated that intra-articular injection of GlcNAc exhibits
chondroprotective effects on experimental osteoarthritis models
(26,27), and the administration of a
GlcNAc-containing beverage improved the symptoms of patients with
knee osteoarthritis in a previous study, possibly by relatively
increasing type II collagen synthesis and reducing the ratio of
CTX-II/CPII (28). The efficacy and
safety of GlcNAc demonstrated in the present study indicates that
this GlcNAc-containing supplement can be safely administered, as it
potently exerts a chondroprotective effect on healthy individuals
by improving the type II collagen metabolism in the cartilage
without any major adverse effects. Therefore, GlcNAc-containing
supplements may be a potential candidate for improved joint health
in healthy individuals without arthritic symptoms.
Acknowledgements
The authors would like to thank Mr. Takashi
Nakagawa, Ms. Kaori Yoshimura and Dr Tetsuro Yamamoto (Total
Technological Consultant Co., Ltd., Tokyo, Japan) for their helpful
discussion and statistical expertise in the preparation of this
manuscript.
References
|
1
|
Ravenda V, Manette C, Lemmens R, Mariani
AM, Struvay N and Reginster JY: Prevalence and impact of
osteoarthritis and osteoporosis on health-related quality of life
among active subjects. Aging Clin Exp Res. 19:55–60. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jinks C, Jordan K and Croft P:
Osteoarthritis as a public health problem: The impact of developing
knee pain on physical function in adults living in the community:
(KNEST 3). Rheumatology (Oxford). 46:877–881. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshimura N, Muraki S, Oka H, Mabuchi A,
En-Yo Y, Yoshida M, Saika A, Yoshida H, Suzuki T, Yamamoto S, et
al: Prevalence of knee osteoarthritis, lumbar spondylosis and
osteoporosis in Japanese men and women: The research on
osteoarthritis/osteoporosis against disability study. J Bone Miner
Metab. 27:620–628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qi C and Changlin H: Effects of moving
training on histology and biomarkers levels of articular cartilage.
J Surg Res. 135:352–363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garnero P, Rousseau JC and Delmas PD:
Molecular basis and clinical use of biochemical markers of bone,
cartilage and synovium in joint diseases. Arthritis Rheum.
43:953–968. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garnero P, Piperno M, Gineyts E, Christgau
S, Delmas PD and Vignon E: Cross sectional evaluation of
biochemical markers of bone, cartilage and synovial tissue
metabolism in patients with knee osteoarthritis: Relations with
disease activity and joint damage. Ann Rheum Dis. 60:619–626. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Poole AR: Biochemical/immunochemical
biomarkers of osteoarthritis: Utility for prediction of incident or
progressive osteoarthritis. Rheum Dis Clin North Am. 29:803–818.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rousseau JC and Delmas PD: Biological
markers in osteoarthritis. Nat Clin Pract Rheumatol. 3:346–356.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garnero P and Delmas PD: Biomarkers in
osteoarthritis. Curr Opin Rheumatol. 15:641–646. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Christgau S, Garnero P, Fledelius C, Moniz
C, Ensig M, Gineyts E, Rosenquist C and Qvist P: Collagen type II
C-telopeptide fragments as an index of cartilage degradation. Bone.
29:209–215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Poole AR, Ionescu M, Fitzcharles MA and
Billinghurst RC: The assessment of cartilage degradation in vivo:
Development of an immunoassay for the measurement in body fluids of
type II collagen cleaved by collagenases. J Immunol Methods.
294:145–153. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shinmei M, Ito K, Matsuyama S, Yoshihara Y
and Matsuzawa K: Joint fluid carboxy-terminal type II procollagen
peptide as a marker of cartilage collagen biosynthesis.
Osteoarthritis Cartilage. 1:121–128. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwenk TL and Costley CD: When food
becomes a drug: Nonanabolic nutritional supplement use in athletes.
Am J Sports Med. 30:907–916. 2002.PubMed/NCBI
|
|
14
|
Gorsline RT and Kaeding CC: The use of
NSAIDs and nutritional supplements in athletes with osteoarthritis:
Prevalence, benefits and consequences. Clin Sports Med. 24:71–82.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ostojic SM, Arsic M, Prodanovic S, Vukovic
J and Zlatanovic M: Glucosamine administration in athletes: Effects
on recovery of acute knee injury. Res Sports Med. 15:113–124. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fenton JI, Chlebek-Brown KA, Peters TL,
Caron JP and Orth MW: Glucosamine HCl reduces equine articular
cartilage degradation in explant culture. Osteoarthritis Cartilage.
8:258–265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gouze JN, Bordji K, Gulberti S, Terlain B,
Netter P, Magdalou J, Fournel-Gigleux S and Ouzzine M:
Interleukin-1beta downregulates the expression of
glucuronosyltransferase I, a key enzyme priming glycosaminoglycan
biosynthesis: Influence of glucosamine on
interleukin-1beta-mediated effects in rat chondrocytes. Arthritis
Rheum. 44:351–360. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura H, Shibakawa A, Tanaka M, Kato T
and Nishioka K: Effects of glucosamine hydrochloride on the
production of prostaglandin E2, nitric oxide and metalloproteases
by chondrocytes and synoviocytes in osteoarthritis. Clin Exp
Rheumatol. 22:293–299. 2004.PubMed/NCBI
|
|
19
|
Derfoul A, Miyoshi AD, Freeman DE and Tuan
RS: Glucosamine promotes chondrogenic phenotype in both
chondrocytes and mesenchymal stem cells and inhibits MMP-13
expression and matrix degradation. Osteoarthritis Cartilage.
15:646–655. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McAlindon TE, Lavalley MP, Gulin JP and
Felson DT: Glucosamine and chondroitin for treatment of
osteoarthritis: A systematic quality assessment and meta-analysis.
JAMA. 283:1469–1475. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reginster JY, Deroisy R, Rovati LC, Lee
RL, Lejeune E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE and
Gossett C: Long-term effects of glucosamine sulphate on
osteoarthritis progression: A randomized, placebo-controlled
clinical trial. Lancet. 357:251–256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pavelká K, Gatterová J, Olejarová M,
Machacek S, Giacovelli G and Rovati LC: Glucosamine sulfate use and
delay of progression of knee osteoarthritis: A 3-year, randomized,
placebo-controlled, double-blind study. Arch Intern Med.
162:2113–2123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Momomura R, Naito K, Igarashi M, Watari T,
Terakado A, Oike S, Sakamoto K, Nagaoka I and Kaneko K: Evaluation
of the effect of glucosamine administration on biomarkers of
cartilage and bone metabolism in bicycle racer. Mol Med Report.
7:742–746. 2013.
|
|
24
|
Shikhman AR, Brinson DC, Valbracht J and
Lotz MK: Differential metabolic effects of glucosamine and
N-acetylglucosamine in human articular chondrocytes. Osteoarthritis
Cartilage. 17:1022–1028. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shikhman AR, Kuhn K, Alaaeddine N and Lotz
M: N-acetylglucosamine prevents IL-1beta-mediated activation of
human chondrocytes. J Immunol. 166:5155–5160. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shikhman AR, Amiel D, D'Lima D, Hwang SB,
Hu C, Xu A, Hashimoto S, Kobayashi K, Sasho T and Lotz MK:
Chondroprotective activity of N-acetylglucosamine in rabbits with
experimental osteoarthritis. Ann Rheum Dis. 64:89–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ozkan FU, Ozkan K, Ramadan S and Guven Z:
Chondroprotective effect of N-acetylglucosamine and hyaluronate in
early stages of osteoarthritis: An experimental study in rabbits.
Bull NYU Hosp Jt Dis. 67:352–357. 2009.PubMed/NCBI
|
|
28
|
Katsuno S, Sato K, Eguchi C, Yoshimura K,
Yamamoto T, Tomonaga A and Nagaoka I: Effects and safety of bilk
beverage containing N-acetyl glucosamine on knee joint pain and
biomarkers of type II collagen metabolism. Jpn Pharmacol Ther.
38:435–445. 2010.
|
|
29
|
Kellgren JH and Lawrence JS: Radiological
assessment of osteo-arthritis. Ann Rheum Dis. 16:494–502. 1957.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cahue S, Sharma L, Dunlop D, Ionescu M,
Song J, Lobanok T, King L and Poole AR: The ratio of type II
collagen breakdown to synthesis and its relationship with the
progression of knee osteoarthritis. Osteoarthr Cartilage.
15:819–823. 2007. View Article : Google Scholar
|
|
31
|
Sharif M, Kirwan J, Charni N, Sandell LJ,
Whittles C and Garnero P: A 5-yr longitudinal study of type IIA
collagen synthesis and total type II collagen degradation in
patients with knee osteoarthritis-association with disease
progression. Rheumatology (Oxford). 46:938–943. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Christgau S, Henrotin Y, Tankó LB, Rovati
LC, Collette J, Bruyere O, Deroisy R and Reginster JY:
Osteoarthritic patients with high cartilage turnover show increased
responsiveness to the cartilage protecting effects of glucosamine
sulfate. Clin Exp Rheumatol. 22:36–42. 2004.PubMed/NCBI
|
|
33
|
Cibere J, Thorne A, Kopec JA, Singer J,
Canvin J, Robinson DB, Pope J, Hong P, Grant E, Lobanok T, et al:
Glucosamine sulfate and cartilage type II collagen degradation in
patients with knee osteoarthritis: Randomized discontinuation trial
results employing biomarkers. J Rheumatol. 32:896–902.
2005.PubMed/NCBI
|
|
34
|
Mazières B, Hucher M, Zaïm M and Garnero
P: Effect of chondroitin sulfate in symptomatic knee
osteoarthritis: A multicentre, randomised, double-blind,
placebo-controlled study. Ann Rheum Dis. 66:639–645. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Julovi SM, Ito H, Nishitani K, Jackson CJ
and Nakamura T: Hyaluronan inhibits matrix metalloproteinase-13 in
human arthritic chondrocytes via CD44 and P38. J Orthop Res.
29:258–264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yatabe T, Mochizuki S, Takizawa M,
Chijiiwa M, Okada A, Kimura T, Fujita Y, Matsumoto H, Toyama Y and
Okada Y: Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1)
in human osteoarthritic chondrocytes. Ann Rheum Dis. 68:1051–1058.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mladenovic Z, Saurel AS, Berenbaum F and
Jacques C: Potential role of hyaluronic acid on bone in
osteoarthritis: Matrix metalloproteinases, aggrecanases and RANKL
expression are partially prevented by hyaluronic acid in
interleukin 1-stimulated osteoblasts. J Rheumatol. 41:945–954.
2014. View Article : Google Scholar : PubMed/NCBI
|